Directions from Nature: How to Halt the Tomato Brown Rugose Fruit Virus
Abstract
1. Introduction: Tomato Crop Model for Viral Immunity
2. ToBRFV Is a Tobamovirus
2.1. Tobamoviruses Are the First Described Viruses
2.2. Pathogenic Tomato Viruses
2.3. The Genome Organisation of ToBRFV
3. ToBRFV Spread, Symptoms, and Host Range
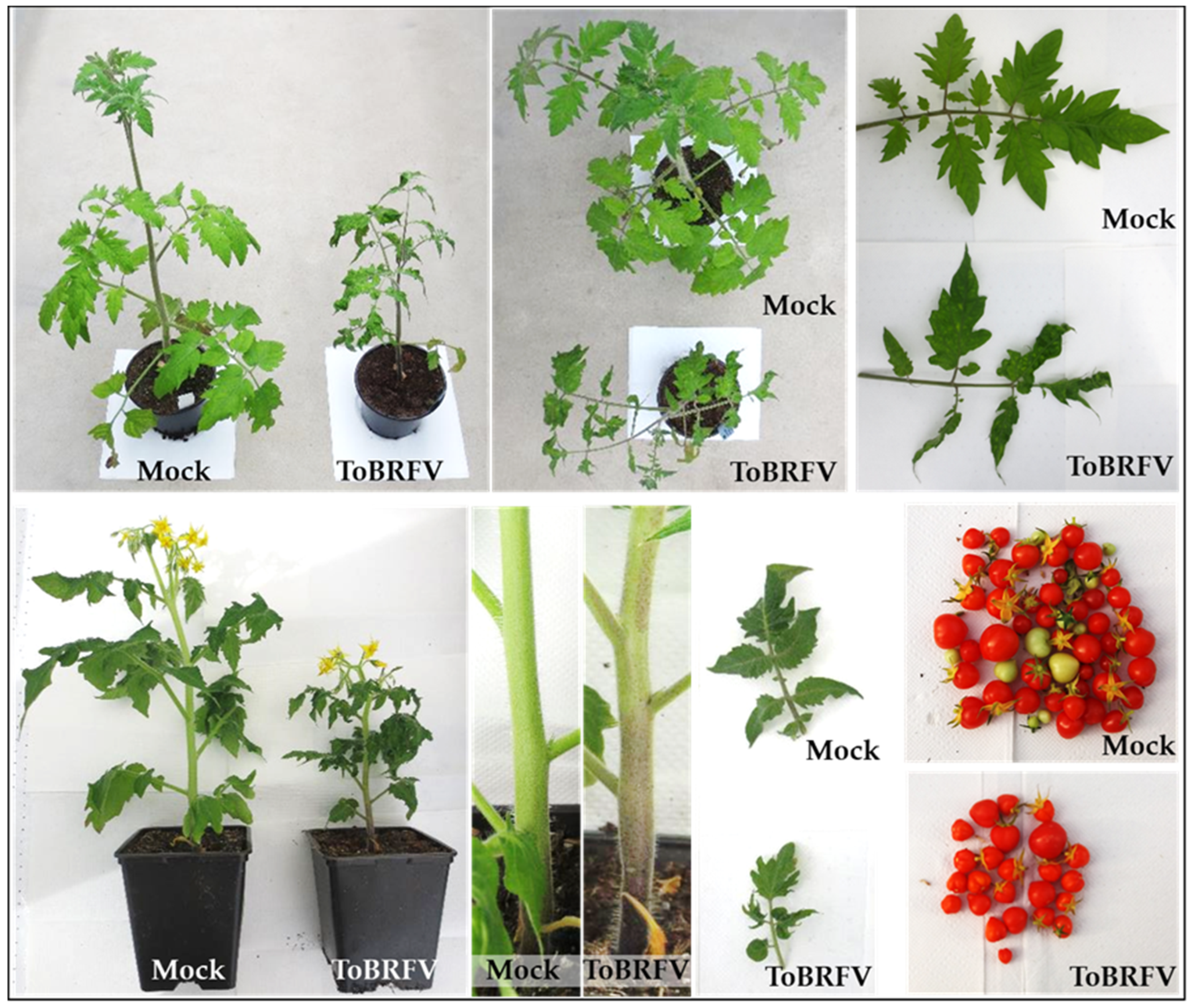
3.1. ToBRFV Disease Symptoms in Tomato
3.2. Additional Host Plants of ToBRFV
3.3. Preventing ToBRFV Distribution
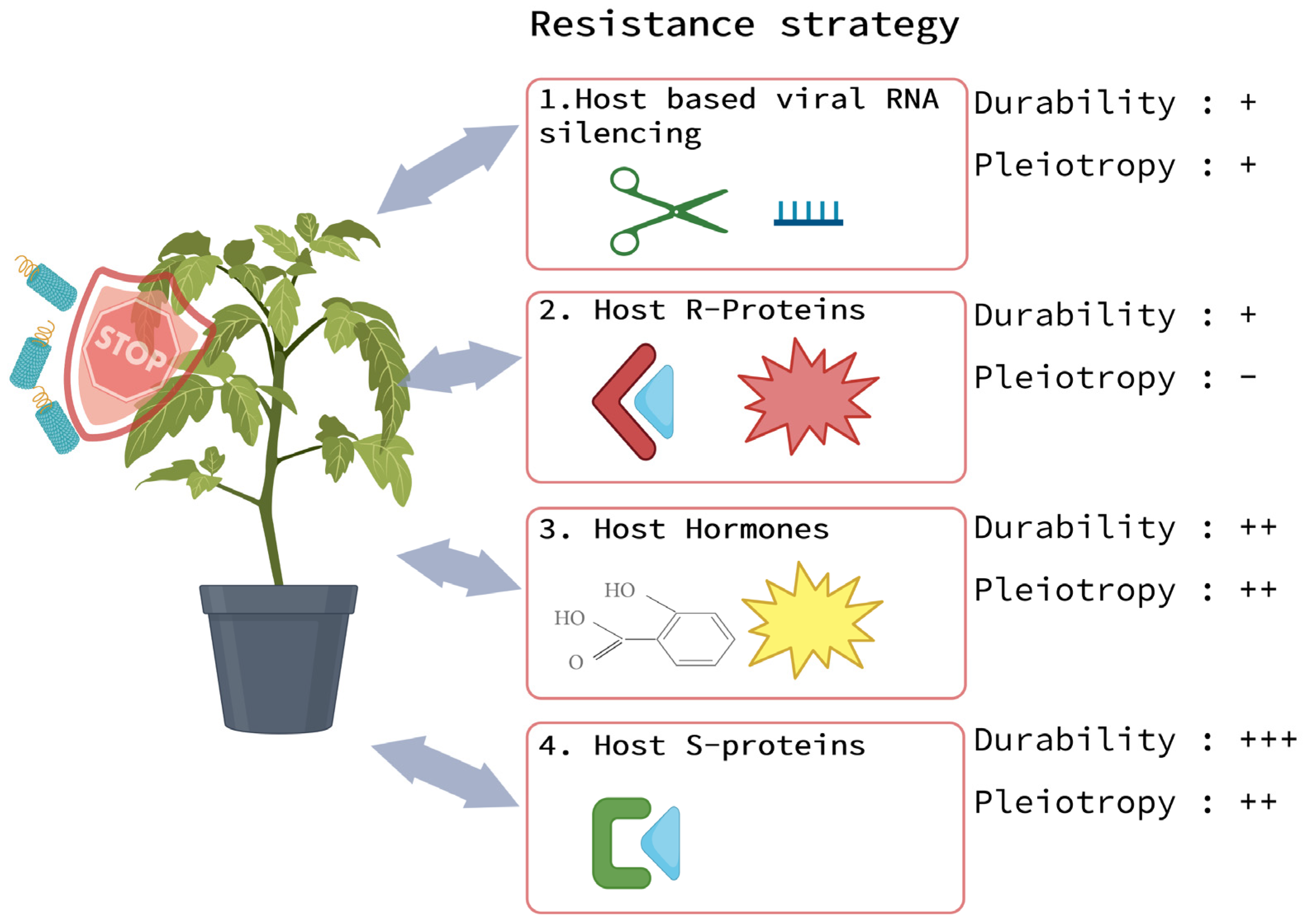
4. Viral Disease Resistance Strategies in Plants
4.1. ToBRFV Resistance Conferred by Host-Based Viral RNA Silencing
4.2. ToBRFV Resistance Conferred by Host Resistance (R) Proteins
4.3. Search for Additional ToBRFV Host Resistance (R) Proteins
4.4. Hormone-Based ToBRFV Resistance
4.5. ToBRFV Resistance Based on Dysfunctional Host Susceptibility (S) Proteins
5. Molecular Factors of Plant Host and Tobamoviruses That Are Instrumental for Viral Proliferation
5.1. Stage 1: Viral Entrance
5.2. Stage 2: Viral Replication and Translation
5.3. Stage 3: Viral Movement and Spread
5.4. Identification of Additional Host Factors Based on Host Processes That Are Manipulated by Tobamoviruses
5.5. Antisense Oligonucleotide Therapeutics: Targeting the Secondary RNA Structures from ToBRFV for Durable Resistance
6. Conclusion and Future Directions towards Durable ToBRFV Tomato Resistance
6.1. The Use of Allelic Polymorphisms of Susceptibility Genes for Durable Resistance
6.2. The Best Strategy for Durable Resistance Is the Absence of Susceptibility Genes
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Yearbook 2021; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Rosli, H.G.; Martin, G.B. Functional genomics of tomato for the study of plant immunity. Brief. Funct. Genom. 2015, 14, 291–301. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, W.G.; Berry, P.E. Solanales. Available online: https://www.britannica.com/plant/Solanales (accessed on 30 March 2023).
- Rivarez, M.P.S.; Vucurovic, A.; Mehle, N.; Ravnikar, M.; Kutnjak, D. Global Advances in Tomato Virome Research: Current Status and the Impact of High-Throughput Sequencing. Front. Microbiol. 2021, 12, 671925. [Google Scholar] [CrossRef] [PubMed]
