Effects of Water Supply Mode on Nitrogen Transformation and Ammonia Oxidation Microorganisms in a Tea Garden
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Design
2.3. Determination of Ammonium Nitrogen and Nitrate Nitrogen in Soil
2.4. Extraction of Total DNA, Real-Time Fluorescent Quantitative PCR
2.5. High-Throughput Sequencing of Soil Ammonia-Oxidizing Microorganisms
2.6. Data Processing
3. Results
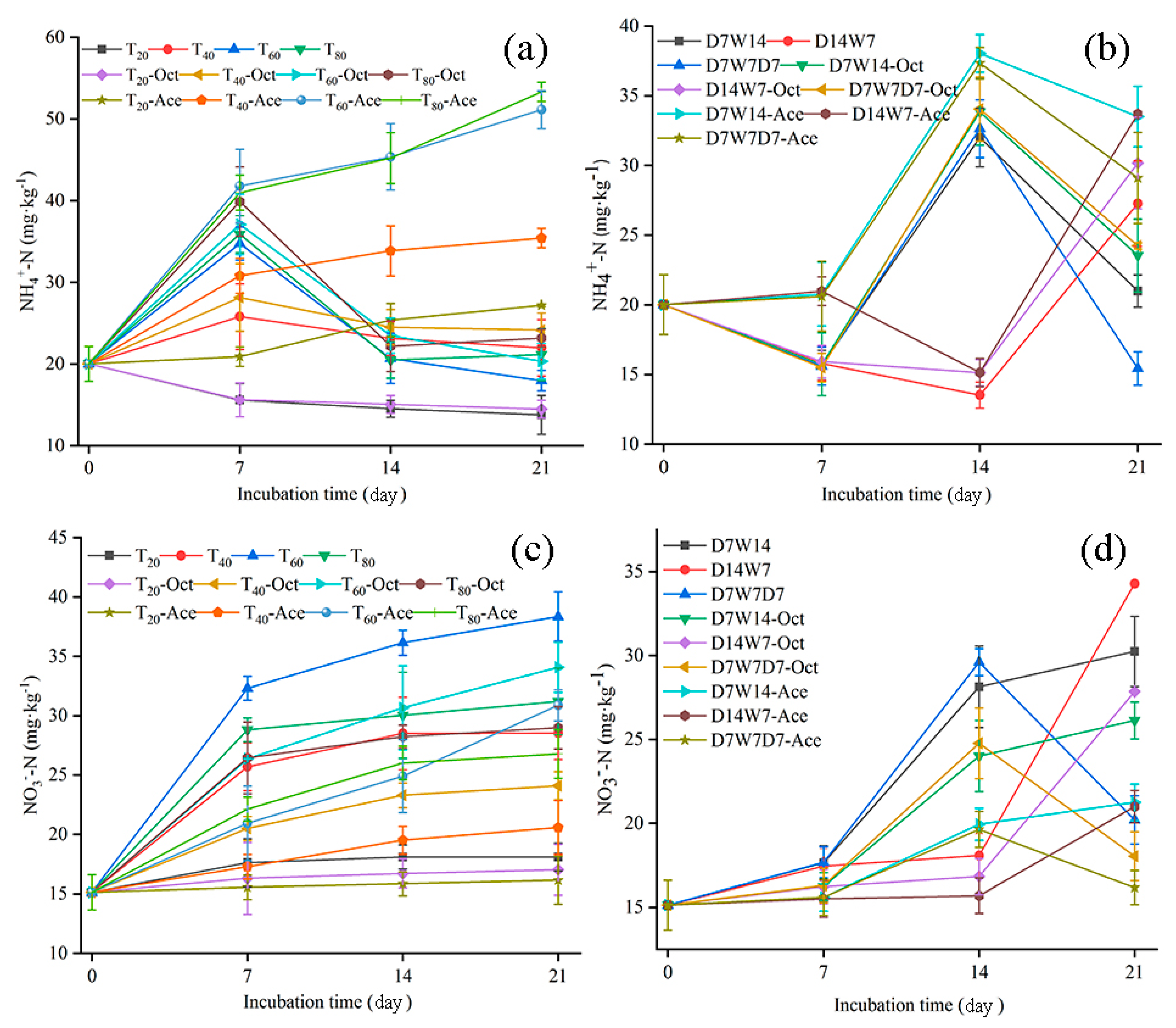
3.1. Effects of Water Supply Model on Soil Mineralization in Tea Garden
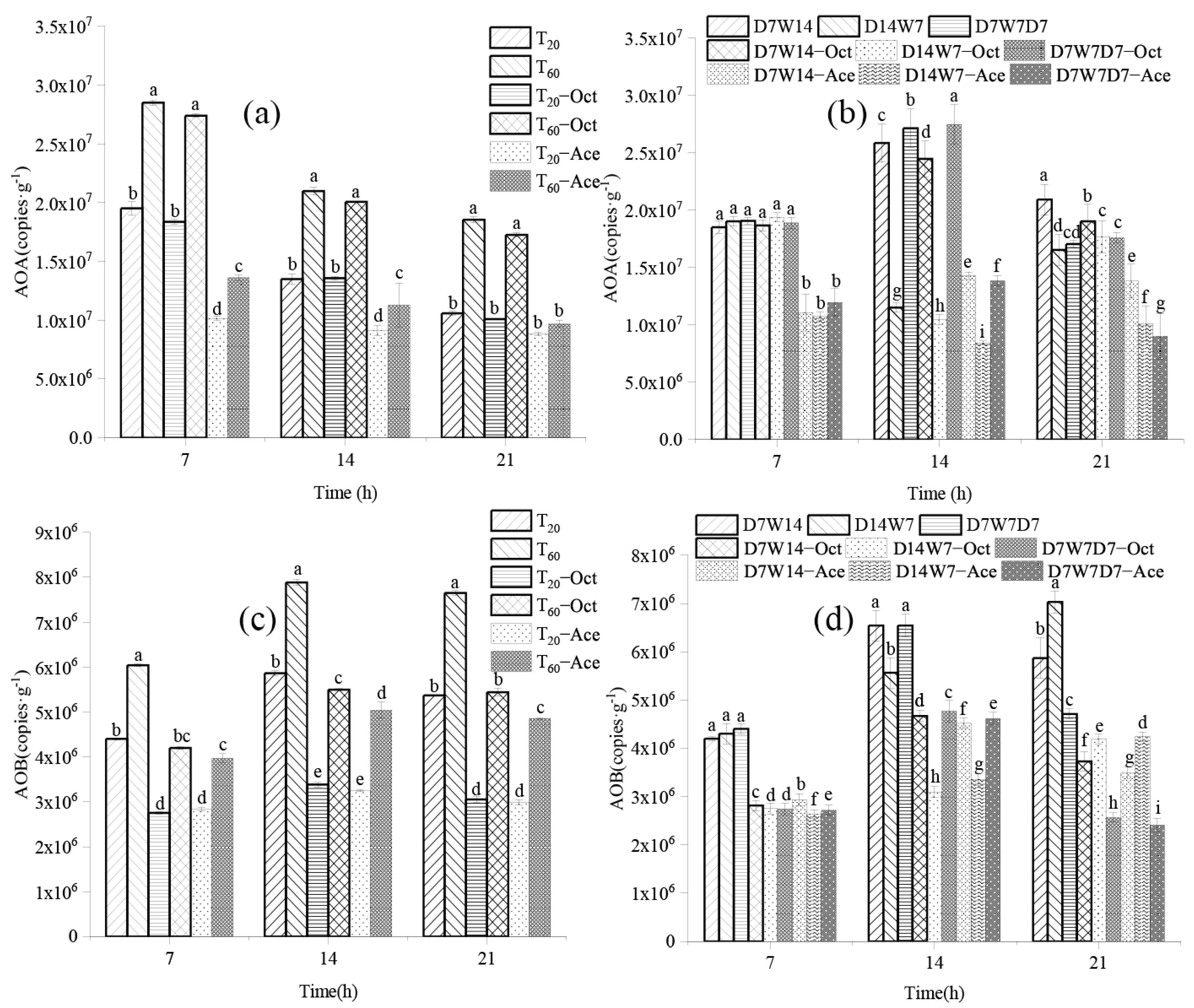
3.2. Relative Contribution of Ammonia-Oxidizing Microorganisms to Soil Nitrate Nitrogen
3.3. Effects of Different Water Supply Modes on Ammonia Oxidizing Microorganisms
3.3.1. Response of Gene Abundance of Ammonia Oxidizing Microorganisms to Different Water Supply Modes
3.3.2. The Community Composition of Ammonia Oxidizing Microorganisms
3.4. The Correlation Analysis
3.4.1. Spearman Correlation Analysis
3.4.2. Redundancy Analysis of Ammonia Oxidizing Microorganisms
4. Discussion
4.1. Effects of Different Moisture Modes on Nitrogen Conversion
4.2. The Driving Effect of Ammonia-Oxidizing Microorganisms on Ammonia Oxidation under Different Moisture Modes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, W.; Ma, L.; Shi, Y.; Ruan, J.; Kemmitt, S. Nitrogen release dynamics and transformation of slow release fertiliser products and their effects on tea yield and quality. J. Sci. Food Agric. 2008, 88, 839–846. [Google Scholar] [CrossRef]
- Yahdjian, L.; Sala, O. Size of precipitation pulses controls nitrogen transformation and losses in an arid patagonian ecosystem. Ecosystems 2010, 13, 575–585. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Han, X.; Yang, S. Influence of drought and dry-wet alternation on nitrogen transformation and low abundance microorganisms in tea garden soil. J. Environ. Biol. 2022, 43, 231–238. [Google Scholar] [CrossRef]
- Saetre, P.; Stark, J. Microbial dynamics and carbon and nitrogen cycling following re-wetting of soils beneath two semi-arid plant species. Oecologia 2005, 142, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, L.; Dai, Y.; Di, H.; He, J. pH-dependent distribution of soil ammonia oxidizers across a large geographical scale as revealed by higher-throughput pyrosequencing. J. Soil Sediments 2013, 13, 1439–1449. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Hu, H.; Kim, Y.; Duan, J.; Luo, C.; Wang, S.; Guo, L.; Zheng, Y. Ammonia oxidizers and denitrifiers in response to reciprocal elevation translocation in an alpine meadow on the Tibetan Plateau. J. Soils Sediments 2014, 14, 1189–1199. [Google Scholar] [CrossRef]
