A Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation, Chemical Modification and Characterization of the Sorbent
2.2. Experimental Design Using Taguchi
2.3. Sorption Tests
2.3.1. Adsorption Isotherm Study
2.3.2. Adsorption Kinetics Study
3. Results and Discussion
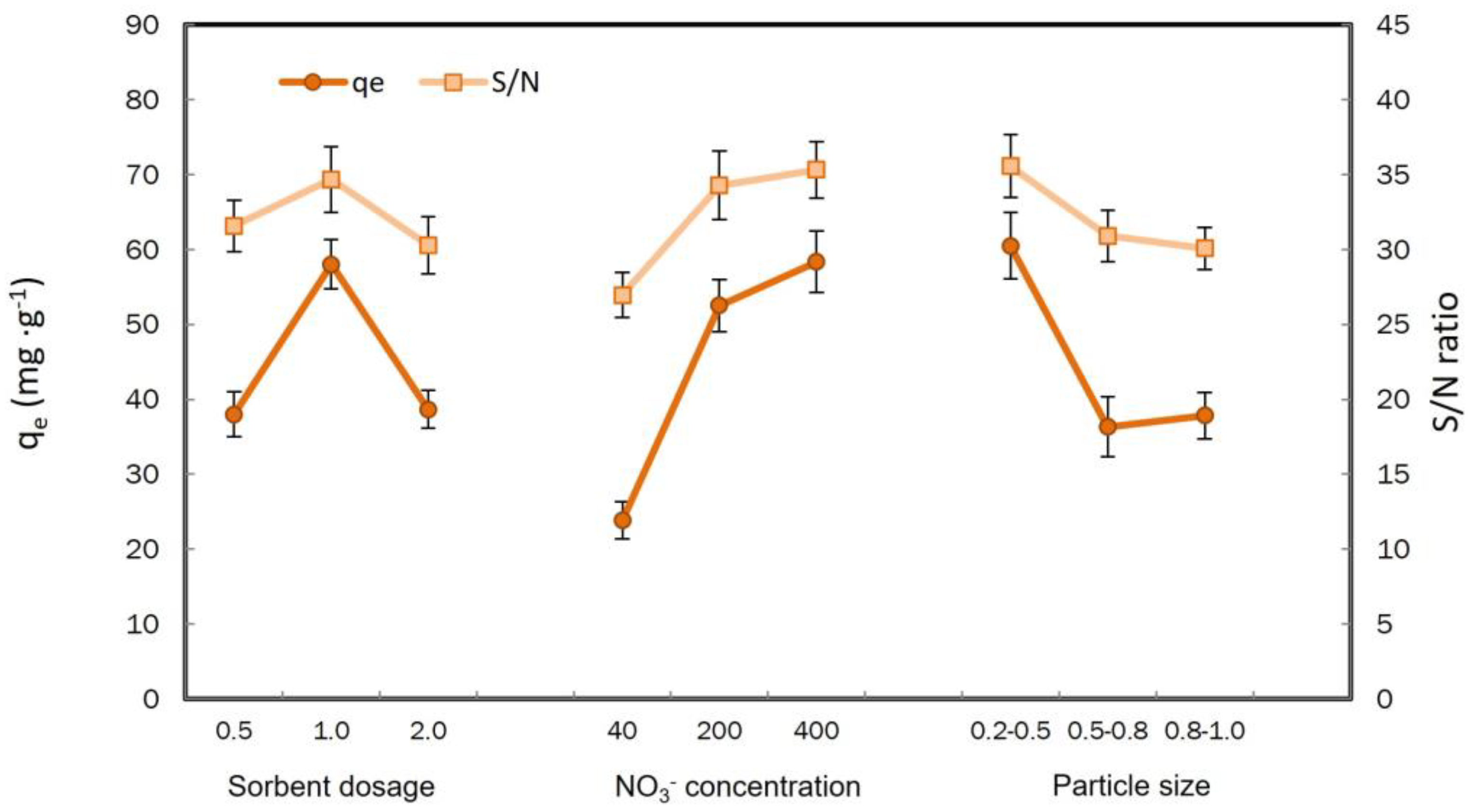
3.1. Optimization of the Taguchi Experiments
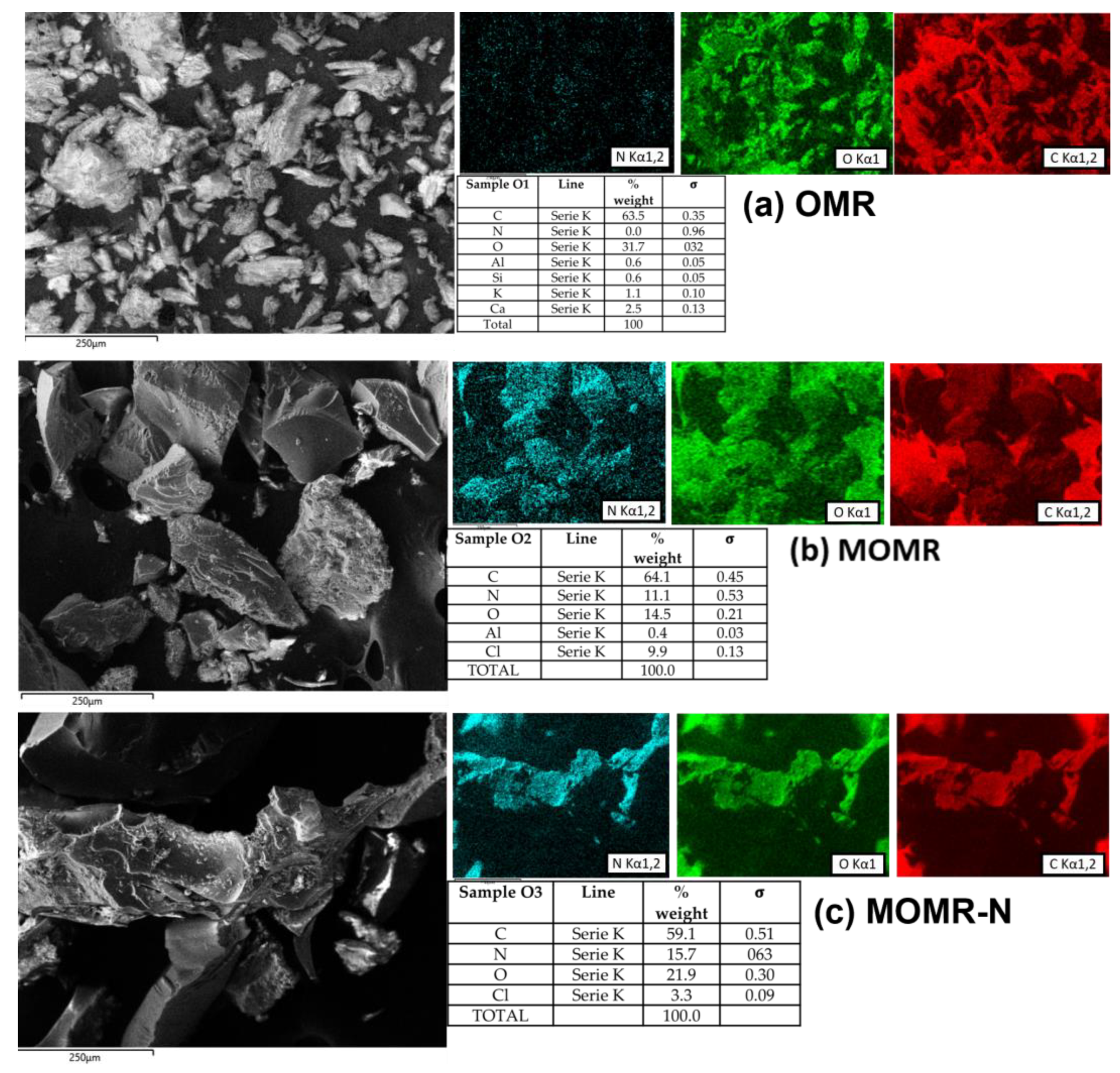
3.2. Biomass Characterization
Field Emission Scanning Electron Microscopy (FESEM)
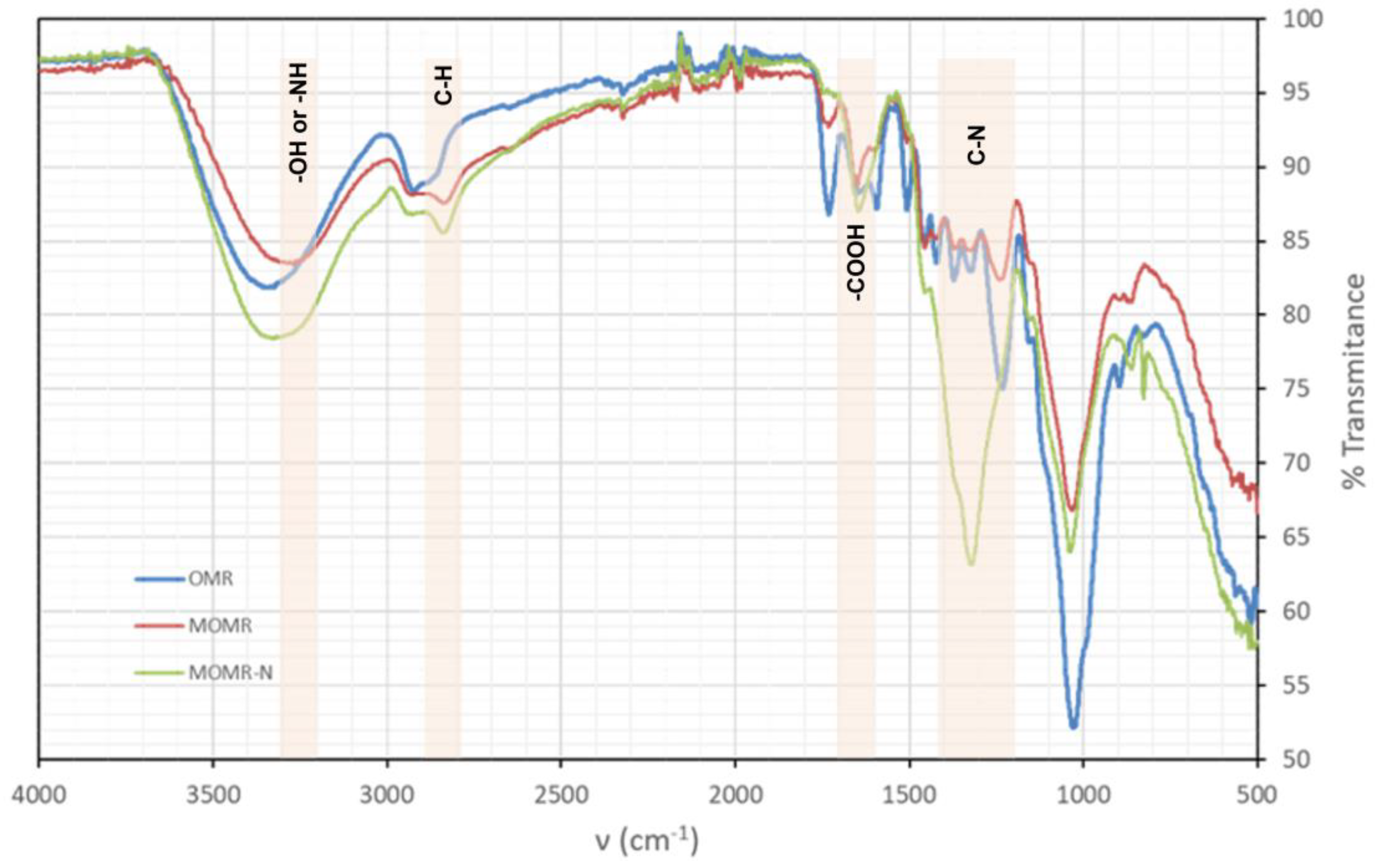
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
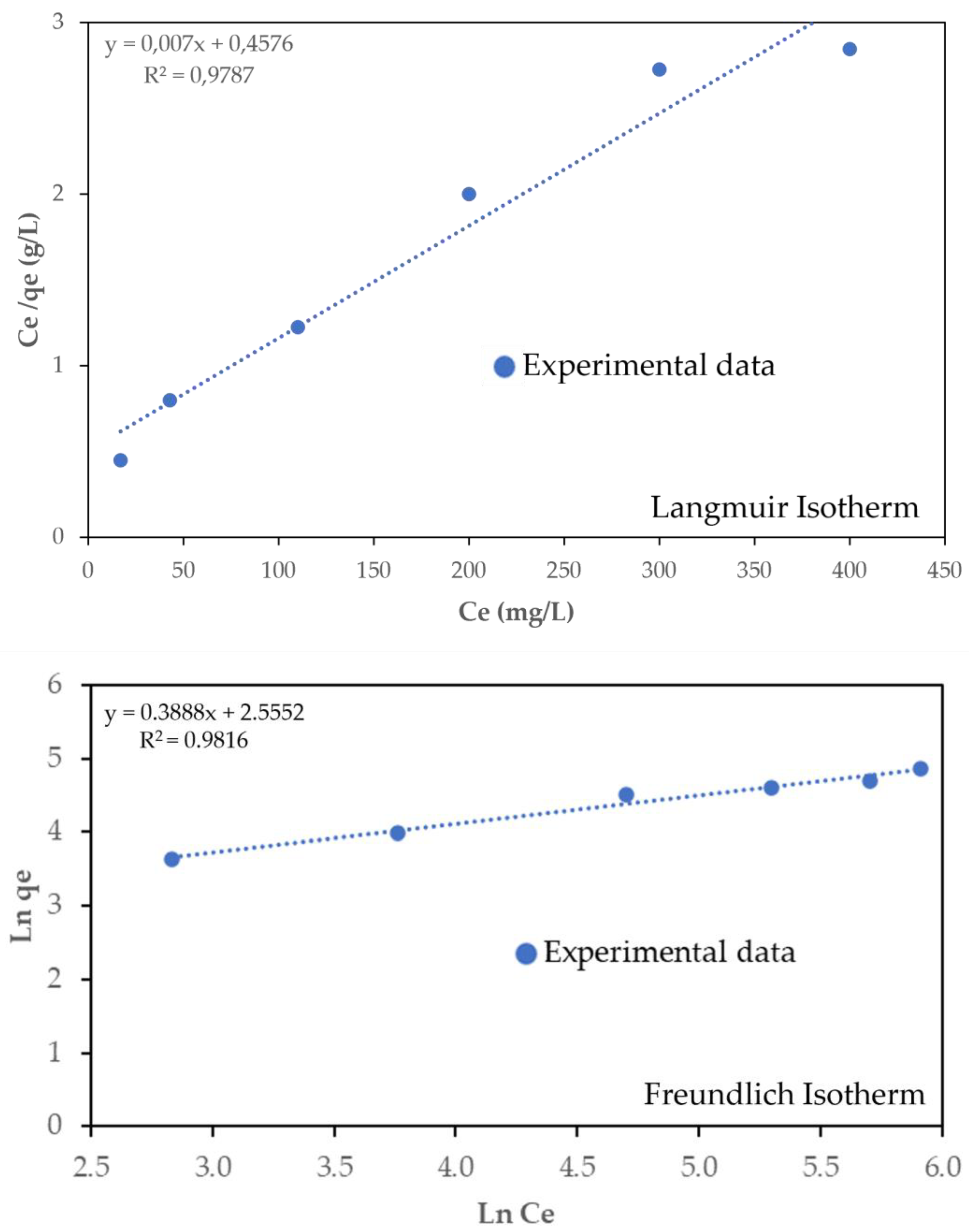
3.4. Sorption Isotherms
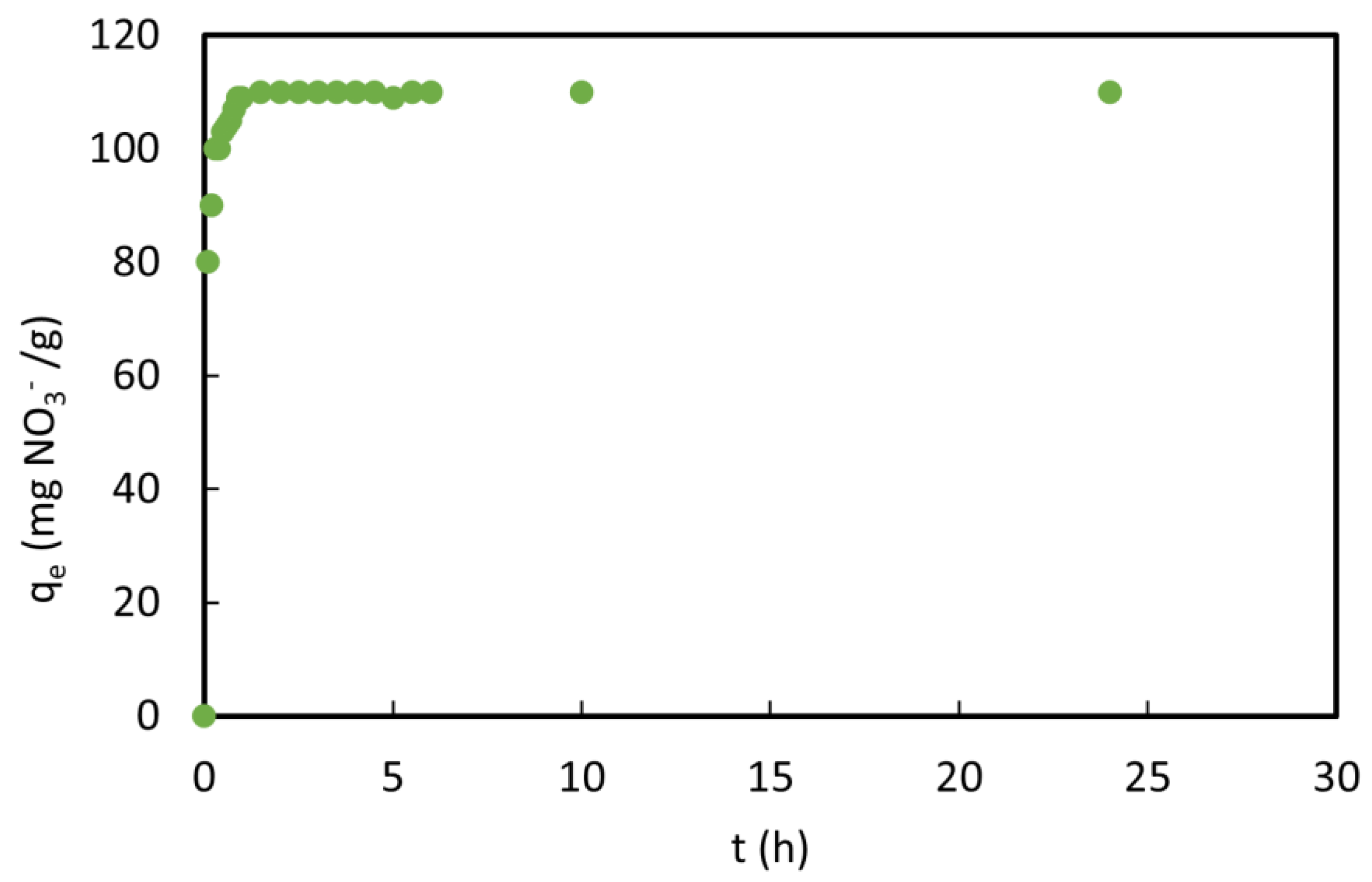
3.5. Sorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abascal, E.; Gómez-Coma, I.; Ortiz, I.; Ortiz, A. Global dignosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2022, 291, 132728. [Google Scholar] [CrossRef]
