Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities
Abstract
1. Introduction
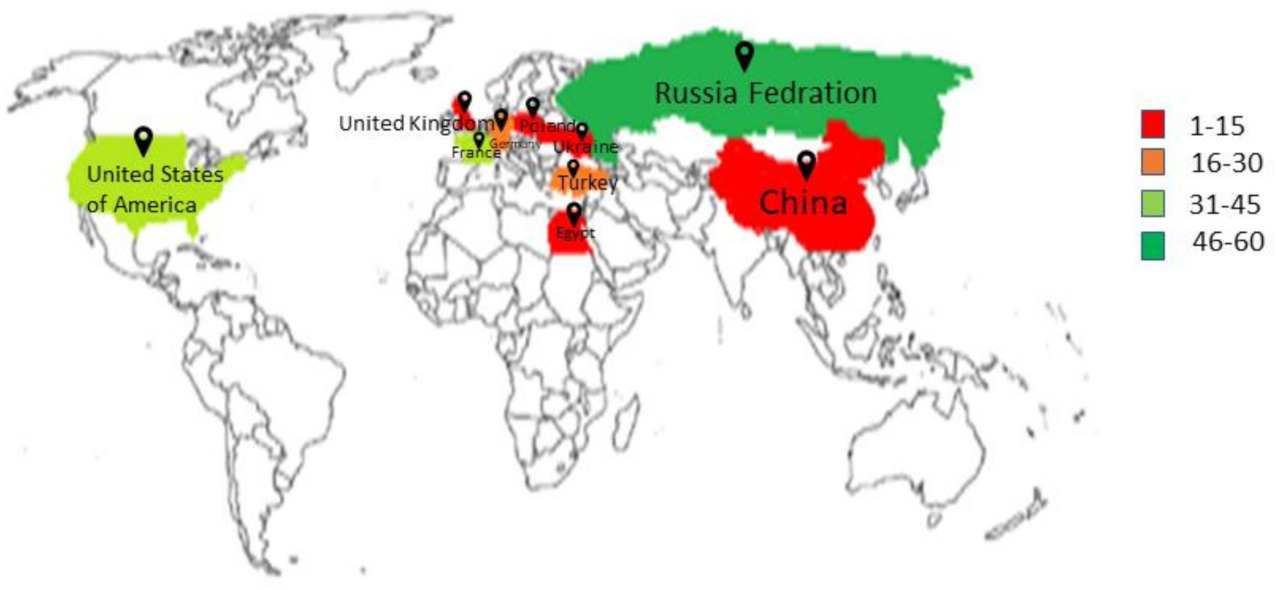
2. Major Sugar Crops Worldwide
3. Sugar Beet Cultivation in the Tropics and Subtropics
3.1. Nutrient Management in Sugar Beet
3.2. Major Pest and Diseases
3.2.1. Curly Top
3.2.2. Rhizomania
3.2.3. Cercospora Leaf Spot
3.2.4. Beet Cyst Nematode
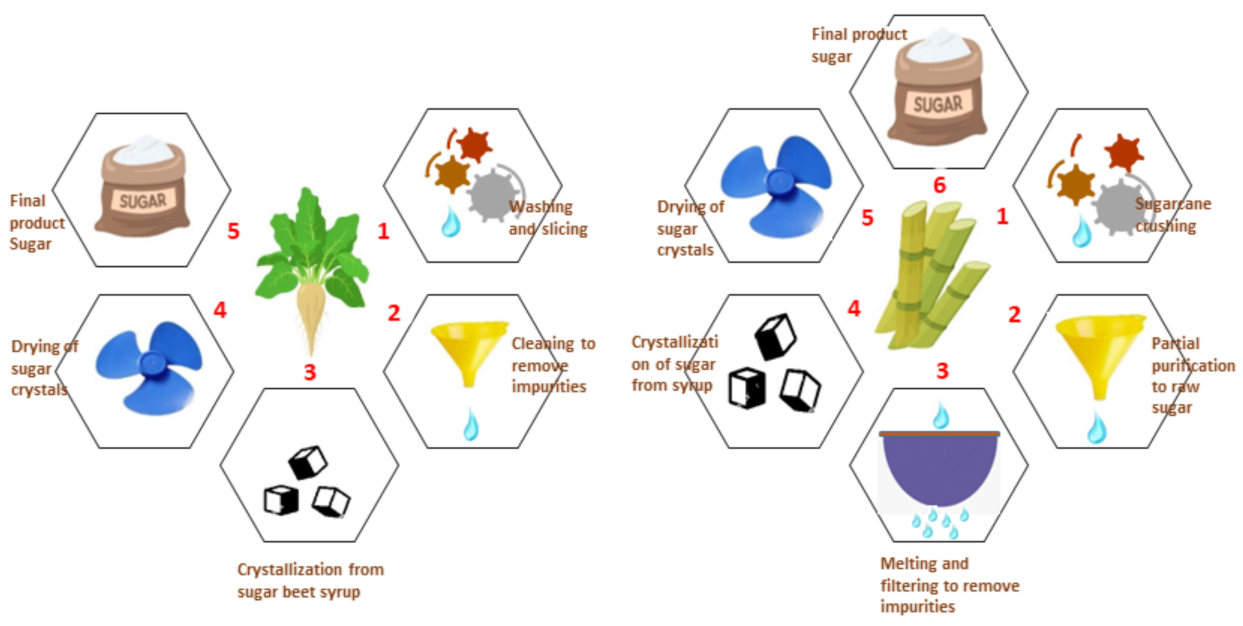
3.3. Harvesting and Processing
3.4. Yield and Quality
4. Challenges of Sugar Beet Cultivation in the Tropics and Subtropics
4.1. Bolting Concerns
4.2. Lack of Farm Mechanization
4.3. Increased Processing Cost
4.4. Marketing
5. Perspectives of Sugar Beet Production in Tropics and Subtropics
5.1. Low Soil Fertility
5.2. Water Scarcity
5.3. Salt-Affected Soils
5.4. Use of Soil for Additional Crops Compared to the Production Technology of Sugarcane
6. Conclusions and Future Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- James, G.; Tate, B. An introduction to sugarcane. In Sugarcane, 2nd ed.; James, G., Ed.; Blackwell Science: Oxford, UK, 2004; pp. 1–19. [Google Scholar]
- Zicari, S.; Zhang, R.; Kaffka, S. Sugar beet. In Integrated Processing Technologies for Food and Agricultural By-Products; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 331–351. [Google Scholar]
- Duraisam, R.; Salelgn, K.; Berekete, A.K. Production of beet sugar and bioethanol from sugar beet and it bagasse: A review. Int. J. Eng. Trends Technol. 2017, 43, 222–233. [Google Scholar] [CrossRef]
