The Impacts of Nitrogen Accumulation, Translocation, and Photosynthesis on Simultaneous Improvements in the Grain Yield and Gluten Quality of Dryland Wheat
Abstract
1. Introduction
2. Materials and Methods
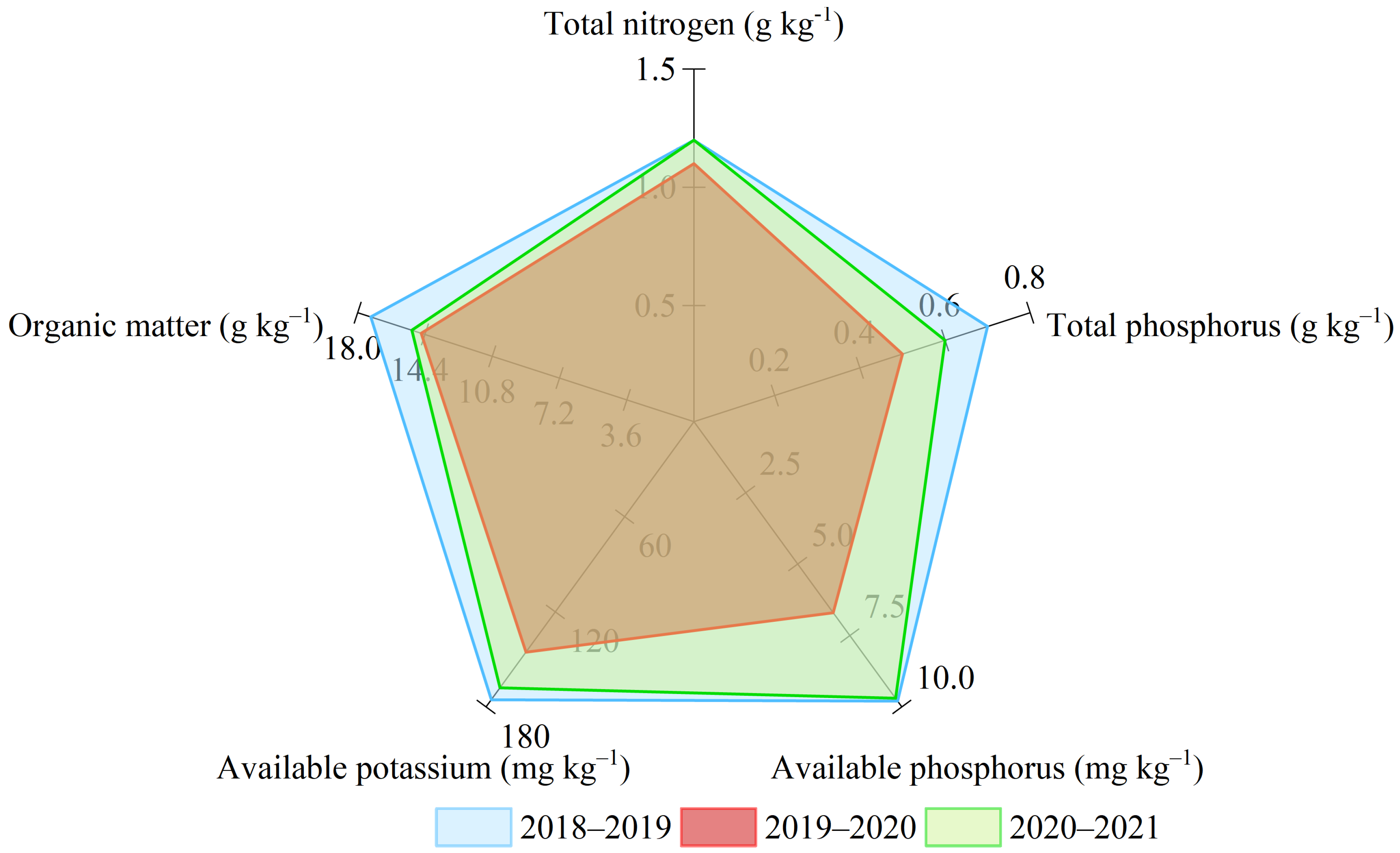
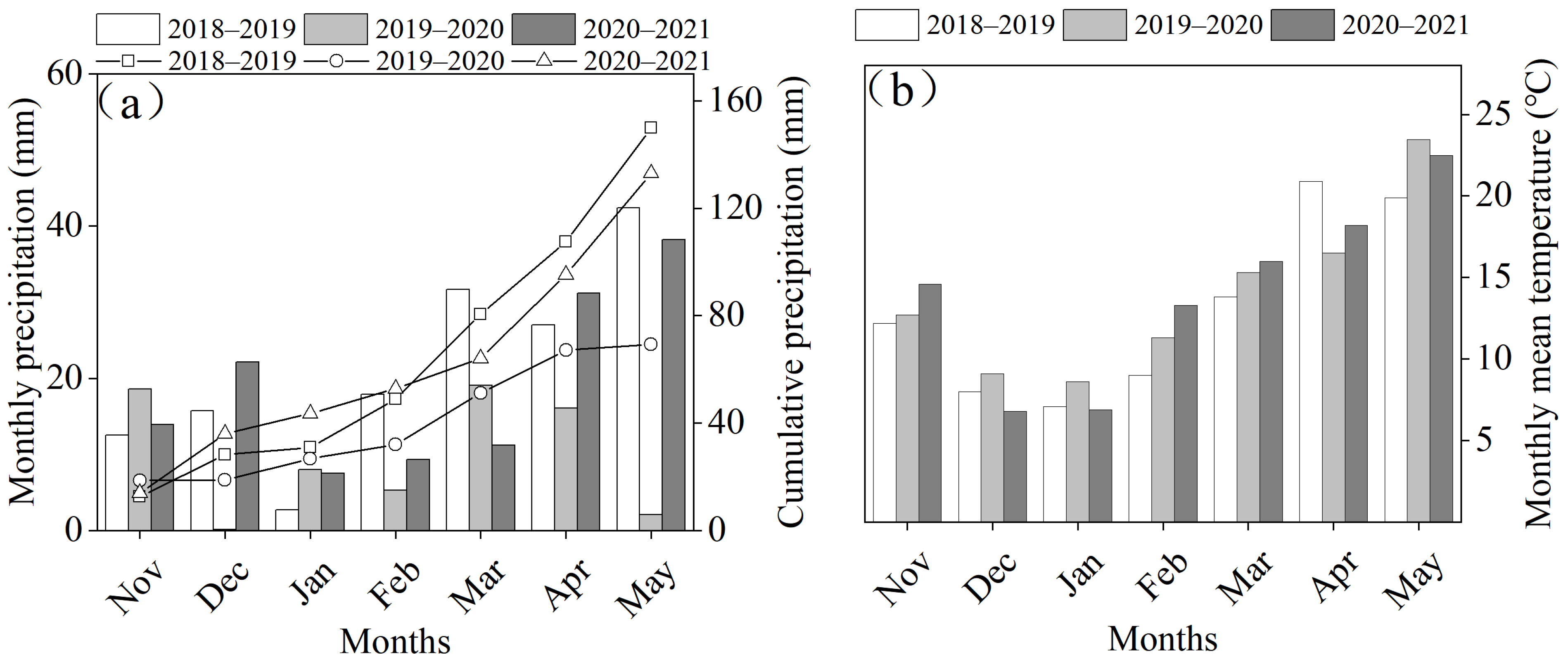
2.1. Experimental Site and Design
2.2. Experimental Material, Design, and Treatments
2.3. Measurements and Calculations
2.3.1. Yield, Yield Components, and Related Traits
2.3.2. NUE-Related Traits
2.3.3. Gluten Quality-Related Traits
2.3.4. Photosynthesis and Dry Matter Accumulation and Translocation
2.4. Statistical Analysis
3. Results
3.1. Combined Variance Analysis of Yield, Gluten Quality, and NUE-Related Traits
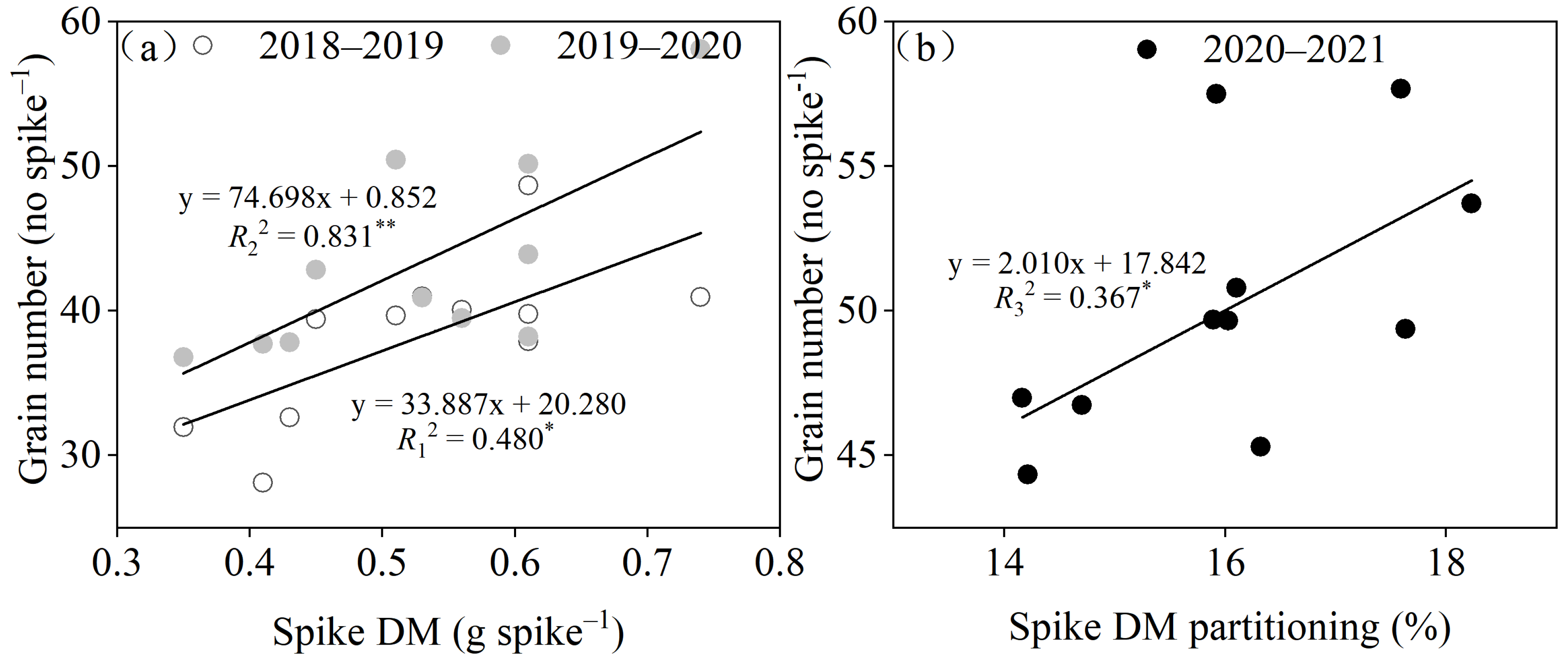
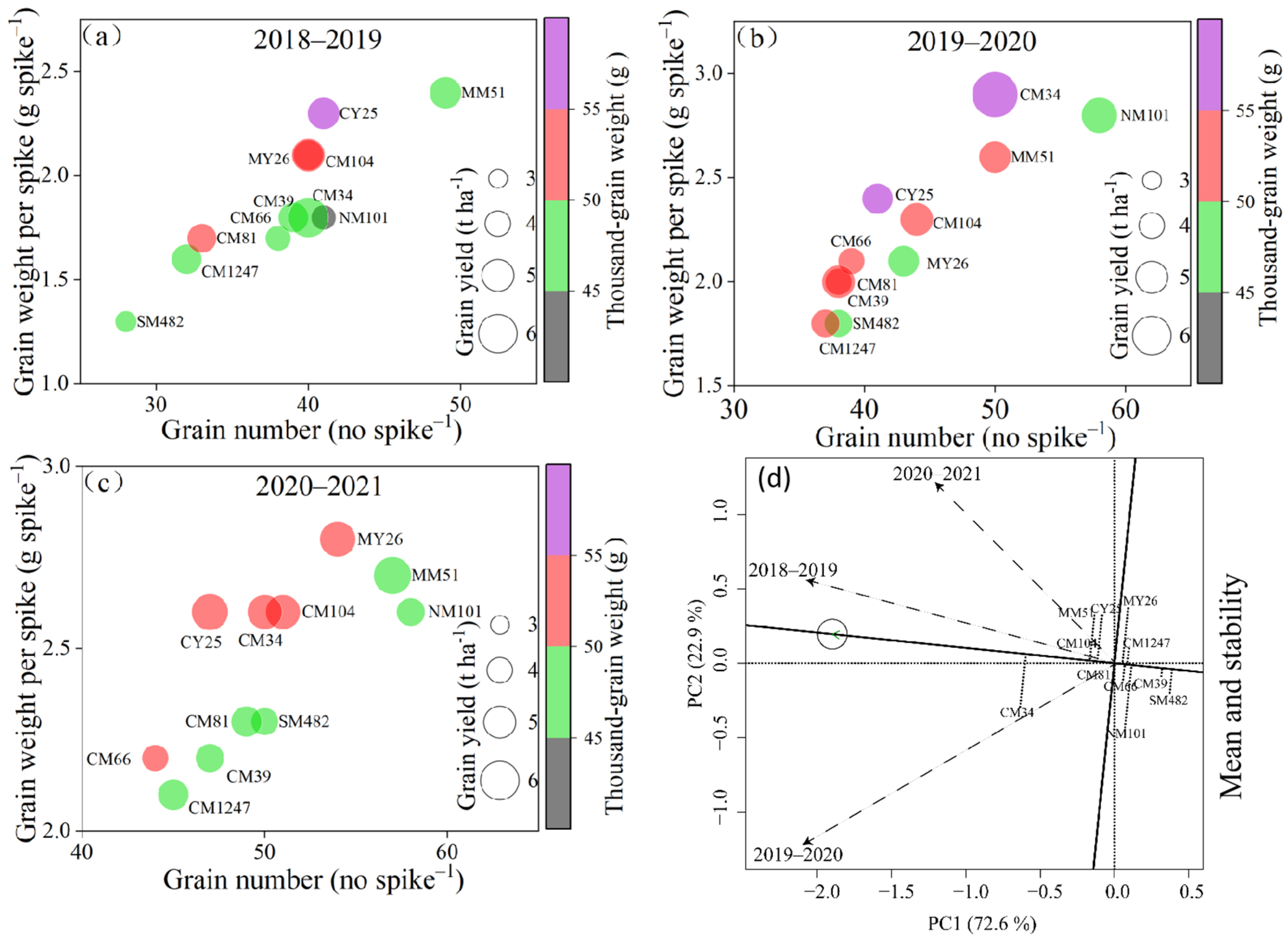
3.2. Yield and Yield-Related Traits
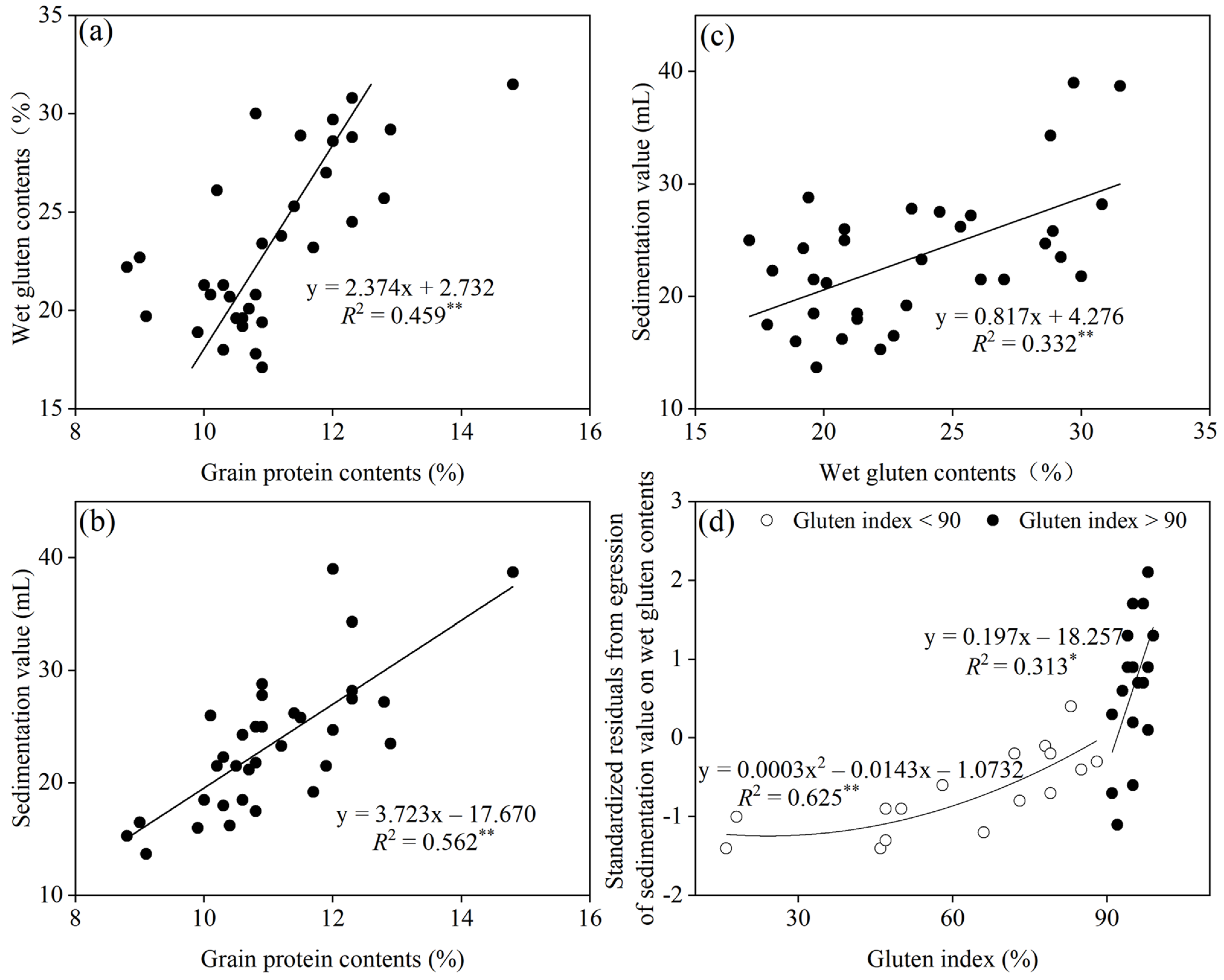
3.3. Gluten Quality-Related Traits
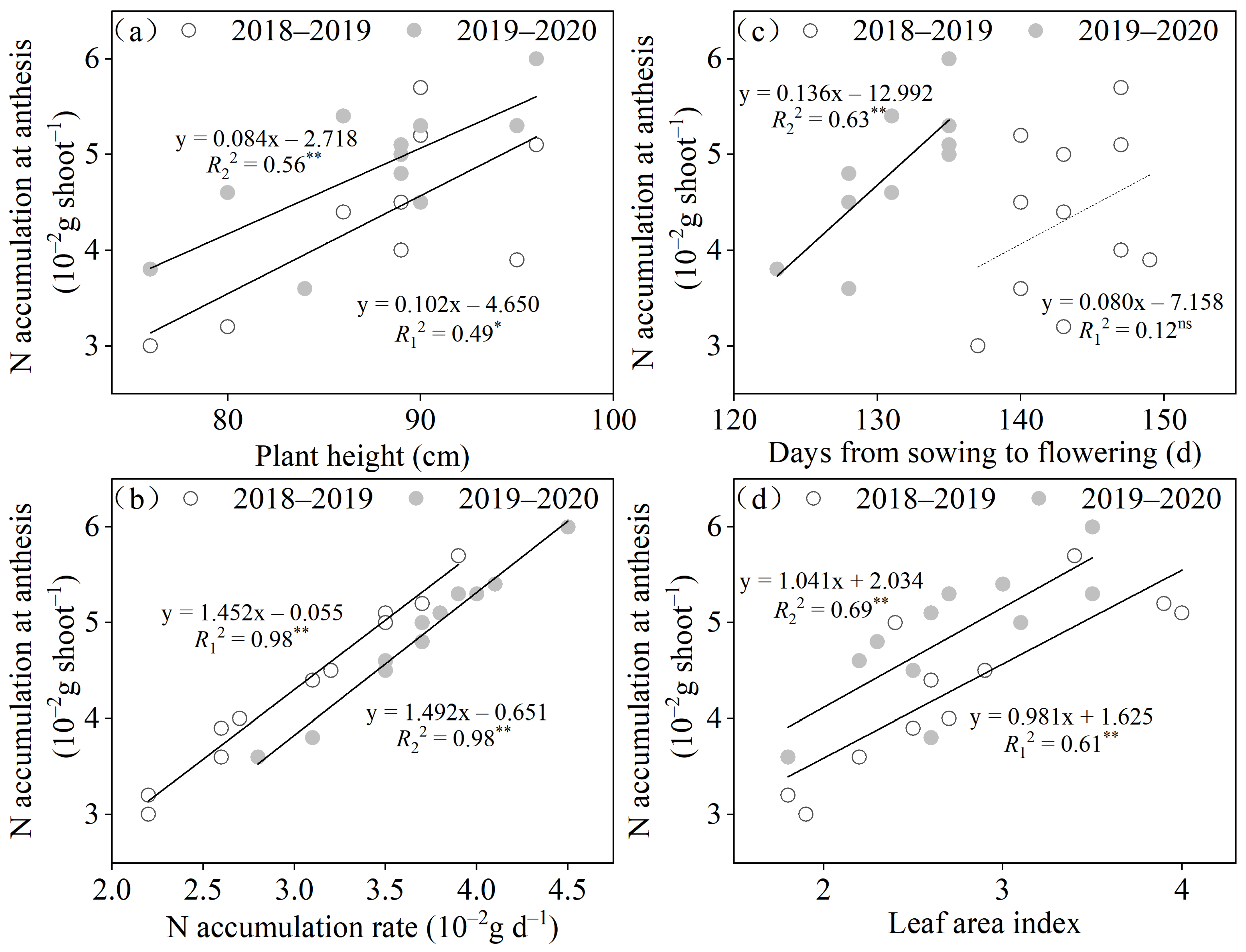
3.4. Relationships between NUE and Yield as Well as Gluten Quality-Related Traits
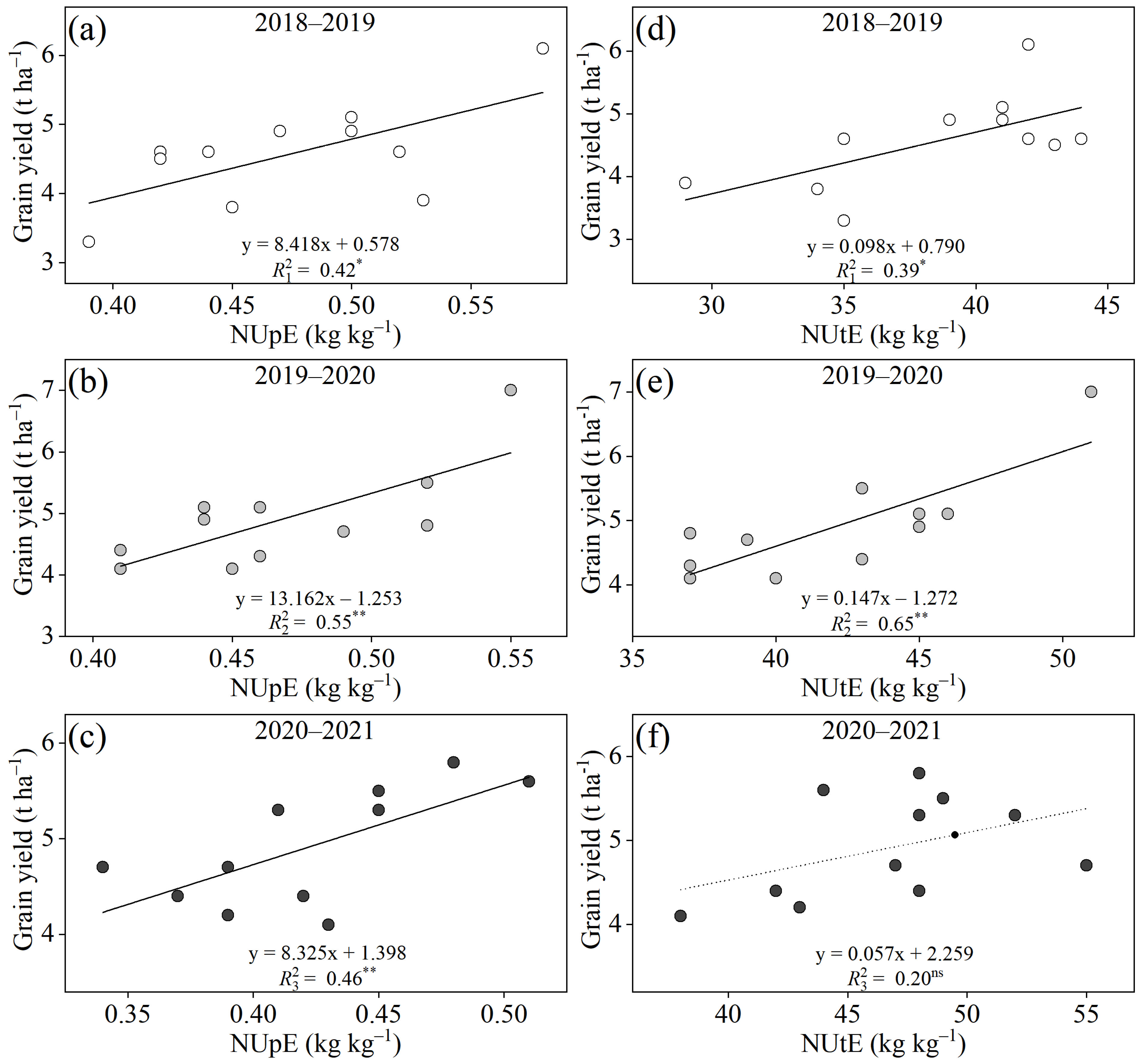
3.5. Critical NUE-Related Traits Associated with High Yield and High Gluten Quality
3.6. Photosynthesis, Dry Matter Accumulation, and Translocation
4. Discussion
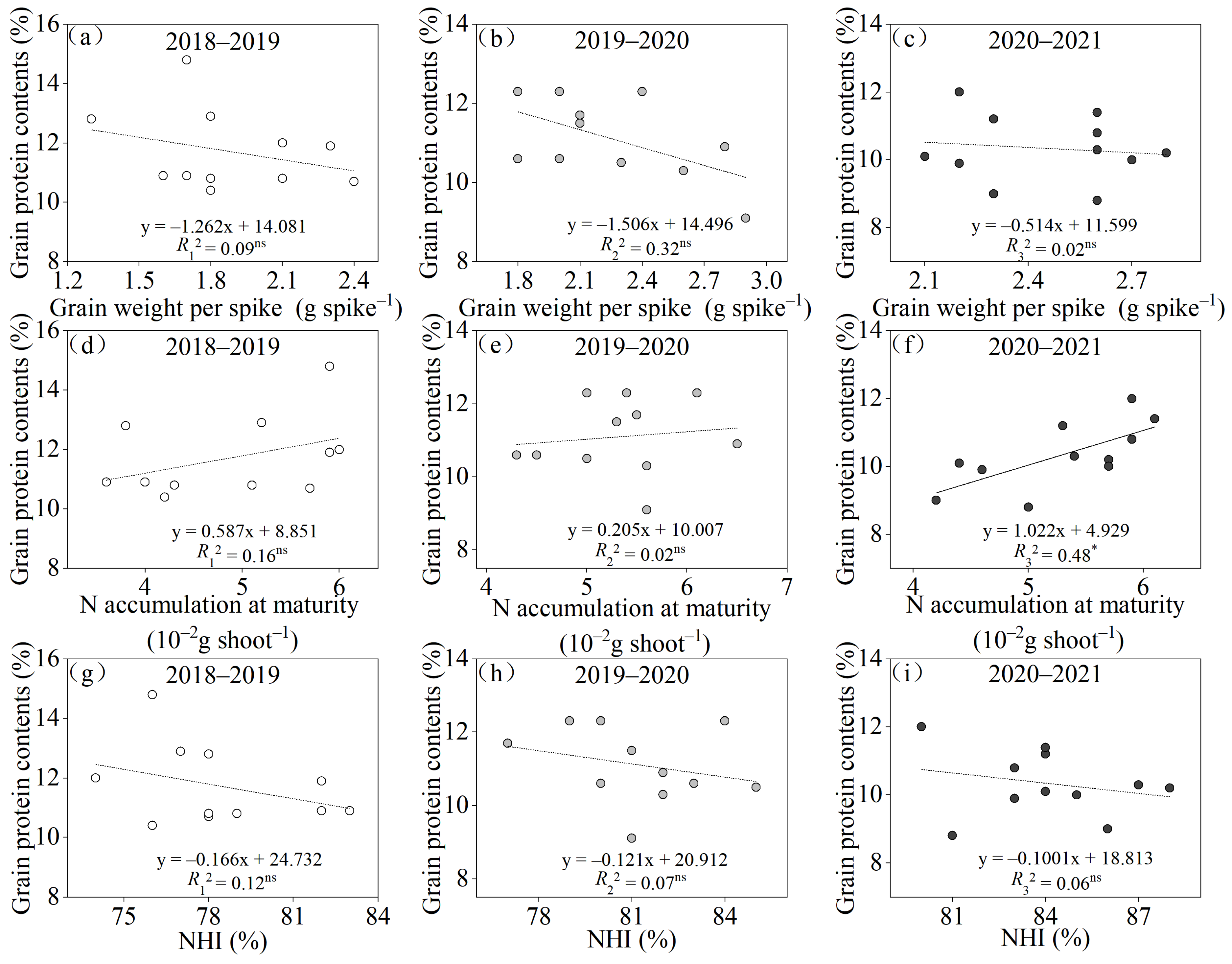
4.1. Variations in Grain Weight per Spike and Individual N Accumulation at the Maturity Stage Affect Simultaneous Improvements in the Grain Yield and Protein Contents
4.2. The Genotype–Environment Interaction Effect on the Gluten Index Was Related to N Translocation Affected by Source N Supply and Sink N Demands
4.3. Photosynthesis Improvements Simultaneously Improved the Grain Yield and Gluten Quality by Increasing N Accumulation or Contributing to N Translocation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baresel, J.P.; Zimmermann, G.; Reents, H.J. Effects of genotype and environment on N uptake and N partition in organically grown winter wheat (Triticum aestivum L.) in Germany. Euphytica 2008, 163, 347–354. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Yu, Z.; Zeng, J.; Xu, D.; Dong, J.; Ma, W. Wheat quality formation and its regulatory mechanism. Front. Plant Sci. 2022, 13, 834654. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yu, Z.; She, M.; Zhao, Y.; Islam, S. Wheat gluten protein and its impacts on wheat processing quality. Front. Agric. Sci. Eng. 2019, 6, 279–287. [Google Scholar] [CrossRef]
