Influence of Fruit Wounding on Subsequent Monilinia laxa Infection of Nectarines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Pathogen Culture and Incubation Conditions
2.3. Experiments
2.3.1. First Experiment: Evaluating a Possible Systemic Reaction to Wounding That May Impact Subsequent M. laxa Infection
2.3.2. Second Experiment: Evaluating the Influence of Fruit on the In Vitro Development of M. laxa
2.3.3. Third Experiment: Evaluating the Influence of the Presence of Wounded Fruits on the Infection of Other Fruits Inoculated with M. laxa
2.3.4. Forth Experiment: Evaluating the Fruit Reaction to Brown Rot at Different Development Stages and Inoculation Times after Wounding
2.4. Exploration of Red Reaction
2.5. Statistical Analysis and Graphic Representations
3. Results
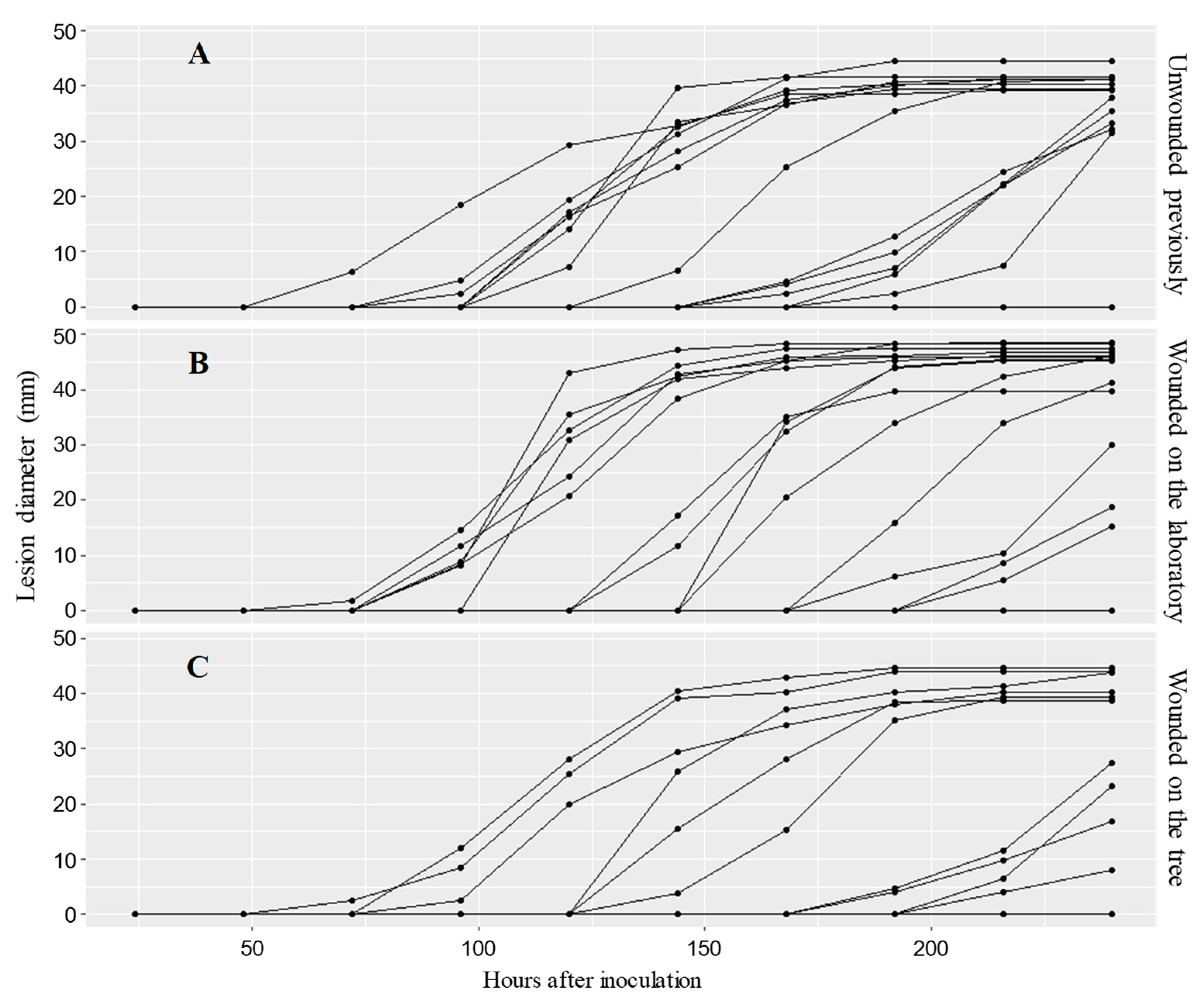
3.1. First Experiment: Evaluating a Possible Systemic Reaction to Wounding That May Impact Subsequent M. laxa Infection
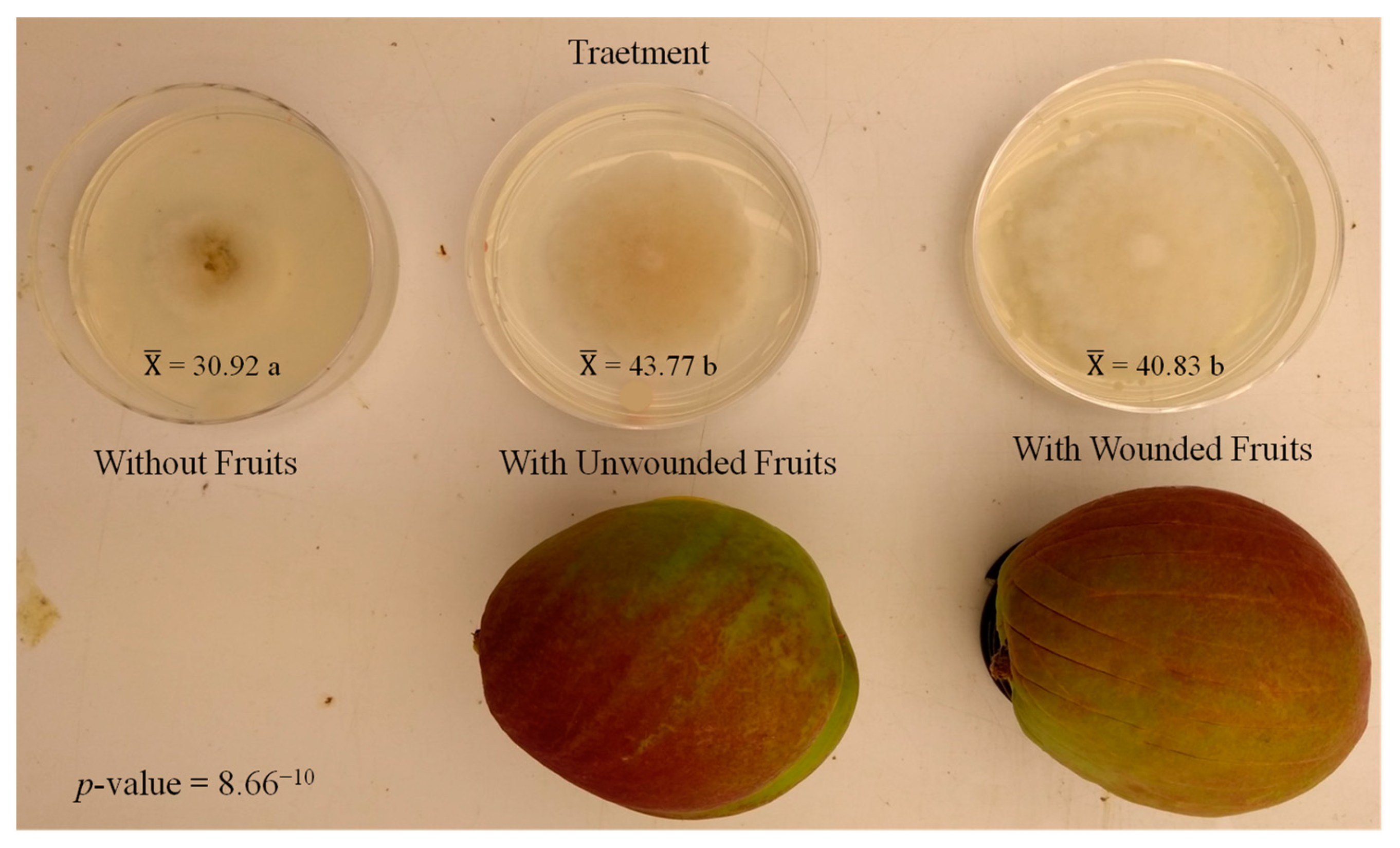
3.2. Second Experiment: Evaluating the Influence of Fruit on M. laxa In Vitro Development
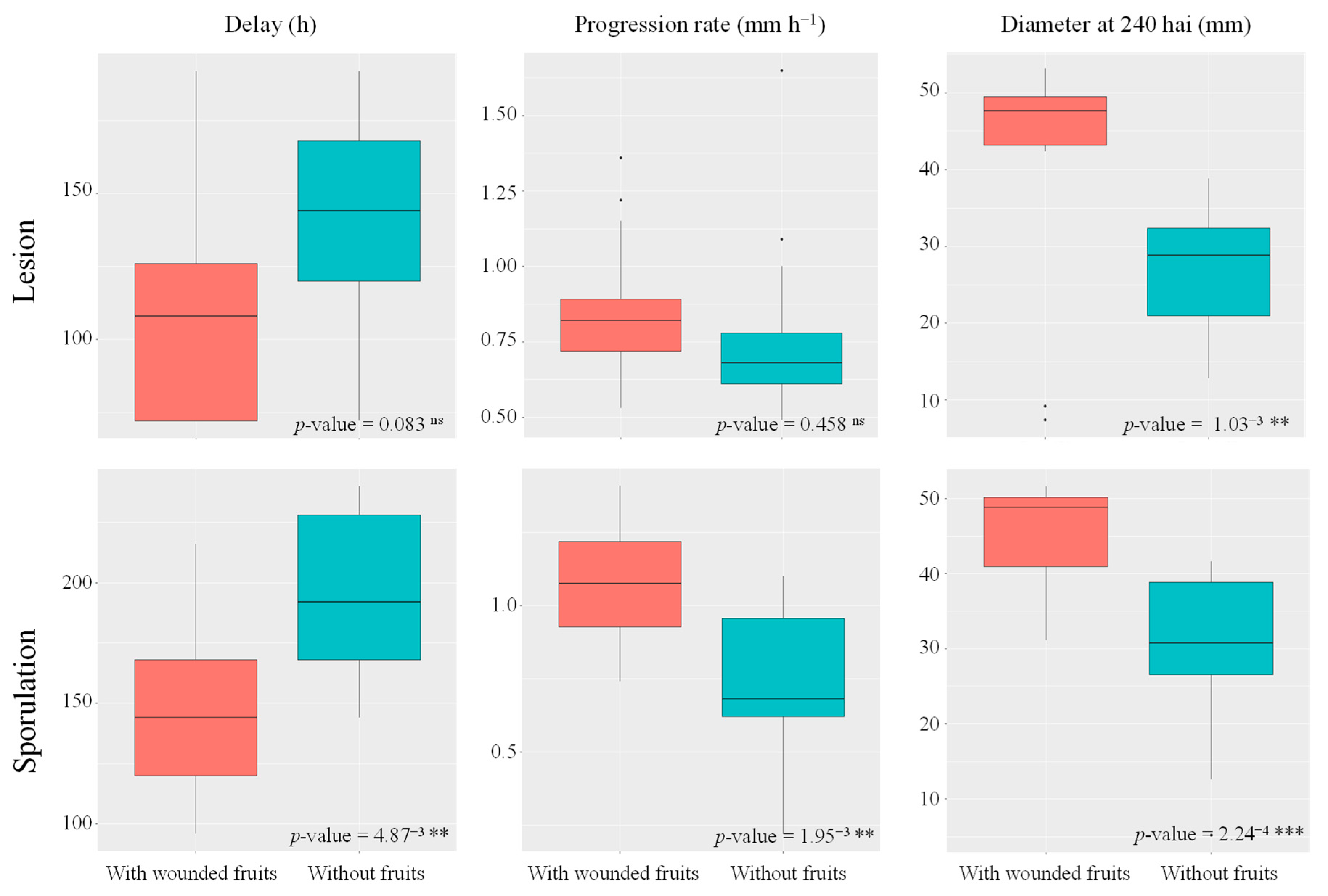
3.3. Third Experiment: Influence of the Presence of Wounded Fruits on the Infection of Other Fruits Inoculated with M. laxa
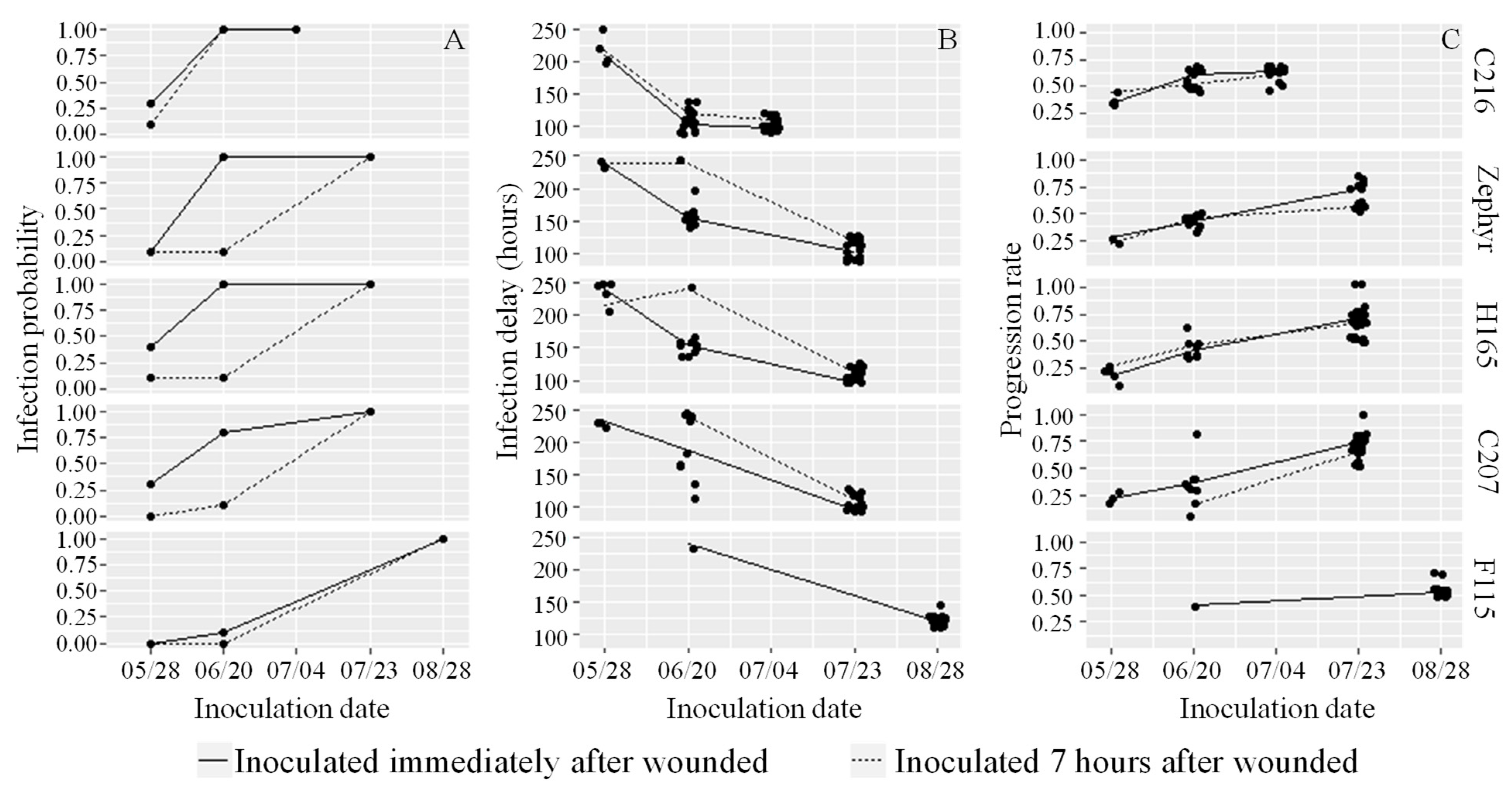
3.4. Fourth Experiment: Fruit Reaction to Brown Rot at Different Development Stages and Inoculation Times after Wounding
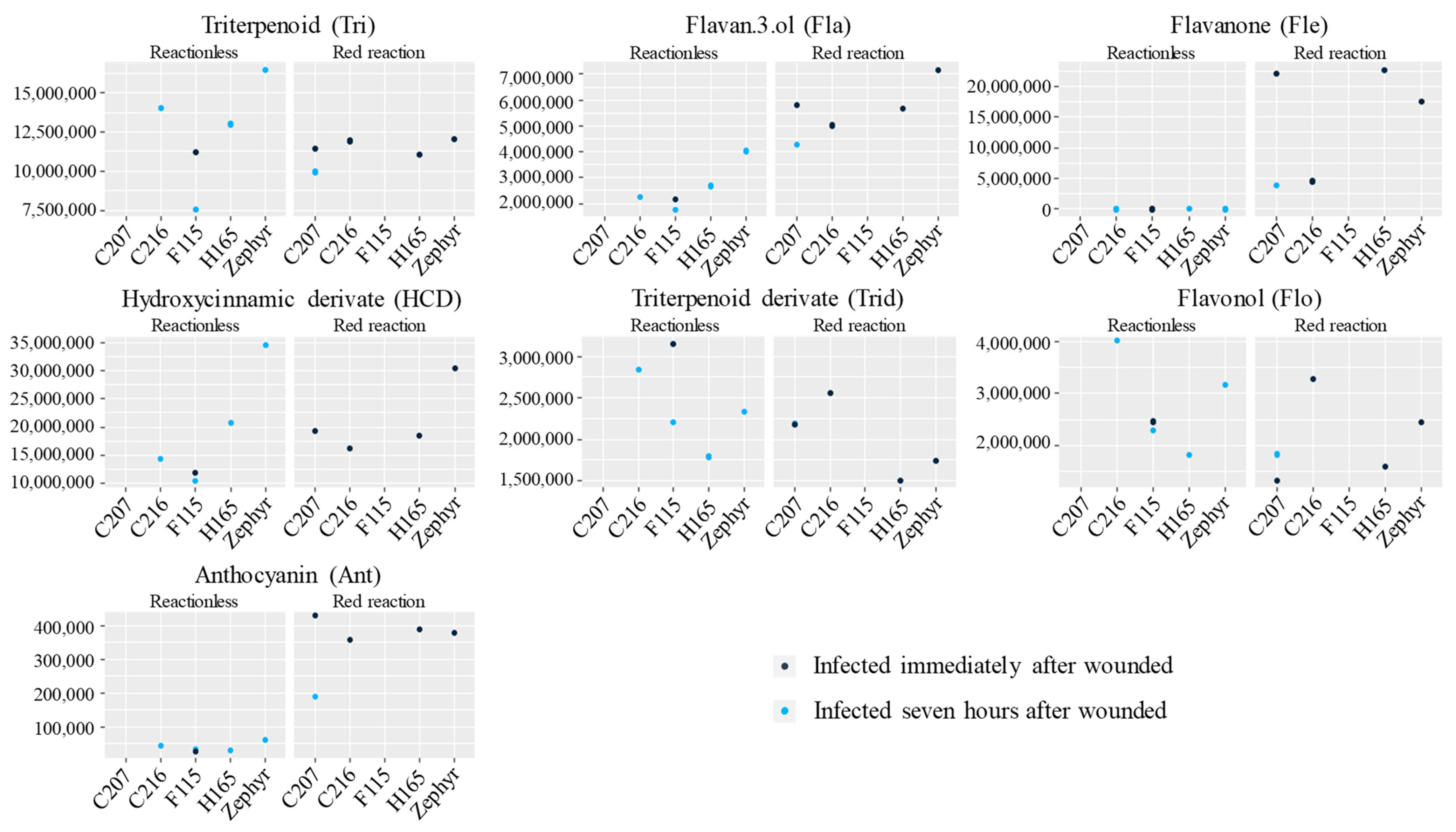
3.5. Exploration of Compounds Present in Red Reaction Zones
4. Discussion
4.1. Wounding Has No Systemic Effect on M. laxa Infection
4.2. Fruit Presence Enhances M. laxa Growth Both In Vitro and in Fruit
4.3. A Delay of 7 h between Wounding and Subsequent Inoculation Decreases Infection in Immature Fruit
4.4. Specialized Metabolites Detected in Red Reaction Zones
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogawa, J.M.; Zehr, E.I.; Bird, G.W.; Ritchie, D.F.; Uriu, K.; Uyemoto, J.K. Compendium of Stone Fruit Diseases, 1st ed.; The American Phytopathological Society: Saint Paul, MN, USA, 1995; 98p, ISBN 9780890541746. [Google Scholar]
- Adaskaveg, J.E.; Schnabel, G.; Forster, H. Diseases of peach caused by fungi and fungal-like organisms: Biology, epidemiology and management. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 352–406. ISBN 9781845933869. [Google Scholar] [CrossRef]
- Hu, M.J.; Cox, K.D.; Schnabel, G.; Luo, C.X. Monilinia species causing brown rot of peach in China. PLoS ONE 2011, 6, e24990. [Google Scholar] [CrossRef] [PubMed]
- May-De-Mio, L.L.; Moreira, L.M.; Monteiro, L.B.; Justiniano Júnior, P.R. Infection of Monilinia fructicola in budding stages and incidence of brown rot on fruits in two peach production systems. Trop. Plant Pathol. 2008, 33, 227–234. [Google Scholar] [CrossRef]
