Biological Activities of Lamiaceae Species: Bio-Guided Isolation of Active Metabolites from Salvia officinalis L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Samples
2.3. Preparation of Extracts and Infusions
2.4. Chromatographic Separation and Isolation of the Active Metabolites from S. officinalis Aqueous Extract
2.5. Activity of Plant Extracts/Infusions, Fractions, and Isolated Compounds in Fibroblasts
2.5.1. Materials and Equipment
2.5.2. Cytotoxicity Assay in NIH/3T3 Fibroblasts
2.5.3. Photoprotection Assay in NIH/3T3 Fibroblasts
2.5.4. Intracellular Reactive Oxygen Species (ROS) Assay
2.6. Scratch Assay
2.7. Statistical Processing of Results
3. Results and Discussion
3.1. In Vitro Cytotoxicity, Photoprotection, and Antioxidant Activity of Plant Extracts/Infusions
3.2. Bio-Guided Investigation of S. officinalis Aqueous Extract
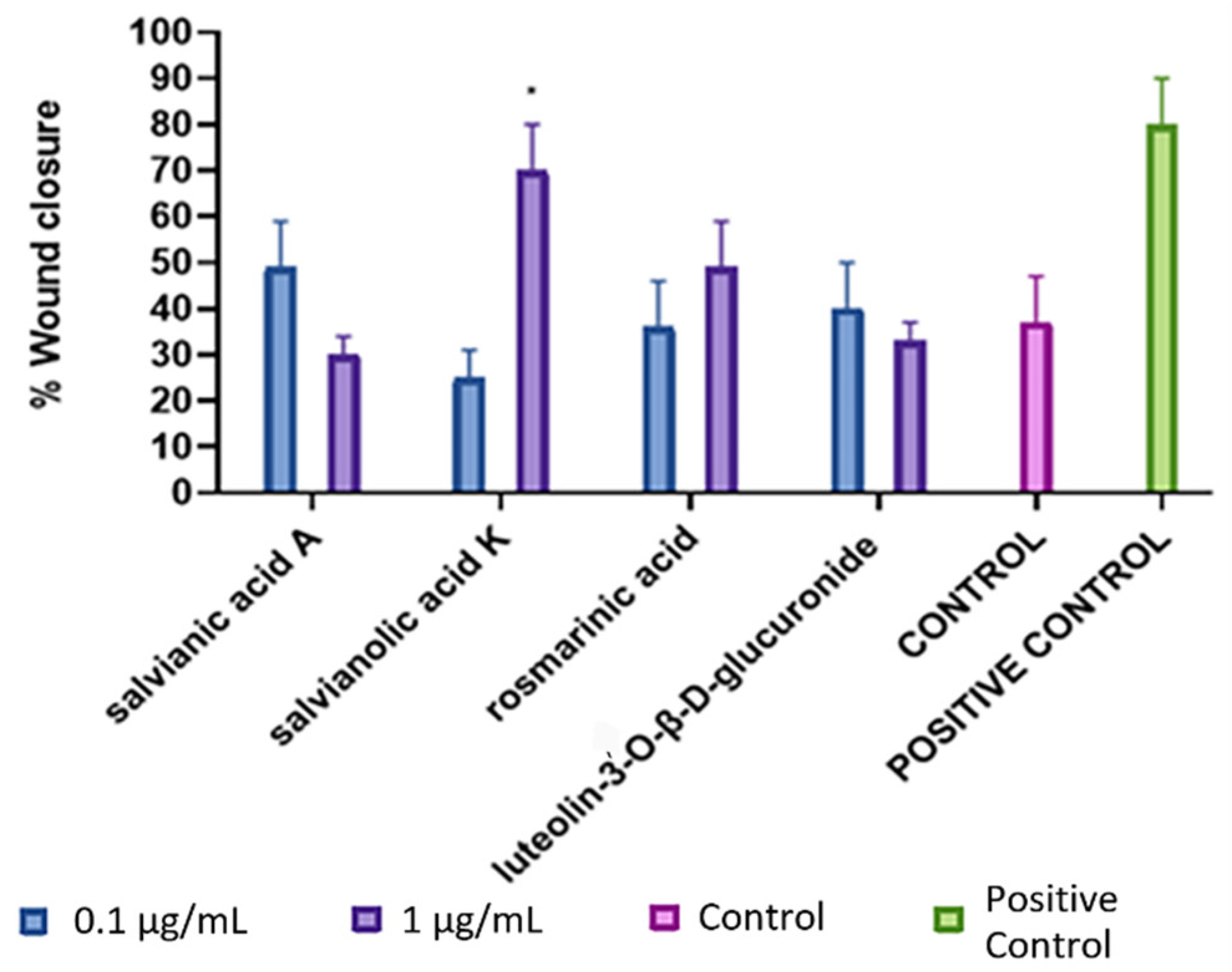
3.3. Wound-Healing Potential of the Isolated Compounds in Scratch Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wong, S.; Mohamed, I.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public. Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.R.; Vallejo, B.; Ruzgas, T.; Björklund, S. The Effect of UVB Irradiation and Oxidative Stress on the Skin Barrier-A New Method to Evaluate Sun Protection Factor Based on Electrical Impedance Spectroscopy. Sensors 2019, 19, 2376. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Castillo, J.; Micol, V. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J. Dermatol. Sci. 2016, 84, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural Substances for Prevention of Skin Photoaging: Screening Systems in the Development of Sunscreen and Rejuvenation Cosmetics. Rejuvenation Res. 2018, 21, 91–101. [Google Scholar] [CrossRef]
- Aghakhani, F.; Kharazian, N.; Lori Gooini, Z. Flavonoid constituents of Phlomis (Lamiaceae) species using liquid chromatography mass spectrometry. Phytochem. Anal. 2018, 29, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, andecological specializations of Salvia and tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products. Assessment Report on Salvia officinalis L., Folium and Salvia officinalis L., Aetheroleum; EMA/HMPC/150801/2015; Committee on Herbal Medicinal Products: London, UK, 2016. [Google Scholar]
- Lamaison, J.L.; Petitjean-Freytet, C.; Carnat, A. Lamiacées médicinales à propriétés antioxydantes, sources potentielles d’acide rosmarinique [Medicinal Lamiaceae with antioxidant properties, a potential source of rosmarinic acid]. Pharm. Acta Helv. 1991, 66, 185–188. [Google Scholar]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P.H. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Hohmann, J.; Zupkó, I.; Rédei, D.; Csányi, M.; Falkay, G.; Máthé, I.; Janicsák, G. Protective effects of the aerial parts of Salvia officinalis, Melissa officinalis and Lavandula angustifolia and their constituents against enzyme-dependent and enzyme-independent lipid peroxidation. Planta Med. 1999, 65, 576–578. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-inflammatory and Antioxidant Agents from Salvia Genus (Lamiaceae): An Assessment of the Current State of Knowledge. Antiinflamm. Antiallergy Agents Med. Chem. 2017, 16, 70–86. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia-a review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products. European Union Herbal Monograph on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., Herba; EMA/HMPC/39455/2015; Committee on Herbal Medicinal Products: London, UK, 2016. [Google Scholar]