- Sánchez-Sastre, L.F.; Alte da Veiga, N.M.; Ruiz-Potosme, N.M.; Hernández-Navarro, S.; Marcos-Robles, J.L.; Martín-Gil, J.; Martín-Ramos, P. Sugar beet agronomic performance evolution in NW Spain in future scenarios of climate change. Agronomy 2020, 10, 91. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Huijbregts, T.; van Swaaij, N.; Jansen, R. Impact of different environments in Europe on yield and quality of sugar beet genotypes. Eur. J. Agron. 2009, 30, 7–26. [Google Scholar] [CrossRef]
- Wimmer, S.; Sauer, J. Profitability Development and Resource Reallocation: The Case of Sugar Beet Farming in Germany. J. Agric. Econ. 2020, 71, 816–837. [Google Scholar] [CrossRef]
- Bahrami, E.M.; Honarvar, M.; Ansari, K.; Jamshidi, B. Measurement of quality parameters of sugar beet juices using near-infrared spectroscopy and chemometrics. J. Food Eng. 2020, 271, 109775. [Google Scholar] [CrossRef]
- Mioduszewska, N.; Pilarska, A.A.; Pilarski, K.; Adamski, M. The influence of the process of sugar beet storage on its biochemical methane potential. Energies 2020, 13, 5104. [Google Scholar] [CrossRef]
- Saldivar, S.O.S. Maize: Foods from Maize. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bareja, B. List of Sugar Crops, Sweeteners Distinguished. Crops Review. 2022. Available online: https://www.cropsreview.com/sugar-crops/ (accessed on 11 April 2023).