- May-De-Mio, L.L.; Garrido, L.R.; Ueno, B.; Fajardo, T.V.M. Doenças da cultura do pessegueiro e métodos de controle. In Pessegueiro; Raseira, M.C.B., Pereira, J.F.M., Carvalho, F.L.C., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 355–432. ISBN 978-85-7035-371-9. [Google Scholar]
- Mari, M.; Casalini, L.; Baraldi, E.; Bertolini, P.; Pratella, G.C. Susceptibility of apricot and peach fruit to Monilinia laxa during phenological stages. Postharvest Biol. Technol. 2003, 30, 105–109. [Google Scholar] [CrossRef]
- Gell, I.; De Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. Eur. J. Plant Pathol. 2008, 121, 487–498. [Google Scholar] [CrossRef]
- Guidarelli, M.; Zubini, P.; Nanni, V.; Bonghi, C.; Rasori, A.; Bertolini, P.; Baraldi, E. Gene expression analysis of peach fruit at different growth stages and with different susceptibility to Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 503–513. [Google Scholar] [CrossRef]
- Raseira, M.C.B.; Franzon, R. Melhoramento genético. In Pessegueiro; Raseira, M.C.B., Pereira, J.F.M., Carvalho, F.L.C., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 57–72. ISBN 978-85-7035-371-9. [Google Scholar]
- Fresnedo-Ramírez, J.; Famula, T.R.; Gradziel, T.M. Application of a Bayesian ordinal animal model for the estimation of breeding values for the resistance to Monilinia fruticola (G.Winter) Honey in progenies of peach [Prunus persica (L.) Batsch]. Breeding Sci. 2017, 67, 110–122. [Google Scholar] [CrossRef]
- Oliveira Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown rot strikes Prunus fruit: An ancient fight almost always lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef]
- Fu, W.; Burrell, R.; Linge, C.S.; Schnabel, G.; Gasic, K. Breeding for brown rot (Monilinia spp.) tolerance in Clemson University Peach Breeding Program. J. Am. Pom. Soc. 2018, 72, 94–100. [Google Scholar] [CrossRef]
- Martínez-García, P.J.; Parfitt, D.E.; Bostock, R.M.; Fresnedo-Ramírez, J.; Vazquez-Lobo, A.; Ogundiwin, E.A.; Gradziel, T.M.; Crisosto, C.H. Application of genomic and quantitative genetic tools to identify candidate resistance genes for brown rot resistance in peach. PLoS ONE 2013, 8, e78634. [Google Scholar] [CrossRef]
- Pacheco, I.; Bassi, D.; Eduardo, I.; Ciacciulli, A.; Pirona, R.; Rossini, L.; Vecchietti, A. QTL mapping for brown rot (Monilinia fructigena) resistance in an intraspecific peach (Prunus persica L. Batsch) F1 progeny. Tree Genet. Genomes 2014, 10, 1223–1242. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Eduardo, I.; Usall, J.; Casals, C.; Arús, P.; Teixidó, N.; Torres, R. Exploring sources of resistance to brown rot in an interspecific almond × peach population. J. Sci. Food Agric. 2019, 99, 4105–4113. [Google Scholar] [CrossRef] [PubMed]
- Scariotto, S.; Dini, M.; Raseira, M.C.B.; Santos, J. Estimates of genetic parameters for brown rot resistance in Prunus persica. Acta Hort. 2021, 1304, 289–297. [Google Scholar] [CrossRef]
- Dini, M.; Scariotto, S.; Raseira, M.C.B.; Ueno, B. Heritability and segregation of resistance to brown rot in peach fruits. Acta Hort. 2021, 1304, 339–346. [Google Scholar] [CrossRef]