- Lustig, A.; Levine, A.J. One hundred years of virology. J. Virol. 1992, 66, 4629–4631. [Google Scholar] [CrossRef] [PubMed]
- Bos, L. Beijerinck’s work on tobacco mosaic virus: Historical context and legacy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Centre, D.S. Corona Crisis from a Historical Perspective. Available online: https://heritage.tudelft.nl/en/exhibitions/corona-chronicles (accessed on 30 March 2023).
- Creager, A.N.; Morgan, G.J. After the double helix: Rosalind Franklin’s research on Tobacco mosaic virus. Isis 2008, 99, 239–272. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global Organization and Proposed Megataxonomy of the Virus World. Microbiol. Mol. Biol. Rev. 2020, 84, e00061-19. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P. Emerging viral diseases of tomato crops. Mol. Plant Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed Transmission of Tobamoviruses: Aspects of Global Disease Distribution; Jimenez-Lopez, J., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Salem, N.M.; Sulaiman, A.; Samarah, N.; Turina, M.; Vallino, M. Localization and Mechanical Transmission of Tomato Brown Rugose Fruit Virus in Tomato Seeds. Plant Dis. 2022, 106, 275–281. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Wood, J.; Garcia-Arenal, F.; Ohshima, K.; Armstrong, J.S. Tobamoviruses have probably co-diverged with their eudicotyledonous hosts for at least 110 million years. Virus Evol. 2015, 1, vev019. [Google Scholar] [CrossRef]
- Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zemach, H.; Belausov, E.; Kamenetsky-Goldstein, R.; Lapidot, M. ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination. Cells 2022, 11, 2864. [Google Scholar] [CrossRef]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef]
- Tomato Brown Rugose Fruit Virus (ToBRFV). Available online: https://gd.eppo.int/taxon/tobrfv/reporting (accessed on 30 March 2023).
- Plant Virus Snapshots: Cucumber Mosaic Virus. Available online: https://www.virtigation.eu/plant-virus-snapshots-cucumber-mosaic-virus/ (accessed on 30 March 2023).