- Srikaeo, K.; Sangkhiaw, J.; Likittrakulwong, W. Productions and functional properties of palm sugars. Walailak J. Sci. Technol. 2019, 16, 897–907. [Google Scholar] [CrossRef]
- Naoura, G.; Emendack, Y.; Nébié, B.; Kirsten, V.B.; Mahamat, A.H.; Nerbewende, S.; Amos, D.N.; Reoungal, D.; Gilles, T.; Haydee, E.L. Characterization of semi-arid Chadian sweet sorghum accessions as potential sources for sugar and ethanol production. Sci. Rep. 2020, 10, 14974. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mohdaly, A.A.A.; Assiri, A.M.; Tadros, M.; Niemeyer, B. Functional characteristics, nutritional value and industrial applications of Madhuca longifolia seeds: An overview. Int. J. Food Sci. Technol. 2016, 53, 2149–2157. [Google Scholar] [CrossRef]
- Pielak, M.; Czarniecka-Skubina, E.; Głuchowski, A. Effect of sugar substitution with steviol glycosides on sensory quality and physicochemical composition of low-sugar apple preserves. Foods 2020, 9, 293. [Google Scholar] [CrossRef]
- Abbasi, Z.; Golabadi, M.; Khayamim, S.; Pessarakli, M. The response of drought-tolerant sugar beet to salinity stress under field and controlled environmental conditions. J. Plant Nutr. 2018, 41, 2660–2672. [Google Scholar] [CrossRef]
- Farooq, N.; Gheewala, S.H. Water use and deprivation potential for sugarcane cultivation in Pakistan. J. Sustain. Energy Environ. 2019, 10, 33–93. [Google Scholar]
- Saclain, S.; Latif, A.; Bala, B.; Mallik, M.; Islam, S. Genetic diversity analysis of tropical sugar beet (Beta vulgaris L.) varieties in Bangladesh using RAPD markers. Genetika 2016, 48, 151–164. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Saleem, A.M. Sugar beet potential to beat sugarcane as a sugar crop in Pakistan. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 36–44. [Google Scholar]
- Statista. 2023. Available online: https://www.statista.com/statistics/264670/top-sugar-beet-producers-worldwide-by-volume/ (accessed on 15 April 2023).
- Brar, N.S.; Dhillon, B.S.; Saini, K.S.; Sharma, P.K. Agronomy of sugar beet cultivation-A review. Agric. Rev. 2015, 36, 184–197. [Google Scholar] [CrossRef]
- Kaloi, M.G.; Mari, A.H.; Bughio, N.; Junejo, S.; Panhwar, R.N.; Bhutto, M.A.; Rajput, M.A.; Arain, S. Growth assessment of exotıc sugar beet varieties in southern-zone of Sindh. Pak. Sugar J. 2019, 34, 33–40. [Google Scholar] [CrossRef]
- Pathak, A.D.; Kapur, R.; Solomon, S.; Kumar, R.; Srivastava, S.; Singh, P.R. Sugar beet: A historical perspective in Indian context. Sugar Technol. 2014, 16, 125–132. [Google Scholar] [CrossRef]
- Baryga, A.; Połeć, B.; Klasa, A. Quality of sugar beets under the effects of digestate application to the soil. Processes 2020, 8, 1402. [Google Scholar] [CrossRef]
- Ahmad, S.; Zubair, M.; Iqbal, N.; Cheema, N.M.; Mahmood, K. Evaluation of sugar beet hybrid varieties under Thal-Kumbi soil series of Pakistan. Int. J. Agric. Biol. 2012, 14, 605–608. [Google Scholar]
- Soleymani, A.; Shahrajabian, M.H. Effects of planting dates and row distance on sugar content, root yield and solar radiation absorption in sugar beet at different plant densities. Romanian Agric. Res. 2017, 34, 145–155. [Google Scholar]
- Pavlů, K.; Chochola, J.; Pulkrábek, J.; Urban, J. Influence of sowing and harvest dates on production of two different cultivars of sugar beet. Plant Soil Environ. 2017, 63, 76–81. [Google Scholar] [CrossRef]
- Zarski, J.; Kuśmierek-Tomaszewska, R.; Dudek, S. Impact of irrigation and fertigation on the yield and quality of sugar beet (Beta vulgaris L.) in a moderate climate. Agronomy 2020, 10, 166. [Google Scholar] [CrossRef]