- Muhr, J.; Franke, J.; Borken, W. Drying-rewetting events reduce C and N losses from a Norway pruce forest floor. Soil Boil. Biochem. 2010, 42, 1303–1312. [Google Scholar] [CrossRef]
- Chen, D.; Mi, J.; Chu, P.; Cheng, J.; Zhang, L.; Pan, Q.; Xie, Y.; Bai, Y. Patterns and drivers of soil microbial communities along a precipitation gradient on the Mongolian Plateau. Landsc. Ecol. 2015, 30, 1669–1682. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Yang, S.; Yang, Z.; Lv, Y. Effect of a 10 degrees C-elevated temperature under different water contents on the microbial community in a tea orchard soil. Eur. J. Soil Biol. 2014, 62, 113–120. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Petersen, D.; Nuccio, E.; Firestone, M. Influence of oxic/anoxic fluctuations on ammonia oxidizers and nitrification potential in a wet tropical soil. FEMS Microbol. Ecol. 2013, 85, 179–194. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, G.; Ju, X.; Liu, R. How nitrification-related N2O is associated with soil ammonia oxidizers in two contrasting soils in China? Sci. Total Environ. 2021, 770, 143212. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Li, Y.; Xu, J.; Brookes, P. Abiotic processes dominate soil organic matter mineralization: Investigating the regulatory gate hypothesis by inoculating a previously fumigated soil with increasing fresh soil inocula. Geoderma 2020, 373, 114400. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Zhu, Q.; Ding, W. Image grey value analysis for estimating the effect of microorganism inoculants on straws decomposition. Comput. Electron. Agric. 2016, 128, 120–126. [Google Scholar] [CrossRef]
- Bernal, S.; Sabater, F.; Butturini, A.; Nin, E.; Sabater, S. Factors limiting denitrification in a Mediterranean riparian forest. Soil Boil. Biochem. 2007, 39, 2685–2688. [Google Scholar] [CrossRef]
- Francisco, S.; Urrutia, O.; Martin, V.; Peristeropoulos, A.; Garcia-Mina, J. Efficiency of urease and nitrification inhibitors in reducing ammonia volatilization from diverse nitrogen fertilizers applied to different soil types and wheat straw mulching. J. Sci. Food Agr. 2011, 91, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.; Bertiller, M.; Cisneros, H.; Olivera, N. Nitrification and ammonia-oxidizing bacteria shift in response to soil moisture and plant litter quality in arid soils from the Patagonian Monte. Pedobiologia 2016, 59, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Park, S.; Lee, B.; Jeong, K. Changes in Nitrogen Mineralization as Affected by Soil Temperature and Moisture. J. Korean Soc. Grassl. Forage Sci. 2018, 38, 196–201. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Xin, X.; Yuan, S.; Wang, R. Effects of belowground litter addition, increased precipitation and clipping on soil carbon and nitrogen mineralization in a temperate steppe. Biogeosciences 2013, 10, 7361–7372. [Google Scholar] [CrossRef]