- Lewis Ivey, M.L.; Sidhu, J. Diagnosing Off-Target 2,4-D and Glyphosate Herbicide Damage to Tomato. Available online: https://www.lsu.edu/agriculture/plant/extension/hcpl-publications/herbicide_injury_tomato_ppcp-veg-004.pdf (accessed on 30 March 2023).
- Zhang, S.K.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A.M. Tomato brown rugose fruit virus: An emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef]
- Scholthof, K.B. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Barone, S.; Lo Bosco, G.; Rangel, E.A.; Davino, S. Spread of Tomato Brown Rugose Fruit Virus in Sicily and Evaluation of the Spatiotemporal Dispersion in Experimental Conditions. Agronomy 2020, 10, 834. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Davino, S. First Report of Tomato Brown Rugose Fruit Virus on Tomato Crops in Italy. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Chanda, B.; Gilliard, A.; Jaiswal, N.; Ling, K.S. Comparative analysis of host range, ability to infect tomato cultivars with Tm-22 gene, and real-time reverse transcription PCR detection of tomato brown rugose fruit virus. Plant Dis. 2021, 105, 3643–3652. [Google Scholar] [CrossRef]
- Gaffar, F.Y.; Koch, A. Catch me if you can! RNA silencing-based improvement of antiviral plant immunity. Viruses 2019, 11, 673. [Google Scholar] [CrossRef]
- Meins, F., Jr.; Si-Ammour, A.; Blevins, T. RNA silencing systems and their relevance to plant development. Annu. Rev. Cell Dev. Biol. 2005, 21, 297–318. [Google Scholar] [CrossRef]
- Csorba, T.; Kontra, L.; Burgyan, J. viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Masuta, C.; Smith, N.A.; Shimura, H. RNA silencing and plant viral diseases. Mol. Plant Microbe Interact. 2012, 25, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Tsuda, S.; Tamai, A.; Meshi, T. Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J. Virol. 2003, 77, 11016–11026. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; van der Hoorn, R.A.L. Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef] [PubMed]
- de Ronde, D.; Butterbach, P.; Kormelink, R. Dominant resistance against plant viruses. Front. Plant Sci. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Lanfermeijer, F.C.; Dijkhuis, J.; Sturre, M.J.; de Haan, P.; Hille, J. Cloning and characterization of the durable tomato mosaic virus resistance gene Tm-22 from Lycopersicon esculentum. Plant Mol. Biol. 2003, 52, 1037–1049. [Google Scholar] [CrossRef]
- Lanfermeijer, F.C.; Warmink, J.; Hille, J. The products of the broken Tm-2 and the durable Tm-22 resistance genes from tomato differ in four amino acids. J. Exp. Bot. 2005, 56, 2925–2933. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kishibayashi, N.; Motoyoshi, F.; Okada, Y. Characterization of Tm-1 gene action on replication of common isolates and a resistance-breaking isolate of TMV. Virology 1987, 161, 527–532. [Google Scholar] [CrossRef]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of Plant Viruses and Disease Management: Relevance of Genetic Diversity and Evolution. Front. Plant Sci. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Weber, H.; Schultze, S.; Pfitzner, A.J. Two amino acid substitutions in the tomato mosaic virus 30-kilodalton movement protein confer the ability to overcome the Tm-22 resistance gene in the tomato. J. Virol. 1993, 67, 6432–6438. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Ma, H.Y.; Wang, L.; Tettey, C.; Zhao, M.S.; Geng, C.; Tian, Y.P.; Li, X.D. Identification of genetic determinants of tomato brown rugose fruit virus that enable infection of plants harbouring the Tm-22 resistance gene. Mol. Plant Pathol. 2021, 22, 1347–1357. [Google Scholar] [CrossRef]
- Hak, H.; Spiegelman, Z. The tomato brown rugose fruit virus movement protein overcomes Tm-22 resistance in tomato while attenuating viral transport. Mol. Plant Microbe Interact. 2021, 34, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ykema, M.; Verweij, C.W.; De la Fuente van Bentem, S.; Perefarres, F.M.P. Tomato Plant Resistant to Tomato Brown Rugose Fruit Virus. US 2021/0238627 A1, 5 August 2021. [Google Scholar]
- Fontanet, L.; Skoneczka, J.; Lionneton, E.; Lederer, J. Resistance in Plants of Solanum lycopersicum to the ToBRFV. WO 2021/245282 A1, 9 December 2021. [Google Scholar]
- Hamelink, R.; Kalisvaart, J.; Rashidi, H. TBRFV Resistant Tomato Plant. WO 2019/110130 A1, 13 June 2019. [Google Scholar]
- Zinger, A.; Lapidot, M.; Harel, A.; Doron-Faigenboim, A.; Gelbart, D.; Levin, I. Identification and Mapping of Tomato Genome Loci Controlling Tolerance and Resistance to Tomato Brown Rugose Fruit Virus. Plants 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, V.; Rotem, Y.; Ecker, R.; Nashilevitz, S.; Barom, N. Resistance in Plants of Solanum lycopersicum to the Tobamovirus Tomato Brown Rugose Fruit Virus. WO 2020/249798 A1, 17 December 2020. [Google Scholar]
- Lindbo, J. Tomato Plants Resistant to ToBRFV, TMV, TOMV and TOMMV and Corresponding Resistance Genes. WO 2022/117884 A1, 9 June 2022. [Google Scholar]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.S. Antiviral Roles of Abscisic Acid in Plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Zhu, F.; Xi, D.H.; Yuan, S.; Xu, F.; Zhang, D.W.; Lin, H.H. Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol. Plant Microbe Interact. 2014, 27, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, Y.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003, 33, 887–898. [Google Scholar] [CrossRef]