- Pasha, I.; Anjum, F.M.; Butt, M.S.; Sultan, J.I. Gluten quality prediction and correlation studies in spring wheats. J. Food Qual. 2007, 30, 438–449. [Google Scholar] [CrossRef]
- Simic, G.; Horvat, D.; Jurkovic, Z.; Drezner, G.; Novoselovic, D.; Dvojkovic, K. The genotype effect on the ratio of wet gluten content to total wheat grain protein. J. Cent. Eur. Agric. 2006, 7, 13–18. [Google Scholar]
- Šekularac, A.; Torbica, A.; Živančev, D.; Tomić, J.; Knežević, D. The influence of wheat genotype and environmental factors on gluten index and the possibility of its use as bread quality predictor. Genetika 2018, 50, 85–93. [Google Scholar] [CrossRef]
- Kaushik, R.; Kumar, N.; Sihag, M.K.; Ray, A. Isolation, characterization of wheat gluten and its regeneration properties. J. Food Sci. Technol. 2015, 52, 5930–5937. [Google Scholar] [CrossRef]
- Rapp, M.; Lein, V.; Lacoudre, F.; Lafferty, J.; Müller, E.; Vida, G.; Bozhanova, V.; Ibraliu, A.; Thorwarth, P.; Piepho, H.P. Simultaneous improvement of grain yield and protein content in durum wheat by different phenotypic indices and genomic selection. Theor. Appl. Genet. 2018, 131, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Godin, C. Yield and grain protein concentration in bread wheat: How to use the negative relationship between the two characters to identify favourable genotypes? Euphytica 2007, 157, 45–57. [Google Scholar] [CrossRef]
- Nigro, D.; Gadaleta, A.; Mangini, G.; Colasuonno, P.; Marcotuli, I.; Giancaspro, A.; Giove, S.L.; Simeone, R.; Blanco, A. Candidate genes and genome-wide association study of grain protein content and protein deviation in durum wheat. Planta 2019, 249, 1157–1175. [Google Scholar] [CrossRef]
- Mosleth, E.F.; Lillehammer, M.; Pellny, T.K.; Wood, A.J.; Riche, A.B.; Hussain, A.; Griffiths, S.; Hawkesford, M.J.; Shewry, P.R. Genetic variation and heritability of grain protein deviation in European wheat genotypes. Field Crops Res. 2020, 255, 107896. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, D.J.; Posner, E.S. Can bread wheat quality be determined by gluten index? J. Cereal Sci. 2012, 56, 115–118. [Google Scholar] [CrossRef]