- Dini, M.; Raseira, M.C.B.; Scariotto, S.; Ueno, B. Breeding peaches for brown rot resistance in Embrapa. Agronomy 2022, 12, 2306. [Google Scholar] [CrossRef]
- Tosetti, R.; Tardelli, F.; Tadiello, A.; Zaffalon, V.; Giorgi, F.M.; Guidi, L.; Trainotti, L.; Bonghi, C.; Tonutti, P. Molecular and biochemical responses to wounding in mesocarp of ripe peach (Prunus persica L. Batsch) fruit. Postharvest Biol. Technol. 2013, 90, 40–51. [Google Scholar] [CrossRef]
- Zhou, L.; Thornburg, R. Wound-inducible genes in plants. In Inducible Gene Expression; Reynolds, P.H.S., Ed.; CAB International: Wallingford, UK, 1999; pp. 127–158. [Google Scholar]
- Bruxelles, G.L.; Roberts, M.R. Signals regulating multiple responses to wounding and herbivores. Crit. Rev. Plant Sci. 2001, 20, 487–521. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Chang, H.S.; Gupta, R.; Wang, X.; Zhou, T.; Luan, S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002, 129, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Venkateswarlu, B. Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives, 1st ed.; Intech: Rijeka, Croatia, 2011; 360p. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef]
- Christopher, M.E.; Miranda, M.; Major, I.T.; Constabel, C.P. Gene expression profiling of systemically wound-induced defenses in hybrid poplar. Planta 2004, 219, 936–947. [Google Scholar] [CrossRef]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagia, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef]
- Koo, A.J.K.; Howea, G.A. The wound hormone jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Trinidade, I.; Santos, D.; Dalmay, T.; Fevereiro, P. Facing the environment: Small RNAs and the regulation of gene expression under abiotic stress in plants. In Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives; Shanker, A.K., Venkateswarlu, B., Eds.; Intech: Rijeka, Croatia, 2011; pp. 113–136. ISBN 978-953-307-672-0. [Google Scholar]
- Leide, J.; Hildebrandt, U.; Hartung, W.; Riederer, M.; Vogg, G. Abscisic acid mediates the formation of a suberized stem scar tissue in tomato fruits. New Phytol. 2012, 194, 402–415. [Google Scholar] [CrossRef]
- Tsaballa, A.; Nikolaidis, A.; Trikka, F.; Ignea, C.; Kampranis, S.C.; Makris, A.M.; Argiriou, A. Use of the de novo transcriptome analysis of silver-leaf nightshade (Solanum elaeagnifolium) to identify gene expression changes associated with wounding and terpene biosynthesis. BMC Genom. 2015, 16, 504. [Google Scholar] [CrossRef]
- Birkenmeier, G.F.; Ryan, C.A. Wound signalling in tomato plants—Evidence that ABA is not a primary signal for defense gene activation. Plant Physiol. 1998, 117, 687–693. [Google Scholar] [CrossRef]