- Yu, M.H.; Zhao, Z.Z.; He, J.X. Brassinosteroid signaling in plant-microbe interactions. Int. J. Mol. Sci. 2018, 19, 4091. [Google Scholar] [CrossRef]
- Shwartz, I.; Levy, M.; Ori, N.; Bar, M. Hormones in tomato leaf development. Dev. Biol. 2016, 419, 132–142. [Google Scholar] [CrossRef]
- Liu, C.; Nelson, R.S. The cell biology of Tobacco mosaic virus replication and movement. Front. Plant Sci. 2013, 4, 12. [Google Scholar] [CrossRef]
- Nishikiori, M.; Mori, M.; Dohi, K.; Okamura, H.; Katoh, E.; Naito, S.; Meshi, T.; Ishikawa, M. A host small GTP-binding protein ARL8 plays crucial roles in tobamovirus RNA replication. PLoS Pathog. 2011, 7, e1002409. [Google Scholar] [CrossRef] [PubMed]
- Kravchik, M.; Shnaider, Y.; Abebie, B.; Shtarkman, M.; Kumari, R.; Kumar, S.; Leibman, D.; Spiegelman, Z.; Gal-On, A. Knockout of SlTOM1 and SlTOM3 results in differential resistance to tobamovirus in tomato. Mol Plant Pathol 2022, 23, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Ferreira, C.; Munro, S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev. Cell 2011, 21, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Khatter, D.; Sindhwani, A.; Sharma, M. Arf-like GTPase Arl8: Moving from the periphery to the center of lysosomal biology. Cell. Logist. 2015, 5, e1086501. [Google Scholar] [CrossRef]
- Garg, S.; Sharma, M.; Ung, C.; Tuli, A.; Barral, D.C.; Hava, D.L.; Veerapen, N.; Besra, G.S.; Hacohen, N.; Brenner, M.B. Lysosomal trafficking, antigen presentation, and microbial killing are controlled by the Arf-like GTPase Arl8b. Immunity 2011, 35, 182–193. [Google Scholar] [CrossRef]
- Yang, M.; Ismayil, A.; Liu, Y. Autophagy in Plant-Virus Interactions. Annu. Rev. Virol. 2020, 7, 403–419. [Google Scholar] [CrossRef]
- Huang, X.; Chen, S.; Yang, X.; Yang, X.; Zhang, T.; Zhou, G. Friend or Enemy: A Dual Role of Autophagy in Plant Virus Infection. Front. Microbiol. 2020, 11, 736. [Google Scholar] [CrossRef]
- Bassüner, R.; Nong, V.; Jung, R.; Saalbach, G.; Müntz, K. The primary structure of the predominating vicilin storage protein subunit from field bean seeds (Vicia faba L. var. minor cv. Fribo). Nucleic Acids Res. 1987, 15, 9609. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Monteiro, S.; Freitas, R.; Santos, C.N.; Chen, Z.; Batista, L.M.; Duarte, J.; Borges, A.; Teixeira, A.R. The role of plant defence proteins in fungal pathogenesis. Mol. Plant Pathol. 2007, 8, 677–700. [Google Scholar] [CrossRef]
- Cândido Ede, S.; Pinto, M.F.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-de-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef]
- Parcy, F.; Valon, C.; Raynal, M.; Gaubier-Comella, P.; Delseny, M.; Giraudat, J. Regulation of gene expression programs during Arabidopsis seed development: Roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 1994, 6, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.E.; Yeh, K.C.; Wu, S.H.; Wang, H.I.; Yeh, H.H. A vicilin-like seed storage protein, PAP85, is involved in tobacco mosaic virus replication. J. Virol. 2013, 87, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Kumar, N.D.; Reggiori, F.; Khan, G. How Viruses Hijack and Modify the Secretory Transport Pathway. Cells 2021, 10, 2535. [Google Scholar] [CrossRef]
- Sanderfoot, A.A.; Assaad, F.F.; Raikhel, N.V. The Arabidopsis Genome. An Abundance of Soluble N-Ethylmaleimide-Sensitive Factor Adaptor Protein Receptors1. Plant Physiol. 2000, 124, 1558–1569. [Google Scholar] [CrossRef]
- Ibrahim, A.; Yang, X.; Liu, C.; Cooper, K.D.; Bishop, B.A.; Zhu, M.; Kwon, S.; Schoelz, J.E.; Nelson, R.S. Plant SNAREs SYP22 and SYP23 interact with Tobacco mosaic virus 126 kDa protein and SYP2s are required for normal local virus accumulation and spread. Virology 2020, 547, 57–71. [Google Scholar] [CrossRef]
- Lipka, V.; Kwon, C.; Panstruga, R. SNARE-ware: The role of SNARE-domain proteins in plant biology. Annu. Rev. Cell Dev. Biol. 2007, 23, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Ohtomo, I.; Ueda, H.; Shimada, T.; Nishiyama, C.; Komoto, Y.; Hara-Nishimura, I.; Takahashi, T. Identification of an allele of VAM3/SYP22 that confers a semi-dwarf phenotype in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Uemura, T.; Ebine, K.; Nishimori, Y.; Ueda, T.; Nakano, A.; Sato, M.H.; Fukao, Y. Interactomics of Qa-SNARE in Arabidopsis thaliana. Plant Cell Physiol 2014, 55, 781–789. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Tan, X.; Xie, K.; Li, L.; Hong, G.; Li, J.; Cheng, Y.; Yan, F.; Chen, J.; et al. The Dual Effect of the Brassinosteroid Pathway on Rice Black-Streaked Dwarf Virus Infection by Modulating the Peroxidase-Mediated Oxidative Burst and Plant Defense. Mol. Plant Microbe Interact. 2019, 32, 685–696. [Google Scholar] [CrossRef]
- Zhu, X.F.; Liu, Y.; Gai, X.T.; Zhou, Y.; Xia, Z.Y.; Chen, L.J.; Duan, Y.X.; Xuan, Y.H. SNARE proteins SYP22 and VAMP727 negatively regulate plant defense. Plant Signal. Behav. 2019, 14, 1610300. [Google Scholar] [CrossRef]