- Triboi, E.; Martre, P.; Girousse, C.; Ravel, C.; Triboi-Blondel, A.M. Unravelling environmental and genetic relationships between grain yield and nitrogen concentration for wheat. Eur. J. Agron. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef]
- Fischer, T. Wheat physiology: A review of recent developments. Crop. Pasture Sci. 2011, 62, 95–114. [Google Scholar] [CrossRef]
- Burnett, A.C.; Serbin, S.P.; Rogers, A. Source: Sink imbalance detected with leaf-and canopy-level spectroscopy in a field-grown crop. Plant Cell Environ. 2021, 44, 2466–2479. [Google Scholar] [CrossRef]
- Zhou, B.; Serret, M.D.; Pie, J.B.; Shah, S.S.; Li, Z. Relative contribution of nitrogen absorption, remobilization, and partitioning to the ear during grain filling in Chinese winter wheat. Front. Plant Sci. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef]
- Gaju, O.; DeSilva, J.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M.J. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crops Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Lammerts, V.B.E.T.; Struik, P.C. Diverse concepts of breeding for nitrogen use efficiency: A review. Agron. Sustain. Dev. 2017, 37, 50. [Google Scholar] [CrossRef]
- Lemaire, G.; Ciampitti, I. Crop mass and N status as prerequisite covariables for unraveling nitrogen use efficiency across genotype-by-environment-by-management scenarios: A review. Plants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Uauy, C.; Brevis, J.C.; Dubcovsky, A.J. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef]
- Zhao, D.; Derkx, A.P.; Liu, D.C.; Buchner, P.; Hawkesford, M.J.; Flemetakis, E.; Flemetakis, E. Overexpression of a NAC transcription factor delays leaf senescence and increases grain nitrogen concentration in wheat. Plant Biol. 2015, 17, 904–913. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; Machet, J.; Jeuffroy, M.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the grain protein concentration–grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Li, C.; Li, M.; Xiong, T.; Tang, Y. Dry matter and nitrogen accumulation, partitioning, and translocation in synthetic-derived wheat cultivars under nitrogen deficiency at the post-jointing stage. Field Crops Res. 2020, 248, 107720. [Google Scholar] [CrossRef]