- Leilah, A.A.; Khan, N. Interactive Effects of Gibberellic Acid and Nitrogen Fertilization on the Growth, Yield, and Quality of Sugar Beet. Agronomy 2021, 11, 137. [Google Scholar] [CrossRef]
- Heidarian, F.; Rokhzadi, A.; Mirahmadi, F. Response of sugar beet to irrigation interval, harvesting time and integrated use of farmyard manure and nitrogen fertilizer. Environ. Exp. Biol. 2018, 16, 169–175. [Google Scholar]
- Afshar, R.K.; Nilahyane, A.; Chen, C.; He, H.; Bart Stevens, W.B.; William, M.I. Impact of conservation tillage and nitrogen on sugarbeet yield and quality. Soil Tillage Res. 2019, 191, 216–223. [Google Scholar] [CrossRef]
- Ghaly, F.; Abd-Hady, M.; Abd-Elhamied, M. Effect of varieties, phosphorus and boron fertilization on sugar beet yield and its quality. J. Soil Sci. Agric. Eng. 2019, 10, 115–122. [Google Scholar] [CrossRef]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The effects of potassium fertilization on water-use efficiency in crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Anabestani, A.; Behjatnia, S.A.A.; Izadpanah, K.; Tabein, S.; Accotto, G.P. Seed transmission of beet curly top virus and beet curly top Iran virus in a local cultivar of petunia in Iran. Viruses 2017, 9, 299. [Google Scholar] [CrossRef]
- Nansen, C.; Stewart, A.N.; Gutierrez, T.A.M.; Wintermantel, W.M.; McRoberts, N.; Gilbertson, R.L. Proximal remote sensing to differentiate nonviruliferous and viruliferous insect vectors–proof of concept and importance of input data robustness. Plant Pathol. 2019, 68, 746–754. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, A.; Ahmad, M.; Ullah, N.; Afridi, U.K.; Bostan, N.; Qureshi, R.; Tawwab, S.; Ahmad, I.; Zubair, M. Characterization of beet necrotic yellow vein virus in Pakistan. J. Plant Pathol. 2018, 100, 357. [Google Scholar] [CrossRef]
- Zare, B.; Niazi, A.; Sattari, R.; Aghelpasand, H.; Zamani, K.; Sabet, M.S.; Moshiri, F.; Darabie, S.; Daneshvar, M.H.; Norouzi, P. Resistance against rhizomania disease via RNA silencing in sugar beet. Plant Pathol. 2015, 64, 35–42. [Google Scholar] [CrossRef]
- Weiland, J.J.; Poudel, R.S.; Flobinus, A.; Cook, D.E.; Secor, G.A.; Bolton, M.D. RNAseq Analysis of Rhizomania-Infected Sugar Beet Provides the First Genome Sequence of Beet Necrotic Yellow Vein Virus from the USA and Identifies a Novel Alphanecrovirus and Putative Satellite Viruses. Viruses 2020, 12, 626. [Google Scholar] [CrossRef]
- Jay, S.; Comar, A.; Benicio, R.; Beauvois, J.; Dutartre, D.; Daubige, G.; Li, W.; Labrosse, J.; Thomas, S.; Henry, N. Scoring cercospora leaf spot on sugar beet: Comparison of UGV and UAV phenotyping systems. Plant Phenom. 2020, 9452123, 18. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Kenter, C.; Holst, C.; Märländer, B. New generation of resistant sugar beet varieties for advanced integrated management of Cercospora leaf spot in central Europe. Front. Plant Sci. 2018, 9, 222. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Vaghefi, N.; Kikkert, J.R. Management of Cercospora leaf spot in conventional and organic table beet production. Plant Dis. 2017, 101, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.; Leucker, M.; Steiner, U. Sensory assessment of Cercospora beticola sporulation for phenotyping the partial disease resistance of sugar beet genotypes. Plant Methods 2019, 15, 133, 1–12. [Google Scholar] [CrossRef]
- Escobar-Avila, I.M.; Cruz-Alvarado, Y.; Tovar-Soto, A.; Subbotin, S.A. First report of sugar beet cyst nematode, Heterodera schachtii on beet root and broccoli in Mexico. Plant Dis. 2019, 103, 1434. [Google Scholar] [CrossRef]