- Yamanaka, T.; Imai, T.; Satoh, R.; Kawashima, A.; Takahashi, M.; Tomita, K.; Kubota, K.; Meshi, T.; Naito, S.; Ishikawa, M. Complete inhibition of tobamovirus multiplication by simultaneous mutations in two homologous host genes. J. Virol. 2002, 76, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Ohta, T.; Takahashi, M.; Meshi, T.; Schmidt, R.; Dean, C.; Naito, S.; Ishikawa, M. TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl. Acad. Sci. USA 2000, 97, 10107–10112. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Naito, S.; Ohno, T. Effects of the tom1 mutation of Arabidopsis thaliana on the multiplication of tobacco mosaic virus RNA in protoplasts. J. Virol. 1993, 67, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dubey, A.K.; Karmakar, R.; Kini, K.R.; Mathew, M.K.; Prakash, H.S. Inhibition of TMV multiplication by siRNA constructs against TOM1 and TOM3 genes of Capsicum annuum. J. Virol. Methods 2012, 186, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Lim, G.X.; Wong, S.M. Profiling of genes related to cross protection and competition for NbTOM1 by HLSV and TMV. PLoS ONE 2013, 8, e73725. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yoshida, T.; Matsuyama, M.; Kouzai, Y.; Kano, A.; Ishibashi, K. Tomato brown rugose fruit virus resistance generated by quadruple knockout of homologs of TOBAMOVIRUS MULTIPLICATION1 in tomato. Plant Physiol. 2022, 189, 679–686. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Numaga, T.; Ohshima, K.; Yano, M.A.; Ohsawa, R.; Goto, D.B.; Naito, S.; Ishikawa, M. Arabidopsis TOBAMOVIRUS MULTIPLICATION (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J. 2003, 22, 335–343. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, H.; Zhang, L.; Liu, Y.; Huang, C.; Yuan, C.; Chen, Z.; Li, K.; Larkin, R.M.; Chen, J.; et al. Two TOBAMOVIRUS MULTIPLICATION 2A homologs in tobacco control asymptomatic response to tobacco mosaic virus. Plant Physiol. 2021, 187, 2674–2690. [Google Scholar] [CrossRef]
- Galichet, A.; Gruissem, W. Protein farnesylation in plants--conserved mechanisms but different targets. Curr. Opin. Plant Biol. 2003, 6, 530–535. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Faulkner, C.; Ritzenthaler, C.; Maule, A.J. Plasmodesmata: Gateways to local and systemic virus infection. Mol. Plant Microbe Interact. 2010, 23, 1403–1412. [Google Scholar] [CrossRef]
- Lucas, W.J.; Wolf, S. Connections between virus movement, macromolecular signaling and assimilate allocation. Curr. Opin. Plant Biol. 1999, 2, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, K.; Watanabe, Y. Tobamovirus replicase coding region is involved in cell-to-cell movement. J. Virol. 2001, 75, 8831–8836. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, K.; Watanabe, Y. RNA helicase domain of tobamovirus replicase executes cell-to-cell movement possibly through collaboration with its nonconserved region. J. Virol. 2003, 77, 12357–12362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueki, S.; Spektor, R.; Natale, D.M.; Citovsky, V. ANK, a host cytoplasmic receptor for the Tobacco mosaic virus cell-to-cell movement protein, facilitates intercellular transport through plasmodesmata. PLoS Pathog. 2010, 6, e1001201. [Google Scholar] [CrossRef]
- Vo, K.T.X.; Kim, C.-Y.; Chandran, A.K.N.; Jung, K.-H.; An, G.; Jeon, J.-S. Molecular insights into the function of ankyrin proteins in plants. J. Plant Biol. 2015, 58, 271–284. [Google Scholar] [CrossRef]
- Tran, P.T.; Citovsky, V. Receptor-like kinase BAM1 facilitates early movement of the Tobacco mosaic virus. Commun. Biol. 2021, 4, 511. [Google Scholar] [CrossRef]
- Rosas-Diaz, T.; Zhang, D.; Fan, P.; Wang, L.; Ding, X.; Jiang, Y.; Jimenez-Gongora, T.; Medina-Puche, L.; Zhao, X.; Feng, Z.; et al. A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc. Natl. Acad. Sci. USA 2018, 115, 1388–1393. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.Y.; Song, H.H.; Hu, X.; Qiu, B.S. Identification of a tobacco protein interacting with tomato mosaic virus coat protein and facilitating long-distance movement of virus. Arch. Virol. 2005, 150, 1993–2008. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Sun, X.; Qian, W.; Zhang, D.; Qiu, B. Characterization of a specific interaction between IP-L, a tobacco protein localized in the thylakoid membranes, and Tomato mosaic virus coat protein. Biochem. Biophys. Res. Commun. 2008, 374, 253–257. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in Plant-Virus Interaction. Front. Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef]
- Liu, C.; Pu, Y.; Peng, H.; Lv, X.; Tian, S.; Wei, X.; Zhang, J.; Zou, A.; Fan, G.; Sun, X. Transcriptome sequencing reveals that photoinduced gene IP-L affects the expression of PsbO to response to virus infection in Nicotiana benthamiana. Physiol. Mol. Plant Pathol. 2021, 114, 101613. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant-virus interaction. Mol. Plant Pathol. 2018, 19, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Q.; Zhang, H.; Jia, Q.; Hong, Y.; Liu, Y. The rubisco small subunit is involved in tobamovirus movement and Tm-22-mediated extreme resistance. Plant Physiol. 2013, 161, 374–383. [Google Scholar] [CrossRef]