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound signalling in plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Delessert, C.; Wilson, I.W.; Van Der Straeten, D.; Dennis, E.S.; Dolferus, R. Spatial and temporal analysis of the local response to wounding. Plant Mol. Biol. 2004, 55, 165–181. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Zelauré, S.L.; De Bolle, M.F.C.; Cammue, B.P.A. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006, 44, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ikegami, A.; Yonemori, K. DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.), contributes to proanthocyanidin regulation. Planta 2010, 232, 1045–1059. [Google Scholar] [CrossRef]
- Hara, K.; Yagi, M.; Kusano, T.; Sano, S. Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol. Gen. Genet. 2000, 263, 30–37. [Google Scholar] [CrossRef]
- Smith, C.M.; Rodriguez-Buey, M.; Karlsson, J.; Campbell, M.M. The response of the poplar transcriptome to wounding and subsequent infection by a viral pathogen. New Phytol. 2004, 164, 123–136. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, L.F.; Dai, L.J.; Yang, S.G.; Tian, W.M. Characterization of HbEREBP1, a wound-responsive transcription factor gene in laticifers of Hevea brasiliensis Muell. Arg. Mol. Biol. Rep. 2012, 39, 3713–3719. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Chen, J.; He, L.; Jiang, Y.; Kuang, J.; Lu, C.; Joyce, D.; Macnish, A.; He, Y.; Lu, W. Expression of PAL and HSPs in fresh-cut banana fruit. Environ. Exp. Bot. 2009, 66, 31–37. [Google Scholar] [CrossRef]
- Su, J.; Tu, K.; Cheng, L.; Tu, S.; Wang, M.; Xu, H.; Zhan, G. Wound-induced H2O2 and resistance to Botrytis cinerea decline with the ripening of apple fruit. Postharvest Biol. Technol. 2011, 62, 64–70. [Google Scholar] [CrossRef]
- Dini, M. Resistência à Podridão-Parda no Pessegueiro. Ph.D. Thesis, Programa de Pós-Graduação em Agronomia, Faculdade de Agronomia Eliseu Maciel, Universidade Federal de Pelotas, Pelotas, Brazil, 2019; 237p. [Google Scholar]
- Lurol, S.; Tabaries, P.; Buffet, B.; Sobas, M.-A.; Portal, A. Lutte post récolte contre Monilia. Application d´eau chaude sur pêche. InfosCTIFL 2009, 250, 32–36. [Google Scholar]
- Lino, L.O.; Confolent, C.; Signoret, V.; Génard, M.; Quilot-Turion, B. Genetic variability in the susceptibility of immature peach fruit to Monilinia laxa is associated with surface conductance but not stomatal density. J. Plant Sci. Phytopathol. 2022, 6, 91–100. [Google Scholar] [CrossRef]
- Lino, L.O.; Quilot-Turion, B.; Dufour, C.; Corre, M.-N.; Lessire, R.; Génard, M.; Poëssel, J.-L. Cuticular waxes of nectarines during fruit development in relation to surface conductance and susceptibility to Monilinia laxa. J. Exp. Bot. 2020, 71, 5521–5537. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2018. Available online: https://www.R-project.org (accessed on 15 June 2018).