- Ghaemi, R.; Pourjam, E.; Safaie, N.; Verstraeten, B.; Mahmoudi, S.B.; Mehrabi, R.; Meyer, T.D.; Kyndt, T. Molecular insights into the compatible and incompatible interactions between sugar beet and the beet cyst nematode. BMC Plant Biol. 2020, 20, 483, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hemayati, S.S.; Akbar, M.R.J.E.; Ghaemi, A.R.; Fasahat, P. Efficiency of white mustard and oilseed radish trap plants against sugar beet cyst nematode. Appl. Soil Ecol. 2017, 119, 192–196. [Google Scholar] [CrossRef]
- Nuaima, H.R.; Ashrafi, S.; Maier, W.; Heuer, H. Fungi isolated from cysts of the beet cyst nematode parasitized its eggs and counterbalanced root damages. J. Pest Sci. 2021, 94, 563–572. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Bieliński, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevsky, A.; Witońska, I.A. Products of sugar beet processing as raw materials for chemicals and biodegradable polymers. RSC Adv. 2018, 8, 3161–3177. [Google Scholar] [CrossRef]
- Curcic, Z.; Ciric, M.; Nagl, N.; Taski-Ajdukovic, K. Effect of sugar beet genotype, planting and harvesting dates and their interaction on sugar yield. Front. Plant Sci. 2018, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.C.; Christine, K. Yield potential of sugar beet–have we hit the ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef]
- Panella, L.; Kaffka, S.R.; Lewellen, R.T.; McGrath, J.M.; Metzger, M.S.; Strausbaugh, C.A. Sugar beet. In Yield Gains in Major U.S. Field Crops; Smith, S., Diers, B., Specht, J., Carver, B., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 33, pp. 357–395. [Google Scholar]
- Kaffka, S.R.; Grantz, D.A. Sugar Crops. In Encyclopedia of Agriculture and Food Systems; Alfen, N.K.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 240–260. [Google Scholar]
- Nilahyane, A.; Chen, C.; Afshar, R.K.; Sutradhar, A.; Stevens, W.B.; William, I.W. Deficit irrigation for sugar beet under conventional and no-till production. Agro. Geosci. Environ. 2020, 3, e20114. [Google Scholar]
- Wakeel, A.; Steffens, D.; Schubert, S. Potassium substitution by sodium in sugar beet (Beta vulgaris) nutrition on K-fixing soils. J. Plant Nutr. Soil Sci. 2010, 173, 127–134. [Google Scholar] [CrossRef]
- Pi, Z.; Stevanato, P.; Yv, L.H.; Geng, G.; Guo, X.L.; Yang, Y.; Peng, C.X.; Kong, X.S. Effects of potassium deficiency and replacement of potassium by sodium on sugar beet plants. Russian J. Plant Physiol. 2014, 61, 224–230. [Google Scholar] [CrossRef]
- Hlisnikovský, L.; Menšík, L.; Křížová, K.; Kunzová, E. The effect of farmyard manure and mineral fertilizers on sugar beet beetroot and top yield and soil chemical parameters. Agronomy 2021, 11, 133. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.S.; Aiming, Q.I.; Zhang, W.; Schulze-Buxloh, G.; Jennings, A.; Hohmann, U.; Müller, A.E.; Hedden, P. Bolting and flowering control in sugar beet: Relationships and effects of gibberellin, the bolting gene B and vernalization. AoB Plants 2010, 2010, plq012. [Google Scholar] [CrossRef] [PubMed]
- Draycott, A.P.; Christenson, D.R. Soil physical conditions. In Nutrients for Sugar Beet Production: Soil-Plant Relationships; CABI Publishing: Wallingford, UK, 2003; pp. 139–152. [Google Scholar]
- Kristjanson, P.; Okike, I.; Tarawali, S.; Singh, B.B.; Manyong, V.M. Farmers’ perceptions of benefits and factors affecting the adoption of improved dual-purpose cowpea in the dry savannas of Nigeria. Agric. Econom. 2005, 32, 195–210. [Google Scholar] [CrossRef]