- Janssen, B.J.; Williams, A.; Chen, J.J.; Mathern, J.; Hake, S.; Sinha, N. Isolation and characterization of two knotted-like homeobox genes from tomato. Plant Mol. Biol. 1998, 36, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, A.; Shimizu, T.; Yoshida, A.; Hamada, K.; Sakurai, K.; Yamaji, Y.; Suzuki, M.; Namba, S.; Hibi, T. NTH201, a novel class II KNOTTED1-like protein, facilitates the cell-to-cell movement of Tobacco mosaic virus in tobacco. Mol. Plant Microbe Interact. 2008, 21, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yamaguchi, M.; Grienenberger, E.; Martone, P.T.; Samuels, A.L.; Mansfield, S.D. The Class II KNOX genes KNAT3 and KNAT7 work cooperatively to influence deposition of secondary cell walls that provide mechanical support to Arabidopsis stems. Plant J. 2020, 101, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Sheng, J.; Hind, G.; Handa, A.K.; Citovsky, V. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 2000, 19, 913–920. [Google Scholar] [CrossRef]
- Lionetti, V.; Raiola, A.; Cervone, F.; Bellincampi, D. How do pectin methylesterases and their inhibitors affect the spreading of tobamovirus? Plant Signal. Behav. 2014, 9, e972863. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Frolova, O.Y.; Skurat, E.V.; Ivanov, P.A.; Gasanova, T.V.; Sheveleva, A.A.; Ravin, N.V.; Makinen, K.M.; Klimyuk, V.I.; Skryabin, K.G.; et al. A novel function for a ubiquitous plant enzyme pectin methylesterase: The enhancer of RNA silencing. FEBS Lett. 2006, 580, 3872–3878. [Google Scholar] [CrossRef]
- Gasanova, T.V.; Skurat, E.V.; Frolova, O.; Semashko, M.A.; Dorokhov Iu, L. Pectin methylesterase as a factor of plant transcriptome stability. Mol. Biol. 2008, 42, 478–486. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lazarowitz, S.G. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl. Acad. Sci. USA 2010, 107, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Shimada-Beltran, H.; Levy, A.; Zheng, J.Y.; Javia, P.A.; Lazarowitz, S.G. The Arabidopsis synaptotagmin SYTA regulates the cell-to-cell movement of diverse plant viruses. Front. Plant Sci. 2014, 5, 584. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Zheng, J.Y.; Lazarowitz, S.G. Synaptotagmin SYTA forms ER-plasma membrane junctions that are recruited to plasmodesmata for plant virus movement. Curr. Biol. 2015, 25, 2018–2025. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tamura, K.; Ueda, H.; Ito, Y.; Nakano, A.; Hara-Nishimura, I.; Shimada, T. Synaptotagmin-associated endoplasmic reticulum-plasma membrane contact sites are localized to immobile er tubules. Plant Physiol. 2018, 178, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Tamura, K.; Fukao, Y.; Shimada, T. Structural and functional relationships between plasmodesmata and plant endoplasmic reticulum-plasma membrane contact sites consisting of three synaptotagmins. New Phytol. 2020, 226, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Obata, F.; Kumagai, T.; Ohno, T. Isolation of mutants of Arabidopsis thaliana in which accumulation of tobacco mosaic virus coat protein is reduced to low levels. Mol. Gen. Genet. 1991, 230, 33–38. [Google Scholar] [CrossRef]
- Kushwaha, N.K.; Hafrén, A.; Hofius, D. Autophagy–virus interplay in plants: From antiviral recognition to proviral manipulation. Mol. Plant Pathol. 2019, 20, 1211–1216. [Google Scholar] [CrossRef]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef]
- Agaoua, A.; Bendahmane, A.; Moquet, F.; Dogimont, C. Membrane Trafficking Proteins: A New Target to Identify Resistance to Viruses in Plants. Plants 2021, 10, 2139. [Google Scholar] [CrossRef]
- Mengistu, A.A.; Tenkegna, T.A. The role of miRNA in plant–virus interaction: A review. Mol. Biol. Rep. 2021, 48, 2853–2861. [Google Scholar] [CrossRef]
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J.; et al. Criteria for annotation of plant MicroRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Baig, M.S.; Khan, J.A. Suppression of cotton leaf curl disease symptoms in Gossypium hirsutum through over expression of host-encoded miRNAs. J. Biotechnol. 2017, 263, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Pignatta, D.; Bendix, C.; Brunkard, J.O.; Cohn, M.M.; Tung, J.; Sun, H.; Kumar, P.; Baker, B. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1790–1795. [Google Scholar] [CrossRef]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic biology approach for plant protection using dsRNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef]
- Manfredonia, I.; Nithin, C.; Ponce-Salvatierra, A.; Ghosh, P.; Wirecki, T.K.; Marinus, T.; Ogando, N.S.; Snijder, E.J.; van Hemert, M.J.; Bujnicki, J.M.; et al. Genome-wide mapping of SARS-CoV-2 RNA structures identifies therapeutically-relevant elements. Nucleic Acids Res. 2020, 48, 12436–12452. [Google Scholar] [CrossRef] [PubMed]
- Zimmern, D. An extended secondary structure model for the TMV assembly origin, and its correlation with protection studies and an assembly defective mutant. EMBO J. 1983, 2, 1901–1907. [Google Scholar] [CrossRef]
- Hermann, T. Small molecules targeting viral RNA. Wiley Interdiscip. Rev. RNA 2016, 7, 726–743. [Google Scholar] [CrossRef]
- Zafirov, D.; Giovinazzo, N.; Bastet, A.; Gallois, J.-L. When a knockout is an Achilles’ heel: Resistance to one potyvirus species triggers hypersusceptibility to another one in Arabidopsis thaliana. Mol. Plant Pathol. 2021, 22, 334–347. [Google Scholar] [CrossRef]
- Duprat, A.; Caranta, C.; Revers, F.; Menand, B.; Browning, K.S.; Robaglia, C. The Arabidopsis eukaryotic initiation factor (iso)4E is dispensable for plant growth but required for susceptibility to potyviruses. Plant J. 2002, 32, 927–934. [Google Scholar] [CrossRef]