- Knogge, W. Molecular basis of specificity in host/fungus interactions. Eur. J. Plant Pathol. 1996, 102, 807–816. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Wilson, C.L.; Franklin, J.D.; Otto, B.E. Fruit volatiles inhibitory to Monilinia fructicola and Botrytis cinerea. Plant Dis. 1987, 71, 316–319. [Google Scholar] [CrossRef]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchitti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2009, 90, 1146–1154. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, H.Y.; Seo, E.; Lee, J.; Kim, S.B.; Oh, S.; Choi, E.; Choi, E.; Lee, S.E.; Choi, D. Current understandings of plant nonhost resistance. Mol. Plant Microbe Interact. 2017, 30, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Biggs, A.R.; Northover, J. Early and late-season susceptibility of peach fruits to Monilinia fructicola. Plant Dis. 1988, 72, 1070–1074. [Google Scholar] [CrossRef]
- Gradziel, T.M. Changes in susceptibility to brown rot with ripening in three clingstone peach genotypes. J. Am. Soc. Hort. Sci. 1994, 119, 101–105. [Google Scholar] [CrossRef]
- Emery, K.M.; Michailides, T.J.; Scherm, H. Incidence of latent infection of immature peach fruit by Monilinia fructicola and relationship to brown rot in Georgia. Plant Dis. 2000, 84, 853–857. [Google Scholar] [CrossRef]
- Dean, B.B.; Kolattukudy, P.E. Synthesis of suberin during wound-healing in jade leaves, tomato fruit, and bean pods. Plant Physiol. 1976, 58, 411–416. [Google Scholar] [CrossRef]
- Spotts, R.A.; Sanderson, P.G.; Lennox, C.L.; Sugar, D.; Cervantes, L.A. Wounding, wound healing and staining of mature pear fruit. Postharvest Biol. Technol. 1998, 13, 27–36. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Nichols, B.; Bauchan, G.; Chao, T.C.; Jurick II, W.M. Wound responses of wild apples suggest multiple resistance mechanism against blue mold decay. Postharvest Biol. Technol. 2016, 117, 132–140. [Google Scholar] [CrossRef]
- Han, C.; Jin, P.; Li, M.; Wang, L.; Zheng, Y. Physiological and transcriptomic analysis validates previous findings of changes in primary metabolism for the production of phenolic antioxidants in wounded carrots. J. Agric. Food Chem. 2017, 65, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Bostock, R.M. Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox? Phytopathology 2007, 97, 269–277. [Google Scholar] [CrossRef]
- Roemmelt, S.; Fischer, T.C.; Halbwirth, H.; Peterek, S.; Schlangen, K.; Speakman, J.B.; Treutter, D.; Forkmann, G.; Stich, K. Effect of dioxygenase inhibitors on the resistance-related flavonoid metabolism of apple and pears: Chemical, biochemical and molecular biological aspects. Eur. J. Hort. Sci. 2003, 68, 129–136. [Google Scholar]
- Flachowsky, H.; Halbwirth, H.; Treutter, D.; Richter, K.; Hanke, M.V.; Szankowski, I.; Gosch, C.; Stich, K.; Fischer, T.C. Silencing of flavanone-3-hydroxylase in apple (Malus × domestica Borkh.) leads to accumulation of flavanones, but not to reduced fire blight susceptibility. Plant Physiol. Biochem. 2012, 51, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Halbwirth, H.; Fischer, T.C.; Roemmelt, S.; Spinellid, F.; Schlangena, K.; Peterekc, S.; Sabatini, E.; Messinae, C.; Speakman, J.B.; Andreotti, C.; et al. Induction of antimicrobial 3-deoxyflavonoids in pome fruit trees controls fire blight. Z. Naturforsch. 2003, 58, 765–770. [Google Scholar] [CrossRef]