- Kramer, E. US Sugar Beet Price Analysis. Research Papers. Master’s Thesis, Southern Illinois University Carbondale, Carbondale, IL, USA, 2016; p. 39. [Google Scholar]
- Ishfaq, M.; Kiran, A.; Ur Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhong, Y.; Wang, Y.; Li, X. Magnesium limitation leads to transcriptional down-tuning of auxin synthesis, transport, and signaling in the tomato root. Front. Plant Sci. 2021, 12, 802399. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Kiran, A.; Wakeel, A.; Tayyab, M.; Li, X. Foliar-applied potassium triggers soil potassium uptake by improving growth and photosynthetic activity of wheat and maize. J. Plant Nutr. 2022. [Google Scholar] [CrossRef]
- Wakeel, A.; Ishfaq, M. Promoting precise and balanced use of fertilizers in Pakistan at farm-gate level. Electron. Int. Fertil. Corresp. 2016, 47, 20–25. [Google Scholar]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of china. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jian-Zhou, C.; Hong-Yang, Z. Comprehensive Evaluation of Different Sugar Beet Varieties by Using Principal Component and Cluster Analyses. J. Phys. Conf. Ser. 2019, 1176, 42021. [Google Scholar] [CrossRef]
- Sidra, M.; Nazir, M.; Zaman, S.B.; Farooq, W. Potential of sugar beet production in Pakistan: A Review. Pak. J. Agric. Res. 2016, 29, 202–211. [Google Scholar]
- Ganapati, R.K.; Rehman, M.M.; Kabiraj, R.C.; Sen, R.; Islam, M.S. Screening of water stress tolerant sugar beet (Beta vulgaris L.) genotypes. Int. J. Sustain. Crop Prod. 2016, 11, 29–35. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Jha, R.; Parida, S.K. Salinity stress response and ‘omics’ approaches for improving salinity stress tolerance in major grain legumes. Plant Cell Rep. 2019, 38, 255–277. [Google Scholar] [CrossRef]
- UN. 2019. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed on 5 April 2023).
- Zhang, J.L.; Shi, H. Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Sadughi, M.; Sharifan, H.; Pessarakli, M. Effects of caspian sea water on sugar beet seed germination. J. Plant Nutr. 2015, 38, 1685–1693. [Google Scholar] [CrossRef]
- Khayamim, S.; Tavkol, A.R.; Sadeghian, S.Y.; Poustini, K.; Roozbeh, F.; Abbasi, Z. Seed germination, plant establishment, and yield of sugar beet genotypes under salinity stress. J. Agric. Sci. Technol. 2014, 16, 779–790. [Google Scholar]
- Wakeel, A.; Sümer, A.; Hanstein, S.; Yan, F.; Schubert, S. In vitro effect of different Na+/K+ ratios on plasma membrane H+-ATPase activity in maize and sugar beet shoot. Plant Physiol. Biochem. 2011, 49, 341–345. [Google Scholar] [CrossRef]
- Lv, X.; Chen, S.; Wang, Y. Advances in understanding the physiological and molecular responses of sugar beet to salt stress. Front. Plant Sci. 2019, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Redox-Dependent Regulation, Redox Control and Oxidative Damage in Plant Cells Subjected to Abiotic Stress. In Plant Stress Tolerance; Methods in Molecular Biology; Sunkar, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 639, pp. 57–70. [Google Scholar] [CrossRef]
- Rashid, A.; Rafique, E. Boron Deficiency in Cotton Grown in Calcareous Soils of Pakistan. In Boron in Plant and Animal Nutrition; Heiner, E., Goldbach, B.R.M.A., Wimmer, P.H., Brown, M.T., Richard, W.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 357–362. [Google Scholar]
- Rashid, A. Boron Deficiency in Soils and Crops in Pakistan: Diagnosis and Management; Pakistan Agricultural Research Council: Islamabad, Pakistan, 2006.