- Fischer, R.A. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agric. Sci. 1985, 105, 447–461. [Google Scholar] [CrossRef]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Z Anal Chem. 1883, 22, 366–375. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, L.; Lei, Y. Correlation among SDS sedimentation value, swelling index of glutenin and solvent retention capacity of spring wheat. Not. Sci. Biol. 2012, 4, 132–135. [Google Scholar] [CrossRef]
- Sadras, V.O.; Slafer, G.A. Environmental modulation of yield components in cereals: Heritabilities reveal a hierarchy of phenotypic plasticities. Field Crops Res. 2012, 127, 215–224. [Google Scholar] [CrossRef]
- Hu, Y.S.; Ren, T.H.; Li, Z.; Tang, Y.Z.; Ren, Z.L.; Yan, B.J. Molecular mapping and genetic analysis of a QTL controlling spike formation rate and tiller number in wheat. Gene 2017, 634, 15. [Google Scholar] [CrossRef]
- Madry, W.; Studnicki, M.; Rozbicki, J. Ontogenetic-based sequential path analysis of grain yield and its related traits in several winter wheat cultivars. Acta Agric. Scand. 2015, 65, 605–618. [Google Scholar] [CrossRef]
- Sadras, V.O.; Savin, R.; Slafer, G.A. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Liu, Y.; Cui, Y.; Zahoor, R.; Jiang, D.; Dai, T. Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and -sensitive wheat cultivars. Plant Physiol. Biochem. 2016, 106, 218–227. [Google Scholar] [CrossRef]
- Brueck, H.; Lammel, J. Impact of fertilizer N application on the grey water footprint of winter wheat in a NW-European temperate climate. Water 2016, 8, 356. [Google Scholar] [CrossRef]
- Carolina, R.; Eliseo, T.; Gemma, M.; Matthew, P.R.; Roger, S.; Foulkes John, M. Optimizing dry-matter partitioning for increased spike growth, grain number and harvest index in spring wheat—Science Direct. Field Crops Res. 2019, 240, 154–167. [Google Scholar]
- Zhang, H.; Richards, R.; Riffkin, P.; Berger, J.; Christy, B.; O’Leary, G.; Acuña, T.B.; Merry, A. Wheat grain number and yield: The relative importance of physiological traits and source-sink balance in Southern Australia. Eur. J. Agron. 2019, 110, 125935. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Hawkesford, M.J.; Barraclough, P.B.; Holdsworth, M.J.; Kerr, S.; Kightley, S.; Shewry, P.R. Identifying traits to improve the nitrogen economy of wheat: Recent advances and future prospects. Field Crops Res. 2009, 114, 329–342. [Google Scholar] [CrossRef]