- Lu, Y.; Foo, L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000, 55, 263–267. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Mailis, T.; Skaltsa, H. Polar Constituents of Salvia willeana (Holmboe) Hedge, Growing Wild in Cyprus. Plants 2018, 7, 18. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ezzat, S.M.; El Bishbishy, M.H.; Mnayer, D.; Sharopov, F.; Kılıç, C.S.; Neagu, M.; Constantin, C.; Sharifi-Rad, M.; Atanassova, M.; et al. Rosmarinus plants: Key farm concepts towards food applications. Phytother. Res. 2020, 34, 1474–1518. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef]
- Shimada, A.; Ueno, H.; Inagaki, M. Glutaminase inhibitory activities of pentacyclic triterpenes isolated from Thymus vulgaris L. Nat. Prod. Res. 2021, 36, 2864–2868. [Google Scholar] [CrossRef]
- Tomou, E.M.; Barda, C.; Skaltsa, H. Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef]
- Dimas, D.; Tomou, E.-M.; Karamanou, C.; Sfiniadakis, I.; Siakavella, K.I.; Liakopoulou, A.; Hatziantoniou, S.; Rallis, M.; Skaltsa, H. Melissa officinalis ssp. altissima extracts: A therapeutic approach targeting psoriasis in mice. J. Ethnopharmacol. 2019, 246, 112208. [Google Scholar]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef]
- Pritsas, A.; Tomou, E.M.; Tsitsigianni, E.; Papaemmanouil, C.D.; Diamantis, D.A.; Chatzopoulou, P.; Tzakos, A.G.; Skaltsa, H. Valorisation of stachysetin from cultivated Stachys iva Griseb. as anti-diabetic agent: A multi-spectroscopic and molecular docking approach. J. Biomol. Struct. Dyn. 2021, 39, 6452–6466. [Google Scholar] [CrossRef] [PubMed]
- Yahara, S.; Satoshiro, M.; Nishioka, I.; Nagasawa, T.; Oura, H. Isolation and characterization of phenolic compounds from Coptidis Rhizoma. Chem. Pharm. Bull. 1985, 33, 527–531. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Heitz, A.; Carnat, A.; Fraisse, D.; Carnat, A.-P.; Lamaison, J.-L. Luteolin 3′-glucuronide, the major flavonoid from Melissa officinalis subsp. officinalis. Fitoterapia 2000, 71, 201–202. [Google Scholar] [CrossRef]
- Zaabat, N.; Hay, A.E.; Michalet, S.; Skandrani, I.; Chekir-Ghedira, L.; Dijoux-Franca, M.G.; Akkal, S. Chemical Composition, Antioxidant, Genotoxique and Antigenotoxic Potentials of Phlomis bovei De Noé Aerial Parts. Iran. J. Pharm. Res. 2020, 19, 282–291. [Google Scholar]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef]
- Psotova, J.; Svobodova, A.; Kolarova, H.; Walterova, D. Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J. Photochem. Photobiol. B. 2006, 84, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campillo, M.; Gabaldon, J.A.; Castillo, J.; Benavente-García, O.; Del Baño, M.J.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J.A. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Jin, F.; Zhang, B.; Yi, T.H. Salvianolic Acid B Protects Normal Human Dermal Fibroblasts Against Ultraviolet B Irradiation-Induced Photoaging Through Mitogen-Activated Protein Kinase and Activator Protein-1 Pathways. Photochem. Photobiol. 2015, 91, 879–886. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, Y.L. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Mu, Y.P.; Zhang, H.; Wang, X.N.; Zhao, C.Q.; Chen, J.M.; Liu, P. Pharmacological Effects of Salvianolic Acid B Against Oxidative Damage. Front. Pharmacol. 2020, 11, 572373. [Google Scholar] [CrossRef] [PubMed]