- Choi, J.S.; Yokozawa, T.; Oura, H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, Prunin. Planta Med. 1990, 57, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Luque, P.; Heredia, A. Sorption and interaction of the flavonoid naringenin on tomato fruit cuticles. J. Agric. Food Chem. 2009, 57, 7560–7564. [Google Scholar] [CrossRef]
- Castillo, J.; Benavente, O.; Del Rio, J.A. Hesperetin 7-O-glucoside and prunin in citrus species (C. aurantium and C. paradisi). A study of their quantitative distribution in immature fruits and as immediate precursors of Neohesperidin and Naringin in C. aurantium. J. Agric. Food Chem. 1993, 41, 1920–1924. [Google Scholar] [CrossRef]
- Del Río, J.A.; Fuster, M.D.; Sabater, F.; Porras, I.; García-Lidón, A.; Ortuño, A. Effect of benzylaminopurine on the flavanones hesperidin, hesperetin 7-O-glucoside, and prunin in tangelo Nova fruits. J. Agric. Food Chem. 1995, 43, 2030–2034. [Google Scholar] [CrossRef]
- Frydoonfar, H.R.; McGrath, D.R.; Spigelman, A.D. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid Naringenin. Color. Dis. 2003, 5, 149–152. [Google Scholar] [CrossRef]
- Mir, I.A.; Tiku, A.B. Chemopreventive and therapeutic potential of “naringenin,” a flavanone present in citrus fruits. Nutr. Cancer. 2014, 67, 27–42. [Google Scholar] [CrossRef]
- Orallo, F.; Camiña, M.; Álvarez, E.; Basaran, H.; Lugnier, C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (+/−)-naringenin. Planta Med. 2005, 71, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Erez, A.; Lavee, S. Prunin identification, biological activity and quantitative change in comparison to Naringenin in dormant peach buds. Plant Physiol. 1969, 44, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. Phenolic compounds in apple leaves after infection with apple scab. Biol. Plantarum. 2011, 55, 725–730. [Google Scholar] [CrossRef]
- Kaur, S. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Shaefer, H.M.; Rentzsch, M.; Breuer, M. Anthocyanins reduce fungal growth in fruits. Nat. Prod. Commun. 2008, 3, 1267–1272. [Google Scholar] [CrossRef]




| Experiment Number | Objective | Methodology | Treatments |
|---|---|---|---|
| 1 | Evaluate the possibility of a systemic reaction to wounding that could affect subsequent M. laxa infection. | M. laxa inoculation in immature Zephyr nectarine fruit after wounding. BR incidence and growth kinetics of lesions and sporulation were scored. | Previously:
|
| 2 | Evaluate the effect of fruit on the in vitro development of M. laxa. | The growth kinetics of the M. laxa colony on open Petri dishes with PDA media placed inside sealed plastic boxes were studied. | Boxes:
|
| 3 | Evaluate the effect of wounded fruits on the infection of other fruits inoculated with M. laxa. | Wounding and immediate inoculation of immature fruit placed inside plastic boxes with M. laxa under two conditions. BR incidence and growth kinetics of lesions and sporulation were scored. | Boxes:
|
| 4 | Evaluate the effect of wounding time before fruit inoculation with M. laxa at different development stages. | Wounding prior to M. laxa fruit inoculation of five nectarine genotypes at different times and fruit development stages. | Genotypes:
|
| p-Value 1 | Lesion Delay | Progression Rate | LD 2 | Sporulation Delay | Sporulation Rate | SpoD 2 |
|---|---|---|---|---|---|---|
| Genotype | 0.0025 ** | 0.2822 ns | <0.0001 *** | <0.0001 *** | 0.0003 *** | <0.0001 *** |
| Inoculation Time | 0.0190 ns | 0.0244 ns | 0.5113 ns | 0.3180 ns | 0.6580 ns | 0.3148 ns |
| Development Stage | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Gen×InocTime | 0.0561 ns | 0.4073 ns | 0.0396 ns | 0.1293 ns | 0.0955 ns | 0.0446 ns |
| Gen×DevStage | 0.0026 ** | <0.0001 *** | <0.0001 *** | 0.0006 *** | 0.1491 ns | <0.0001 *** |
| InocTime×DevStage | <0.0001 *** | 0.6532 ns | <0.0001 *** | 0.0127 ns | 0.5323 ns | <0.0001 *** |
| Gen×InocTime×DevStage | 0.02403 ns | 0.5149 ns | 0.3433 ns | 0.0008 *** | 0.8843 ns | 0.5836 ns |
| Family 1 | Compound | λ (nm) | RT (min) | λ max (nm) | Abbrev. | p-Value 3 | |
|---|---|---|---|---|---|---|---|
| Compound | Family | ||||||
| Tri | Trihydroxy-urs-12-en-28-oic acid 1 2 | 210 | 124.3 | 198 | thu1 | 0.030 ns | 0.482 ns |
| Trihydroxy-urs-12-en-28-oic acid 2 2 | 210 | 124.7 | 198 | thu2 | 0.049 ns | ||
| Oleanolic acid | 210 | 131.9 | 198 | Ole | 0.496 ns | ||
| Ursolic acid | 210 | 132.6 | 198 | Urs | 0.676 ns | ||
| Fla | Flavan-3-ol 1 | 280 | 25.3 | 253/278 | Fla1 | 1.54−4 *** | 1.20−3 ** |
| Procyanidin B1 | 280 | 27.7 | 254/278 | ProcyaB1 | 0.254 ns | ||
| Flavan-3-ol 2 | 280 | 28.9 | 256/278 | Fla2 | 5.16−3 ** | ||
| Flavan-3-ol 3 | 280 | 30.2 | 255/278 | Fla3 | 9.08−3 ** | ||
| Catechin | 280 | 34.1 | 251/278 | Cat | 8.95−4 *** | ||
| Fle | Flavanone 1 | 280 | 32.0 | 259/285 | Fle1 | Red reaction | Red reaction |
| Flavanone 2 | 280 | 47.2 | 253/282 | Fle2 | Red reaction | ||
| Eriodictyol-7-glucoside | 280 | 66.0 | 283 | E7Glu | Red reaction | ||
| Flavanone 3 | 280 | 66.5 | 218/283 | Fle3 | Red reaction | ||
| Naringenine-7-glucoside (syn.: Prunin) | 280 | 76.5 | 212/282 | N7Glu | Red reaction | ||
| Flavanone 4 | 280 | 99.7 | 284 | Fle4 | Red reaction | ||
| HCD | cis-Neochlorogenic acid | 315 | 20.2 | 266/316 | c3CQ | 9.19−3 ** | 0.644 ns |
| Neochlorogenic acid | 315 | 23.2 | 217/324 | t3CQ | 0.012 ns | ||
| Hydroxycinnamic derivative 1 | 315 | 39.0 | 253/314 | HCD1 | Red reaction | ||
| Chlorogenic acid | 315 | 43.1 | 217/326 | t5CQ | 0.739 ns | ||
| cis-Chlorogenic acid | 315 | 54.2 | 284/317 | c5CQ | 0.733 ns | ||