- Slafer, G.A.; Elia, M.; Savin, R.; García, G.A.; Terrile, I.I.; Ferrante, A.; Miralles, D.J.; González, F.G. Fruiting efficiency: An alternative trait to further rise wheat yield. Food Energy Secur. 2015, 4, 92–109. [Google Scholar] [CrossRef]
- Feng, S.; Gu, S.; Zhang, H.; Wang, D. Root vertical distribution is important to improve water use efficiency and grain yield of wheat. Field Crops Res. 2017, 214, 131–141. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Christopher, J.T.; Hammer, G.L.; Devoil, P. Experimental and modelling studies of drought-adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosyst. 2010, 144, 458–462. [Google Scholar] [CrossRef]
- Mosleth, E.F.; Wan, Y.; Lysenko, A.; Chope, G.A.; Penson, S.P.; Shewry, P.R.; Hawkesford, M.J. A novel approach to identify genes that determine grain protein deviation in cereals. Plant Biotechnol. J. 2015, 13, 625–635. [Google Scholar] [CrossRef]
- Worland, B.; Robinson, N.; Jordan, D.; Schmidt, S.; Godwin, I. Post-anthesis nitrate uptake is critical to yield and grain protein content in Sorghum bicolor. J. Plant Physiol. 2017, 216, 118–124. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Xu, X.; Liu, N.; Zhu, D.; Wang, Z.; Yan, Y. Effect of high-nitrogen fertilizer on gliadin and glutenin subproteomes during kernel development in wheat (Triticum aestivum L.). Crop J. 2020, 8, 38–52. [Google Scholar] [CrossRef]
- Charmet, G.; Robert, N.; Branlard, G.; Linossier, L.; Martre, P.; Triboi, E. Genetic analysis of dry matter and nitrogen accumulation and protein composition in wheat kernels. Theor. Appl. Genet. 2005, 111, 540–550. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Lopez-Bellido, R.; Hawkesford, M.J. Genotypic variation in the uptake, partitioning and remobilisation of nitrogen during grain-filling in wheat. Field Crops Res. 2014, 156, 242–248. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef]