- Kasimu, R.; Tanaka, K.; Tezuka, Y.; Gong, Z.-N.; Li, J.-X.; Basnet, P.; Namba, T.; Kadota, S. Comparative Study of Seventeen Salvia Plants: Aldose Reductase Inhibitory Activity of Water and MeOH Extracts and Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Water Extracts. Chem. Pharm. Bull. 1998, 46, 500–504. [Google Scholar] [CrossRef]
- Ayoub, I.M.; George, M.Y.; Menze, E.T.; Mahmoud, M.; Botros, M.; Essam, M.; Ashmawy, I.; Shendi, P.; Hany, A.; Galal, M.; et al. Insights into the neuroprotective effects of Salvia officinalis L. and Salvia microphylla Kunth in the memory impairment rat model. Food Funct. 2022, 13, 2253–2268. [Google Scholar] [CrossRef]
- Küba, M.C.; Türkoğlu, A.; Oğuz, A.; Tuncer, M.C.; Kaya, Ş.; Başol, Ö.; Bilge, H.; Tatlı, F. Comparison of local rosmarinic acid and topical dexpanthenol applications on wound healing in a rat experimental wound model. Folia Morphol. 2021, 80, 618–624. [Google Scholar] [CrossRef]






| Plant Sample | Origin | Extract | Abbreviation |
|---|---|---|---|
| Melissa officinalis subsp. altissima (Sm.) Arcang. | wild—Crete | EtOAc/Aqueous | MOE/MOW |
| Rosmarinus officinalis L. | wild—Mt. Pelion | EtOAc/Aqueous | ROE/ROW |
| Salvia officinalis L. | wild—Kozani | EtOAc/Aqueous | SOE/SOW |
| Sideritis cypria Post | cultivated—Cyprus | MeOH/Infusion | SCM/SCI |
| Sideritis euboea Heldr. | cultivated—ELGO Dimitra | MeOH/Infusion | SEM/SEI |
| Sideritis perfoliata L. subsp. perfoliata | cultivated—Cyprus | MeOH/Infusion | SPM/SPI |
| Sideritis scardica Griseb. | cultivated—ELGO Dimitra | MeOH/Infusion | SSM/SSW |
| Sideritis sipylea Boiss. | wild—Samos Island | MeOH/Infusion | SSMe/SSI |
| Stachys iva Griseb. | cultivated—ELGO Dimitra | MeOH/Infusion | SIM/SII |
| Thymus vulgaris L. | wild—Mt. Pelion | EtOH/Aqueous | TVE/TVW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsigianni, E.; Tomou, E.-M.; Almpani, C.; Rallis, M.C.; Skaltsa, H. Biological Activities of Lamiaceae Species: Bio-Guided Isolation of Active Metabolites from Salvia officinalis L. Agronomy 2023, 13, 1224. https://doi.org/10.3390/agronomy13051224
Tsitsigianni E, Tomou E-M, Almpani C, Rallis MC, Skaltsa H. Biological Activities of Lamiaceae Species: Bio-Guided Isolation of Active Metabolites from Salvia officinalis L. Agronomy. 2023; 13(5):1224. https://doi.org/10.3390/agronomy13051224
Chicago/Turabian StyleTsitsigianni, Eleni, Ekaterina-Michaela Tomou, Chara Almpani, Michail Ch. Rallis, and Helen Skaltsa. 2023. "Biological Activities of Lamiaceae Species: Bio-Guided Isolation of Active Metabolites from Salvia officinalis L." Agronomy 13, no. 5: 1224. https://doi.org/10.3390/agronomy13051224
APA StyleTsitsigianni, E., Tomou, E.-M., Almpani, C., Rallis, M. C., & Skaltsa, H. (2023). Biological Activities of Lamiaceae Species: Bio-Guided Isolation of Active Metabolites from Salvia officinalis L. Agronomy, 13(5), 1224. https://doi.org/10.3390/agronomy13051224