| Hydroxycinnamic derivative 2 | 315 | 56.6 | 280/311 | HCD2 | 0.437 ns | ||
| 5-p-Coumaroylquinic acid | 315 | 57.4 | 253/311 | pCQ | 9.90−3 ** | ||
| 3,5-Dicaffeoylquinic acid | 315 | 79.1 | 263/328 | x3.5diCQ | 0.334 ns | ||
| Hydroxycinnamic derivative 3 | 315 | 99.2 | 265/330 | HCD3 | Red reaction | ||
| Hydroxycinnamic derivative 4 | 315 | 100.6 | 221/329 | HCD4 | Red reaction | ||
| Trid | p-coumaroyl-2,3-dihydroxy-urs-12-en-28-oic acid 1 2 | 315 | 125.9 | 252/289 | cdhu1 | 0.245 ns | 0.193 ns |
| p-coumaroyl-2,3-dihydroxy-urs-12-en-28-oic acid 2 2 | 315 | 126.3 | 295/319 | cdhu2 | 0.058 ns | ||
| p-coumaroyl-2,3-dihydroxy-urs-12-en-28-oic acid 3 2 | 315 | 126.5 | 250/311 | cdhu3 | 0.133 ns | ||
| p-coumaroyl-2,3-dihydroxy-urs-12-en-28-oic acid 4 2 | 315 | 126.8 | 251/285 | cdhu4 | 0.098 ns | ||
| p-coumaroyl-2,3-dihydroxy-urs-12-en-28-oic acid 5 2 | 315 | 127.6 | 298/308 | cdhu5 | 0.150 ns | ||
| p-coumaroyl-2,3-dihydroxy-urs-12-en-28-oic acid 6 2 | 315 | 128.4 | 249/308 | cdhu6 | 0.103 ns | ||
| feruloyl-2,3-dihydroxy-urs-12-en-28-oic acid 2 | 315 | 129.0 | 282/322 | fdhu | 0.681 ns | ||
| 3β-p-coumaroyloxy-urs-12-en-28-oic acid 1 2 | 315 | 135.2 | 253/286 | cou1 | 0.856 ns | ||
| 3β-p-coumaroyloxy-urs-12-en-28-oic acid 2 2 | 315 | 135.4 | 250/307 | cou2 | 0.953 ns | ||
| 3β-p-coumaroyloxy-urs-12-en-28-oic acid 3 2 | 315 | 135.7 | 257/314 | cou3 | 0.852 ns | ||
| 3β-p-coumaroyloxy-urs-12-en-28-oic acid 4 2 | 315 | 135.9 | 255/313 | cou4 | 0.818 ns | ||
| Flo | Quercetin-3-galactoside | 350 | 84.4 | 255/354 | Q3Gal | 0.228 ns | 0.239 ns |
| Quercetin-3-glucoside | 350 | 86.2 | 255/354 | Q3Glu | 0.234 ns | ||
| Quercetin-3-rutinoside | 350 | 86.8 | 256/354 | Q3Rut | 0.251 ns | ||
| Kaempferol-3-galactoside | 350 | 92.9 | 265/347 | K3Gal | 0.273 ns | ||
| Kaempferol-3-glucoside | 350 | 95.1 | 265/347 | K3Glu | 0.436 ns | ||
| Kaempferol-3-rutinoside | 350 | 95.6 | 266/345 | K3Rut | 0.331 ns | ||
| Ant | Cyanidin-3-glucoside | 520 | 62.8 | 279/518 | Cya3Glu | 8.34−5 *** | 1.41−4 *** |
| Cyanidin-3-rutinoside | 520 | 64.8 | 276/525 | Cya3Rut | 0.135 ns | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, M.; Raseira, M.d.C.B.; Corre, M.-N.; Signoret, V.; Quilot-Turion, B. Influence of Fruit Wounding on Subsequent Monilinia laxa Infection of Nectarines. Agronomy 2023, 13, 1235. https://doi.org/10.3390/agronomy13051235
Dini M, Raseira MdCB, Corre M-N, Signoret V, Quilot-Turion B. Influence of Fruit Wounding on Subsequent Monilinia laxa Infection of Nectarines. Agronomy. 2023; 13(5):1235. https://doi.org/10.3390/agronomy13051235
Chicago/Turabian StyleDini, Maximiliano, Maria do Carmo Bassols Raseira, Marie-Noëlle Corre, Véronique Signoret, and Bénédicte Quilot-Turion. 2023. "Influence of Fruit Wounding on Subsequent Monilinia laxa Infection of Nectarines" Agronomy 13, no. 5: 1235. https://doi.org/10.3390/agronomy13051235