- Rashid, A.; Rafique, E. Boron deficiency diagnosis and management in field crops in calcareous soils of Pakistan: A mini review. J. Boron. 2017, 2, 142–152. [Google Scholar]
- Wu, Z.; Wang, X.; Song, B.; Zhao, X.; Du, J.; Huang, W. Responses of Photosynthetic Performance of Sugar Beet Varieties to Foliar Boron Spraying. Sugar Technol. 2021, 23, 1332–1339. [Google Scholar] [CrossRef]
- Tayyab, M.; Wakeel, A.; Sanaullah, M.; Zahir, M.; Mubarak, M.U.; Ijaz, M.; Ishfaq, M. Foliar application of boron enhances sugar beet yield and industrial sugar content by promoting indigenous soil-boron uptake. Pak. J. Bot. 2022, 55, 1295–1303. [Google Scholar] [CrossRef]
- Abido, W.A.E. Sugar beet productivity as affected by foliar spraying with methanol and boron. Int. J. Agric. 2012, 4, 287. [Google Scholar]
- Aslam, M.; Mahmood, I.H.; Qureshi, R.H.; Nawaz, S.; Akhtar, J. Salinity tolerance of rice as affected by boron nutrition. Pak. J. Soil Sci. 2002, 21, 110–118. [Google Scholar]
- Dong, X.; Sun, L.; Guo, J.; Lili, L.; Han, G.; Wang, B. Exogenous boron alleviates growth inhibition by NaCl stress by reducing Cl- uptake in sugar beet (Beta vulgaris). Plant Soil. 2021, 464, 423–439. [Google Scholar] [CrossRef]
- Hřivna, L.; Joany, K.H.; Machálková, L.; Burešová, I.; Sapáková, E.; Kučerová, J.; Šottníková, V. Effect of foliar nutrition of potassium and silicon on yield and quality of sugar beet in unusual windy conditions in 2014 and 2015. Listy Cukrov. Repar. 2017, 133, 182. [Google Scholar]
- Bonilla, I.; Cadahia, C.; Carpena, O.; Hernando, V. Effects of boron on nitrogen metabolism and sugar levels of sugar beet. Plant Soil 1980, 57, 3–9. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Shaaban, A. Integrative applications of nitrogen, zinc, and boron to nutrients-deficient soil improves sugar beet productivity and technological sugar contents under semi-arid conditions. J. Plant Nutr. 2020, 43, 1935–1950. [Google Scholar] [CrossRef]
- Hauer, M.; Koch, H.J.; Krüssel, S.; Mittler, S.; Märländer, B. Integrated control of Heterodera schachtii Schmidt in Central Europe by trap crop cultivation, sugar beet variety choice and nematicide application. Appl. Soil Ecol. 2016, 99, 62–69. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Heck, R.J.; Bill-Deen, B. Long-term rotation and tillage effects on soil structure and crop yield. Soil Tillage Res. 2013, 127, 85–91. [Google Scholar] [CrossRef]
- Bennett, J.A.; Gary, D.; Bending, G.D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, D.C.; Bradley, K.W. Carryover of common corn and soybean herbicides to various cover crop species. Weed Technol. 2017, 31, 21–31. [Google Scholar] [CrossRef]
- EUROSTAT. Statistics of Crop Production. 2020. Available online: http://ec.europa.eu/eurostat/web/agriculture/data/database (accessed on 18 April 2023).
- Trimpler, K.; Stockfisch, N.; Märländer, B. Efficiency in sugar beet cultivation related to field history. Eur. J. Agron. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- McQuilken, M.P.; Whipps, J.M.; Cooke, R.C. Control of damping-off in cress and sugar-beet by commercial seed-coating with Pythium Oligandrum. Plant Pathol. 1990, 39, 452–462. [Google Scholar] [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Wakeel, A.; Ishfaq, M. Potash Use and Dynamics in Agriculture; Springer: Singapore, 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayyab, M.; Wakeel, A.; Mubarak, M.U.; Artyszak, A.; Ali, S.; Hakki, E.E.; Mahmood, K.; Song, B.; Ishfaq, M. Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities. Agronomy 2023, 13, 1213. https://doi.org/10.3390/agronomy13051213
Tayyab M, Wakeel A, Mubarak MU, Artyszak A, Ali S, Hakki EE, Mahmood K, Song B, Ishfaq M. Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities. Agronomy. 2023; 13(5):1213. https://doi.org/10.3390/agronomy13051213
Chicago/Turabian StyleTayyab, Muhammad, Abdul Wakeel, Muhammad Umair Mubarak, Arkadiusz Artyszak, Sajid Ali, Erdogan Esref Hakki, Khalid Mahmood, Baiquan Song, and Muhammad Ishfaq. 2023. "Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities" Agronomy 13, no. 5: 1213. https://doi.org/10.3390/agronomy13051213
APA StyleTayyab, M., Wakeel, A., Mubarak, M. U., Artyszak, A., Ali, S., Hakki, E. E., Mahmood, K., Song, B., & Ishfaq, M. (2023). Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities. Agronomy, 13(5), 1213. https://doi.org/10.3390/agronomy13051213