- Hajas, L.; Scherf, K.A.; Török, K.; Bugyi, Z.; Schall, E.; Poms, R.E.; Koehler, P.; Tömösközi, S. Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis. Food Chem. 2018, 267, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, F.; Hu, H.; Jiang, S.; Muhammad, A.; Shao, Y.; Sun, C.; Tian, Z.; Jiang, D.; Dai, T. Improved leaf nitrogen reutilisation and Rubisco activation under short-term nitrogen-deficient conditions promotes photosynthesis in winter wheat (Triticum aestivum L.) at the seedling stage. Funct Plant Biol. 2018, 45, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.B.; Pozniak, C.; Fahima, T.; Krugman, T. Grain protein content and thousand kernel weight QTLs identified in a durum × wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2020, 133, 119–131. [Google Scholar] [CrossRef] [PubMed]


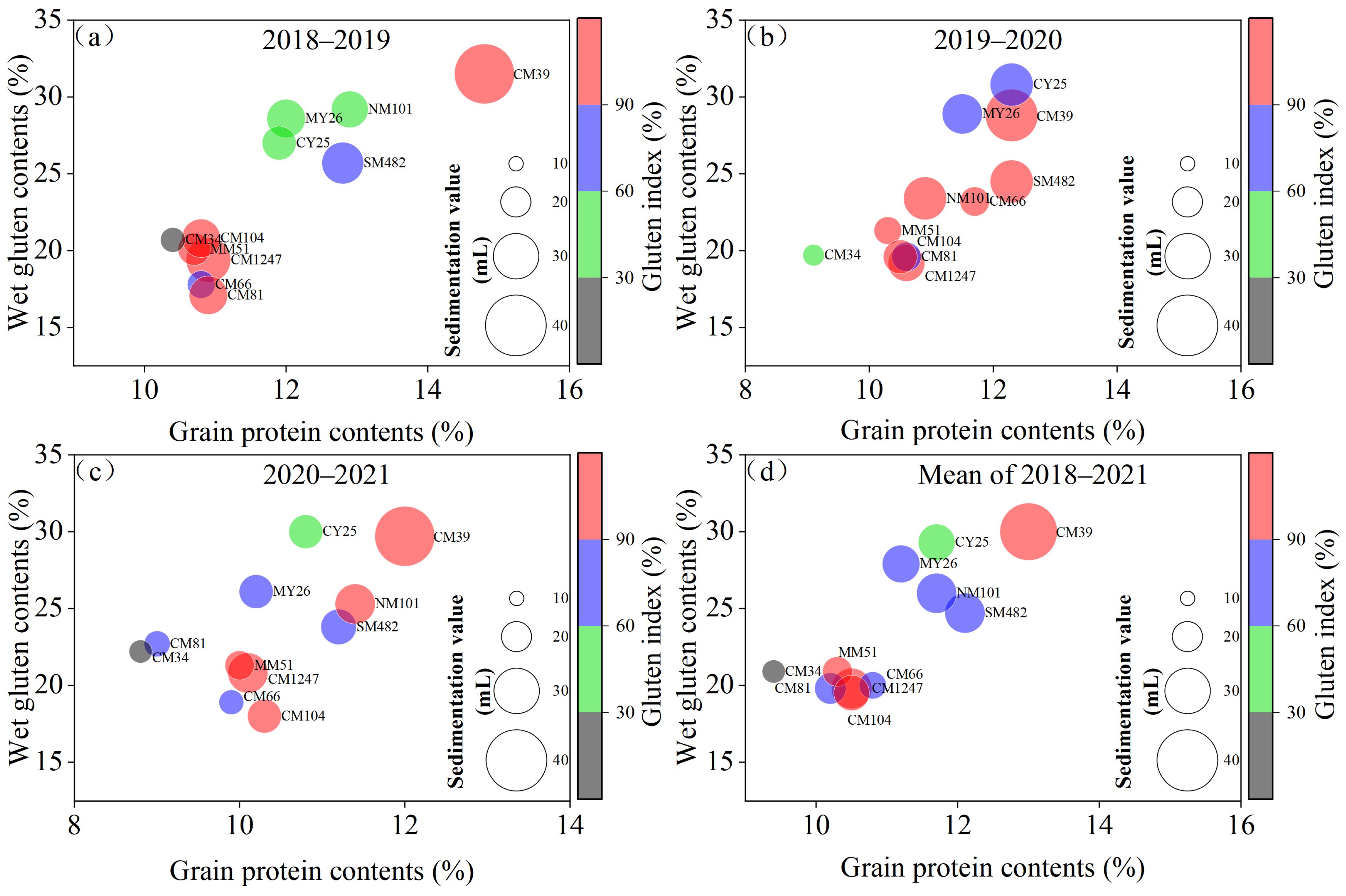


| Genotype | Breeding Groups | Dwarfing Genes | Grain Yield (t ha−1) | Wet Gluten Content (%) | Gluten Index (%) | Harves Index | Yield and Gluten Quality Property | |
|---|---|---|---|---|---|---|---|---|
| G1 | SW482 | L | Rht 8 | 3.2 | 31.7 | 88 | 0.46 | Low yield and low wet gluten content |
| G2 | CW39 | AD | Rht 8 | 3.2 | 36.0 | 97 | 0.39 | Low yield and high wet gluten content |
| G3 | CW1247 | L | Rht 8 | 3.4 | 28.7 | 98 | 0.48 | Low yield and low wet gluten content |
| G4 | CW66 | AD | Rht 8 | 3.4 | 30.0 | 67 | 0.43 | Low yield and low wet gluten content |
| G5 | NW101 | L | Rht D1b | 2.9 | 40.4 | 54 | 0.34 | Low yield and high wet gluten content |
| G6 | MY26 | AD | Rht 8 | 3.3 | 37.3 | 73 | 0.46 | Low yield and high wet gluten content |
| G7 | CW81 | L | Rht 8 | 3.1 | 30.3 | 84 | 0.47 | Low yield and low wet gluten content |
| G8 | CB25 | S × AD | Rht 8 | 4.1 | 38.2 | 52 | 0.47 | High yield and high wet gluten content |
| G9 | MW51 | AD | Rht D1b | 3.9 | 27.4 | 87 | 0.47 | High yield and low wet gluten content |
| G10 | CW104 | S × AD | Rht 8 | 3.9 | 30.2 | 89 | 0.49 | High yield and low wet gluten content |
| G11 | CW34 | S × AD | Rht 8 | 4.2 | 28.3 | 35 | 0.49 | High yield and low wet gluten content |
| Indicators | Mean Values | F-Value | ||||
|---|---|---|---|---|---|---|
| 2018–2019 | 2019–2020 | 2020–2021 | G | Y | G × Y | |
| Grain yield (t ha−1) | 4.6 a | 4.9 a | 4.9 a | 85 ** | 35 ** | 20 ** |
| Fertile spikes (no plant−1) | 1.4 a | 1.2 b | 1.1 c | 37 ** | 199 ** | 14 ** |
| Grain number (no spike−1) | 38 c | 43 b | 50 a | 114 ** | 628 ** | 20 ** |