- Kiese, R.; Hewett, B.; Butterbach-Bahl, K. Seasonal dynamic of gross nitrification and N2O emission at two tropical rainforest sites in Queensland, Australia. Plant Soil 2008, 309, 105–117. [Google Scholar] [CrossRef]
- Guntiñas, M.; Leirós, M.; Trasar-Cepeda, C.; Gil-Sotres, F. Effects of moisture and temperature on net soil nitrogen mineralization: A laboratory study. Eur. J. Soil. Biol. 2012, 48, 73–80. [Google Scholar] [CrossRef]
- Butcher, K.; Nasto, M.; Norton, J.; Stark, J. Physical mechanisms for soil moisture effects on microbial carbon-use efficiency in a sandy loam soil in the western United States. Soil Biol. Biochem. 2020, 150, 107969. [Google Scholar] [CrossRef]
- Weerts, A.; Kandhai, D.; Bouten, W.; Sloot, P. Tortuosity of an unsaturated sandy soil estimated using gas diffusion and bulk soil electrical conductivity. Soil Sci. Soc. Am. J. 2001, 65, 1577–1584. [Google Scholar] [CrossRef]
- Rex, D.; Clough, T.; Lanigan, G.; Jansen-Willems, A.; Condron, L.; Richards, K.; Müller, C. Gross N transformations vary with soil moisture and time following urea deposition to a pasture soil. Geoderma 2021, 386, 114904. [Google Scholar] [CrossRef]
- Gleeson, D.; Müller, C.; Banerjee, S.; Ma, W.; Siciliano, S.; Murphy, D. Response of ammonia oxidizing archaea and bacteria to changing water filled pore space. Soil Biol. Biochem. 2010, 42, 1888–1891. [Google Scholar] [CrossRef]
- Sun, L.; Xia, Z.; Sang, C.; Wang, X.; Bai, E. Soil resource status affects the responses of nitrogen processes to changes in temperature and moisture. Biol. Fert. Soils 2019, 55, 629–641. [Google Scholar] [CrossRef]
- Klein, C.; Van Logtestijn, R. Denitrification in grassland soils in The Netherlands in relation to irrigation, N-application rate, soil water content and soil temperature. Soil Biol. Biochem. 1996, 28, 231–237. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, J.; Zhao, B.; Cheng, Z.; Li, L. Water balance and nitrate leaching losses under intensive crop production with Ochric Aquic Cambosols in North China Plain. Environ. Int. 2005, 31, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Funakawa, S.; Kosaki, T. Effect of repeated drying-rewetting cycles on microbial biomass carbon in soils with different climatic histories. Appl. Soil Ecol. 2017, 120, 1–7. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Dijkstra, F.; Augustine, D.; Brewer, P.; von Fischer, C. Nitrogen cycling and water pulses in semiarid grasslands: Are microbial and plant processes temporally asynchronous? Oecologia 2012, 170, 799–808. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, L.; Bi, Q.; Dai, P.; Sun, D.; Sun, C.; Liu, W.; Lu, L.; Ni, W.; Lin, X. Comparative effects of 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) on ammonia-oxidizing bacteria and archaea in a vegetable soil. Appl. Microbiol. Biotechnol. 2015, 99, 477–487. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Shen, J.; He, J. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Lu, L.; Jia, Z. Urease gene-containing Archaea dominate autotrophic ammonia oxidation in two acid soils. Environ. Microbiol. 2013, 15, 1795–1809. [Google Scholar] [CrossRef]
- Pereira, E.; Poly, F.; Guillaumaud, N.; van Elsas, J.; Salles, J. Fluctuations in ammonia oxidizing communities across agricultural soils are driven by soil structure and pH. Front. Microbiol. 2012, 3, 77. [Google Scholar]
- Nicol, G.; Leininger, S.; Schlepe, C.; Prosser, J. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Song, H.; Che, Z.; Cao, W.; Huang, T.; Wang, J.; Dong, Z. Changing roles of ammonia-oxidizing bacteria and archaea in a continuously acidifying soil caused by over-fertilization with nitrogen. Environ. Sci. Pollut. Res. 2016, 23, 11964–11974. [Google Scholar] [CrossRef]
- He, J.; Hu, H.; Zhang, L. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- Li, X.; Ying, J.; Ying, C.; Zhang, L.; Gao, Y.; Bai, Y. Effects of nitrogen addition on the abundance and composition of soil ammonia oxidizers in Inner Mongolia grassland. Acta Ecol. Sin. 2011, 31, 174–178. [Google Scholar] [CrossRef]
- Di, H.; Cameron, K.; Shen, J.; Winefield, C.; O’Callaghan, M.; Bowatte, S.; He, J. Ammonia oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. Fems Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Roesser, M.; Müller, V. Osmoadaptation in bacteria and archaea: Common principles and differences. Environ. Microbiol. 2001, 3, 743–754. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Barnard, R.; Osborne, C.; Firestone, M. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Wallenstein, M. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Thion, C.; Prosser, J. Differential response of non-adapted ammonia oxidising archaea and bacteria to drying rewetting stress. FEMS Microbiol. Ecol. 2014, 90, 380–389. [Google Scholar] [PubMed]


| Water Supply Mode | No-Inhibitor Culture | Acetylene Culture | 1-Octyne Culture | |
|---|---|---|---|---|