- Ruffel, S.; Dussault, M.H.; Palloix, A.; Moury, B.; Bendahmane, A.; Robaglia, C.; Caranta, C. A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J. 2002, 32, 1067–1075. [Google Scholar] [CrossRef]
- Lapidot, M.; Karniel, U.; Gelbart, D.; Fogel, D.; Evenor, D.; Kutsher, Y.; Makhbash, Z.; Nahon, S.; Shlomo, H.; Chen, L.; et al. A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota. PLoS Genet. 2015, 11, e1005538. [Google Scholar] [CrossRef] [PubMed]
- Koeda, S.; Onouchi, M.; Mori, N.; Pohan, N.S.; Nagano, A.J.; Kesumawati, E. A recessive gene pepy-1 encoding Pelota confers resistance to begomovirus isolates of PepYLCIV and PepYLCAV in Capsicum annuum. Theor. Appl. Genet. 2021, 134, 2947–2964. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Ishii, Y.; Taniguchi, J.-i.; Waliullah, S.; Kobayashi, K.; Yaeno, T.; Yamaoka, N.; Nishiguchi, M. Conferring virus resistance in tomato by independent RNA silencing of three tomato homologs of Arabidopsis TOM1. Arch. Virol. 2018, 163, 1357–1362. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, J.H.; Zhou, X.P. A TOM1 homologue is required for multiplication of Tobacco mosaic virus in Nicotiana benthamiana. J. Zhejiang Univ. Sci. B 2007, 8, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, J.; Ludeking, D.J.W.; Roovers, A.J.M. Gene leading to ToBRFV resistance in S. lycopersicum. WO 2022/013452 A1, 20 January 2022. [Google Scholar]
- Chen, L.; Zhang, L.; Li, D.; Wang, F.; Yu, D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, E1963–E1971. [Google Scholar] [CrossRef]
- Fichtenbauer, D.; Xu, X.M.; Jackson, D.; Kragler, F. The chaperonin CCT8 facilitates spread of tobamovirus infection. Plant Signal. Behav. 2012, 7, 318–321. [Google Scholar] [CrossRef]
- Ouibrahim, L.; Mazier, M.; Estevan, J.; Pagny, G.; Decroocq, V.; Desbiez, C.; Moretti, A.; Gallois, J.-L.; Caranta, C. Cloning of the Arabidopsis rwm1 gene for resistance to Watermelon mosaic virus points to a new function for natural virus resistance genes. Plant J. 2014, 79, 705–716. [Google Scholar] [CrossRef]
- Lin, J.W.; Ding, M.P.; Hsu, Y.H.; Tsai, C.H. Chloroplast phosphoglycerate kinase, a gluconeogenetic enzyme, is required for efficient accumulation of Bamboo mosaic virus. Nucleic Acids Res. 2007, 35, 424–432. [Google Scholar] [CrossRef]
- Poque, S.; Pagny, G.; Ouibrahim, L.; Chague, A.; Eyquard, J.P.; Caballero, M.; Candresse, T.; Caranta, C.; Mariette, S.; Decroocq, V. Allelic variation at the rpv1 locus controls partial resistance to Plum pox virus infection in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 159. [Google Scholar] [CrossRef]
- Orjuela, J.; Deless, E.F.; Kolade, O.; Chéron, S.; Ghesquière, A.; Albar, L. A recessive resistance to rice yellow mottle virus is associated with a rice homolog of the CPR5 gene, a regulator of active defense mechanisms. Mol. Plant Microbe Interact. 2013, 26, 1455–1463. [Google Scholar] [CrossRef]
- Carrasco, J.L.; Ancillo, G.; Castelló, M.J.; Vera, P. A novel DNA-binding motif, hallmark of a new family of plant transcription factors. Plant Physiol. 2005, 137, 602–606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, J.; Oh, C.-S.; Kang, B.-C. Translation elongation factor 1B (eEF1B) is an essential host factor for Tobacco mosaic virus infection in plants. Virology 2013, 439, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Neriya, Y.; Keima, T.; Iwabuchi, N.; Koinuma, H.; Hagiwara-Komoda, Y.; Ishikawa, K.; Himeno, M.; Maejima, K.; Yamaji, Y.; et al. EXA1, a GYF domain protein, is responsible for loss-of-susceptibility to plantago asiatica mosaic virus in Arabidopsis thaliana. Plant J. 2016, 88, 120–131. [Google Scholar] [CrossRef]
- Yusa, A.; Neriya, Y.; Hashimoto, M.; Yoshida, T.; Fujimoto, Y.; Hosoe, N.; Keima, T.; Tokumaru, K.; Maejima, K.; Netsu, O.; et al. Functional conservation of EXA1 among diverse plant species for the infection by a family of plant viruses. Sci. Rep. 2019, 9, 5958. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.N.; Carr, J.P. The GCD10 subunit of yeast eIF-3 binds the methyltransferase-like domain of the 126 and 183 kDa replicase proteins of tobacco mosaic virus in the yeast two-hybrid system. J. Gen. Virol. 2000, 81, 1587–1591. [Google Scholar] [CrossRef]
- Kramer, S.R.; Goregaoker, S.P.; Culver, J.N. Association of the Tobacco mosaic virus 126 kDa replication protein with a GDI protein affects host susceptibility. Virology 2011, 414, 110–118. [Google Scholar] [CrossRef]
- Conti, G.; Rodriguez, M.C.; Manacorda, C.A.; Asurmendi, S. Transgenic Expression of Tobacco mosaic virus Capsid and Movement Proteins Modulate Plant Basal Defense and Biotic Stress Responses in Nicotiana tabacum. Mol. Plant-Microbe Interact. 2012, 25, 1370–1384. [Google Scholar] [CrossRef]
- Zou, L.J.; Deng, X.G.; Han, X.Y.; Tan, W.R.; Zhu, L.J.; Xi, D.H.; Zhang, D.W.; Lin, H.H. Role of Transcription Factor HAT1 in Modulating Arabidopsis thaliana Response to Cucumber mosaic virus. Plant Cell Physiol. 2016, 57, 1879–1889. [Google Scholar] [CrossRef]
- Whitham, S.A.; Quan, S.; Chang, H.S.; Cooper, B.; Estes, B.; Zhu, T.; Wang, X.; Hou, Y.M. Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J. 2003, 33, 271–283. [Google Scholar] [CrossRef]
- Carr, T.; Wang, Y.; Huang, Z.; Yeakley, J.M.; Fan, J.-B.; Whitham, S.A. Tobamovirus infection is independent of HSP101 mRNA induction and protein expression. Virus Res. 2006, 121, 33–41. [Google Scholar] [CrossRef]