| Thousand-grain weight (g) | 49 b | 52 a | 49 b | 79 ** | 94 ** | 13 ** |
| Grain weight per spike (g spike−1) | 1.9 c | 2.3 b | 2.5 a | 119 ** | 561 ** | 25 ** |
| Sedimentation value (mL) | 24.8 a | 23.7 ab | 22.6 b | 137 ** | 19 ** | 8 ** |
| Grain protein contents (%) | 11.7 a | 11.1 b | 10.3 c | 146 ** | 246 ** | 16 ** |
| Wet gluten contents (%) | 23.4 a | 23.6 a | 23.5 a | 164 ** | 0 ns | 12 ** |
| Gluten index (%) | 74 b | 87 a | 76 b | 1008 ** | 392 ** | 90 ** |
| N accumulation at anthesis (10−2 g shoot−1) | 4.3 b | 4.9 a | 3.4 c | 98 ** | 578 ** | 20 ** |
| Post-anthesis N accumulation (10−2 g shoot−1) | 0.5 b | 0.5 b | 1.9 a | 35 ** | 1175 ** | 13 ** |
| N accumulation at maturity (10−2 g shoot−1) | 4.9 b | 5.4 a | 5.3 a | 97 ** | 52 ** | 13 ** |
| NUpE (kg kg−1) | 0.47 a | 0.47 a | 0.42 b | 27 ** | 63 ** | 15 ** |
| N translocation efficiency (%) | 76 b | 79 a | 75 b | 21 ** | 198 ** | 32 ** |
| NUtE (kg kg−1) | 39 c | 42 b | 47 a | 196 ** | 689 ** | 27 ** |
| NHI (%) | 78 c | 81 b | 84 a | 58 ** | 597 ** | 36 ** |
| Cropping Season | Genotype | N Content (%) | Leaf Area (cm2) | Pn (µmol m−2 s−1) | Tr (mmol m−2 s−1) | Ci (µmol m−2 s−1) | WUE (10−3 mol mol−1) |
|---|---|---|---|---|---|---|---|
| 2019–2020 | SM482 | 3.6 de | 32 e | 18.4 b | 2.9 de | 405 a | 6.5 c |
| CM39 | 3.6 de | 38 d | 17.9 c | 2.3 f | 252 e | 7.9 b | |
| CM1247 | 3.4 f | 29 f | 16.7 e | 2.7 e | 254 e | 6.1 d | |
| CM66 | 3.8 b | 48 b | 16.2 f | 4.9 a | 380 b | 3.3 g | |
| NM101 | 3.3 g | 56 a | 17.1 d | 3.4 b | 228 f | 2.0 h | |
| MY26 | 3.8 bc | 36 d | 17.2 d | 3.5 b | 250 e | 4.9 f | |
| CM81 | 3.6 e | 31 ef | 17.0 d | 3.2 c | 211 f | 5.3 e | |
| CY25 | 3.7 cd | 43 c | 20.4 a | 2.3 f | 308 c | 8.6 a | |
| MM51 | 3.6 de | 37 d | 18.6 b | 2.2 f | 258 e | 8.5 a | |
| CM104 | 4.1 a | 32 ef | 17.2 d | 2.2 f | 285 d | 8.0 b | |
| CM34 | 3.4 f | 47 b | 16.6 e | 2.9 d | 211 f | 5.8 d | |
| 2020–2021 | SM482 | 3.4 bd | 36 f | 24.6 a | 4.7 d | 220 ab | 5.2 c |
| CM39 | 3.3 d | 36 ef | 16.8 f | 5.7 ab | 162 f | 2.9 f | |
| CM1247 | 3.5 b | 29 h | 19.3 de | 3.8 e | 130 g | 5.1 c | |
| CM66 | 3.3 d | 37 ef | 21.2 c | 6.0 a | 205 bd | 3.6 e | |
| NM101 | 2.9 e | 51 a | 20.2 ce | 5.5 bc | 159 f | 3.7 e | |
| MY26 | 3.7 a | 41 cd | 24.5 a | 4.0 e | 230 a | 6.1 b | |