- Tokazhanov, G.; Ramazanova, E.; Hamid, S.; Bae, S.; Lee, W. Advances in catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: A review. Chem. Eng. J. 2020, 384, 123252. [Google Scholar] [CrossRef]
- Weilgelhofer, G.; Hein, T. Efficiency and detrimental side effects of denitrifying bioreactors for nitrate reduction in drainage water. Environ. Sci. Pollut. Res. 2015, 22, 13534–13545. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward osmosis technology for water treatment: Recent advances and future perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Palko, J.W.; Oyarzun, D.I.; Ha, B.; Stadermann, M.; Santiago, J.G. Nitrate removal from water using electrostatic regeneration of functionalized adsorbent. Chem. Eng. J. 2018, 334, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Fanning, J.C. The chemical reduction of nitrate in aqueous solution. Coord. Chem. Rev. 2000, 199, 159–179. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biothechn. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Xu, J.; Pu, Y.; Qi, W.; Yang, X.J.; Tang, Y.; Wan, P.; Fisher, A. Chemical removal of nitrate from water by aluminum-iron alloys. Chemosphere 2017, 166, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- An, B.; He, H.; Duan, B.; Deng, J.; Liu, Y. Selective reduction of nitrite to nitrogen gas by CO2 anion radical from the activation of oxalate. Chemosphere 2021, 278, 130388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhuang, S.T. Removal of various pollutants from water and wastewaters