- Gorovits, R.; Moshe, A.; Ghanim, M.; Czosnek, H. Recruitment of the host plant heat shock protein 70 by Tomato yellow leaf curl virus coat protein is required for virus infection. PLoS ONE 2013, 8, e70280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, H.; Brandizzi, F.; Verchot, J.; Wang, A. The UPR branch IRE1-bZIP60 in plants plays an essential role in viral infection and is complementary to the only UPR pathway in yeast. PLoS Genet. 2015, 11, e1005164. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Ogata, T.; Deguchi, M.; Nagai, S.; Tamai, A.; Meshi, T.; Kawakami, S.; Watanabe, Y.; Matsushita, Y.; Nyunoya, H. Over-expression of putative transcriptional coactivator KELP interferes with Tomato mosaic virus cell-to-cell movement. Mol. Plant Pathol. 2009, 10, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sheshukova, E.V.; Komarova, T.V.; Pozdyshev, D.V.; Ershova, N.M.; Shindyapina, A.V.; Tashlitsky, V.N.; Sheval, E.V.; Dorokhov, Y.L. The intergenic interplay between aldose 1-epimerase-like protein and pectin methylesterase in abiotic and biotic stress control. Front. Plant Sci. 2017, 8, 1646. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, D.D.; Liu, Y.; Schiff, M.; Dinesh-Kumar, S. P58IPK, a plant ortholog of double-stranded RNA-dependent protein kinase PKR inhibitor, functions in viral pathogenesis. Dev. Cell 2003, 4, 651–661. [Google Scholar] [CrossRef]
- Vijayapalani, P.; Maeshima, M.; Nagasaki-Takekuchi, N.; Miller, W.A. Interaction of the trans-frame potyvirus protein P3N-PIPO with host protein PCaP1 facilitates potyvirus movement. PLoS Pathog. 2012, 8, e1002639. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lüpken, T.; Habekuss, A.; Hensel, G.; Steuernagel, B.; Kilian, B.; Ariyadasa, R.; Himmelbach, A.; Kumlehn, J.; Scholz, U. PROTEIN DISULFIDE ISOMERASE LIKE 5-1 is a susceptibility factor to plant viruses. Proc. Natl. Acad. Sci. USA 2014, 111, 2104–2109. [Google Scholar] [CrossRef]
- Amari, K.; Boutant, E.; Hofmann, C.; Schmitt-Keichinger, C.; Fernandez-Calvino, L.; Didier, P.; Lerich, A.; Mutterer, J.; Thomas, C.L.; Heinlein, M. A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog. 2010, 6, e1001119. [Google Scholar] [CrossRef]
- Dunoyer, P.; Thomas, C.; Harrison, S.; Revers, F.; Maule, A. A cysteine-rich plant protein potentiates Potyvirus movement through an interaction with the virus genome-linked protein VPg. J. Virol. 2004, 78, 2301–2309. [Google Scholar] [CrossRef]
- Naderpour, M.; Lund, O.S.; Santana, G.; Blair, M.; Johansen, E. Potyviral VPG-interacting proteins and bean common mosaic virus resistance in Phaseolus vulgaris L. BIC 2010, 53, 44–45. [Google Scholar]
- Feng, Z.; Xue, F.; Xu, M.; Chen, X.; Zhao, W.; Garcia-Murria, M.J.; Mingarro, I.; Liu, Y.; Huang, Y.; Jiang, L. The ER-membrane transport system is critical for intercellular trafficking of the NSm movement protein and tomato spotted wilt tospovirus. PLoS Pathog. 2016, 12, e1005443. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, M.; Yamazaki, M.; Rakwal, R.; Kishi-Kaboshi, M.; Miyao, A.; Hirochika, H. The NAC transcription factor RIM1 of rice is a new regulator of jasmonate signaling. Plant J. 2010, 61, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Maio, F.; Arroyo-Mateos, M.; Bobay, B.G.; Bejarano, E.R.; Prins, M.; van den Burg, H.A. A lysine residue essential for geminivirus replication also controls nuclear localization of the tomato yellow leaf curl virus rep protein. J. Virol. 2019, 93, e01910–e01918. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Taniyama, T.; Yamanaka, T.; Ishikawa, M.; Naito, S. Isolation of a Mutant ofArabidopsis thalianaCarrying Two Simultaneous Mutations Affecting Tobacco Mosaic Virus Multiplication within a Single Cell. Virology 1998, 243, 472–481. [Google Scholar] [CrossRef]
- Pena, E.J.; Ferriol, I.; Sambade, A.; Buschmann, H.; Niehl, A.; Elena, S.F.; Rubio, L.; Heinlein, M. Experimental virus evolution reveals a role of plant microtubule dynamics and TORTIFOLIA1/SPIRAL2 in RNA trafficking. PLoS ONE 2014, 9, e105364. [Google Scholar] [CrossRef]
- Ye, C.; Verchot, J. Role of unfolded protein response in plant virus infection. Plant Signal. Behav. 2011, 6, 1212–1215. [Google Scholar] [CrossRef]
- Ye, C.; Dickman, M.B.; Whitham, S.A.; Payton, M.; Verchot, J. The Unfolded Protein Response Is Triggered by a Plant Viral Movement Protein. Plant Physiol. 2011, 156, 741–755. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Damme, M.; Zois, R.; Verbeek, M.; Bai, Y.; Wolters, A.-M.A. Directions from Nature: How to Halt the Tomato Brown Rugose Fruit Virus. Agronomy 2023, 13, 1300. https://doi.org/10.3390/agronomy13051300
van Damme M, Zois R, Verbeek M, Bai Y, Wolters A-MA. Directions from Nature: How to Halt the Tomato Brown Rugose Fruit Virus. Agronomy. 2023; 13(5):1300. https://doi.org/10.3390/agronomy13051300
Chicago/Turabian Stylevan Damme, Mireille, Romanos Zois, Martin Verbeek, Yuling Bai, and Anne-Marie A. Wolters. 2023. "Directions from Nature: How to Halt the Tomato Brown Rugose Fruit Virus" Agronomy 13, no. 5: 1300. https://doi.org/10.3390/agronomy13051300
APA Stylevan Damme, M., Zois, R., Verbeek, M., Bai, Y., & Wolters, A.-M. A. (2023). Directions from Nature: How to Halt the Tomato Brown Rugose Fruit Virus. Agronomy, 13(5), 1300. https://doi.org/10.3390/agronomy13051300