| CM81 | 3.4 bc | 38 de | 19.1 e | 5.3 c | 193 de | 3.6 e | |
| CY25 | 3.4 bc | 43 c | 25.3 a | 5.0 d | 197 ce | 5.1 c | |
| MM51 | 3.4 bc | 37 ef | 22.6 b | 4.7 d | 186 e | 4.8 d | |
| CM104 | 3.3 cd | 33 g | 20.9 c | 3.0 f | 164 f | 6.8 a | |
| CM34 | 2.8 e | 47 b | 20.3 cd | 5.5 bc | 211 bc | 3.7 e |
| Genotype | 2019–2020 | 2020–2021 | ||
|---|---|---|---|---|
| Stem DM Translocation (g Plant−1) | Grain DM Accumulation (g Plant−1) | Stem DM Translocation (g Plant−1) | Grain DM Accumulation (g Plant−1) | |
| SM482 | 1.3 cd | 2.1 f | 1.0 ad | 1.4 g |
| CM39 | 1.3 cd | 2.3 d | 0.8 de | 1.7 d |
| CM1247 | 1.0 e | 1.9 h | 0.7 e | 1.5 f |
| CM66 | 1.1 e | 1.9 h | 0.9 bd | 1.8 c |
| NM101 | 1.7 a | 2.8 b | 1.0 ac | 1.8 c |
| MY26 | 1.2 d | 2.2 e | 0.9 bd | 2.0 a |
| CM81 | 1.4 c | 2.1 f | 0.8 de | 1.6 e |
| CY25 | 1.3 cd | 2.9 a | 1.1 a | 2.0 a |
| MM51 | 1.6 b | 2.5 c | 1.1 ab | 1.8 c |
| CM104 | 1.1 e | 2.0 g | 0.9 ce | 1.7 d |
| CM34 | 1.3 cd | 2.5 c | 1.1 a | 1.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, H.; Chen, R.; Yang, H.; Zheng, T.; Huang, X.; Fan, G. The Impacts of Nitrogen Accumulation, Translocation, and Photosynthesis on Simultaneous Improvements in the Grain Yield and Gluten Quality of Dryland Wheat. Agronomy 2023, 13, 1283. https://doi.org/10.3390/agronomy13051283
Chen Y, Chen H, Chen R, Yang H, Zheng T, Huang X, Fan G. The Impacts of Nitrogen Accumulation, Translocation, and Photosynthesis on Simultaneous Improvements in the Grain Yield and Gluten Quality of Dryland Wheat. Agronomy. 2023; 13(5):1283. https://doi.org/10.3390/agronomy13051283
Chicago/Turabian StyleChen, Yufeng, Haolan Chen, Renhua Chen, Hongkun Yang, Ting Zheng, Xiulan Huang, and Gaoqiong Fan. 2023. "The Impacts of Nitrogen Accumulation, Translocation, and Photosynthesis on Simultaneous Improvements in the Grain Yield and Gluten Quality of Dryland Wheat" Agronomy 13, no. 5: 1283. https://doi.org/10.3390/agronomy13051283
APA StyleChen, Y., Chen, H., Chen, R., Yang, H., Zheng, T., Huang, X., & Fan, G. (2023). The Impacts of Nitrogen Accumulation, Translocation, and Photosynthesis on Simultaneous Improvements in the Grain Yield and Gluten Quality of Dryland Wheat. Agronomy, 13(5), 1283. https://doi.org/10.3390/agronomy13051283