| Constant humidity mode | soil water content was set to 20% WHC | T20 | T20-Ace | T20-Oct |
| soil water content was set to 40% WHC | T40 | T40-Ace | T40-Oct | |
| soil water content was set to 60% WHC | T60 | T60-Ace | T60-Oct | |
| soil water content was set to 80% WHC | T80 | T80-Ace | T80-Oct | |
| Dry–wet alternation mode | 20% WHC for 1–7 d, 60% WHC for 8–21 d | D7W14 | D7W14-Ace | D7W14-Oct |
| 20% WHC for 1–14 d, 60% WHC for 15–21 d | D14W7 | D14W7-Ace | D14W7-Oct | |
| 20% WHC for 1–7 d, 60% WHC for 8–14 d, 20% WHC for 15–21 d | D7W7D7 | D7W7D7-Ace | D7W7D7-Oct | |
| Target Gene | Primer Name | Sequence(5’-3’) | Length of Amplicon (bp) |
|---|---|---|---|
| AOA | amoA F | 5’-STAATGGTCTGGCTTAGACG-3’ | 635 |
| amoA R | 5’-GCGGCCATCCATCTGTATGT-3’ | ||
| AOB | amoA1F | 5’-GGGGTTTCTACTGGTGGT-3’ | 491 |
| amoA2R | 5’-CCCCTCKGSAAAGCCTTCTTC-3’ |
| Water Supply Mode | Relative Contribution Rate(%) | ||
|---|---|---|---|
| AOA | AOB | ||
| Constant humidity mode | T20 | 45.62 | 54.38 |
| T40 | 44.22 | 55.78 | |
| T60 | 42.75 | 57.25 | |
| T80 | 49.72 | 50.28 | |
| Dry–wet alternation mode | D7W14 | 54.34 | 45.66 |
| D14W7 | 51.63 | 48.37 | |
| D7W7D7 | 46.17 | 53.83 | |
| Water Supply Mode | NH4+ | NO3− | PNR | AOA | AOB | |
|---|---|---|---|---|---|---|
| Constant humidity mode | NH4+ | 1 | 0.571 * | 0. 643 ** | 0.938 ** | 0.631 * |
| NO3− | 1 | 0.955 ** | 0.640 * | 0.849 ** | ||
| PNR | 1 | 0.615 * | 0.724 ** | |||
| AOA | 1 | 0.406 | ||||
| AOB | 1 | |||||
| Dry–wet alternation mode | NH4+ | 1 | 0.572 ** | 0.645 ** | 0.689 ** | 0.626 ** |
| NO3− | 1 | 0.915 ** | 0.786 ** | 0.748 ** | ||
| PNR | 1 | 0.658 ** | 0.649 ** | |||
| AOA | 1 | 0.185 | ||||
| AOB | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Hou, J.; Zhou, B.; Han, X. Effects of Water Supply Mode on Nitrogen Transformation and Ammonia Oxidation Microorganisms in a Tea Garden. Agronomy 2023, 13, 1279. https://doi.org/10.3390/agronomy13051279
Wang H, Hou J, Zhou B, Han X. Effects of Water Supply Mode on Nitrogen Transformation and Ammonia Oxidation Microorganisms in a Tea Garden. Agronomy. 2023; 13(5):1279. https://doi.org/10.3390/agronomy13051279
Chicago/Turabian StyleWang, Heng, Jian Hou, Bo Zhou, and Xiaoyang Han. 2023. "Effects of Water Supply Mode on Nitrogen Transformation and Ammonia Oxidation Microorganisms in a Tea Garden" Agronomy 13, no. 5: 1279. https://doi.org/10.3390/agronomy13051279
APA StyleWang, H., Hou, J., Zhou, B., & Han, X. (2023). Effects of Water Supply Mode on Nitrogen Transformation and Ammonia Oxidation Microorganisms in a Tea Garden. Agronomy, 13(5), 1279. https://doi.org/10.3390/agronomy13051279