by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Rosique, M.; Angosto, J.M.; Guibal, E.; Roca, M.J.; Fernández-López, J.A. Factorial design methodological approach for enhanced cadmium ions bioremoval by Opuntia biomass. CLEAN Soil Air Water 2016, 44, 959–966. [Google Scholar] [CrossRef]
- Saavedra, M.I.; Doval Miñarro, M.; Angosto, J.M.; Fernández-López, J.A. Reuse potential of residues of artichoke (Cynara scolymus L.) from industrial canning processing as sorbent of heavy metals in multimetallic effluents. Ind. Crops Prod. 2019, 141, 111751. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Rachna, S.A. Water purification by using adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Dovi, E.; Kani, A.N.; Aryee, A.A.; Jie, M.; Li, J.J.; Li, Z.H.; Qu, L.B.; Han, R.P. Decontamination of bisphenol A and congo red dye from solution by using CTAB functionalized walnut shell. Environ. Sci. Pollut. Res. 2021, 28, 28732–28749. [Google Scholar] [CrossRef]
- Kani, A.N.; Dovi, E.; Mptani, F.M.; Aryee, A.A.; Han, R.; Li, Z.; Qu, L. Pollutant decontamination by polyethyleneimineengineered agricultural waste materials: A review. Environ. Chem. Lett. 2022, 20, 705–729. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Angosto, J.M.; Avilés, M.D. Biosorption of hexavalent chromium from aqueous medium with Opuntia biomass. Sci. World J. 2014, 2014, 670249. [Google Scholar] [CrossRef] [Green Version]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive removal of nickel (II) ions from aqueous environment: A review. J. Environ. Manag. 2016, 179, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Liu, Y.; Wang, J.L. Mechanistic insight into the adsorption of diclofenac by MIL-100: Experiments and theoretical calculations. Environ. Pollut. 2019, 253, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Lamine, A.B.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of crystal violet on biomass from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modelling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Zue, H.; Gao, X.; Sliem, M.; Mobarak, M.; Dong, R.; Wang, X.; Fu, K.; Li, Q.; Li, Z. Efficient adsorption of anionic azo dyes on porous heterostuctured MXene/biomass activated carbon composites: Experiments, characterization, and theoretical analysis via advanced statistical physis models. Chem. Eng. J. 2023, 451, 138735. [Google Scholar]
- Tong, D.; Zhuang, J.; Lee, J.; Buchanan, J.; Chen, X. Concurrent transport and removal of nitrate, phosphate and pesticides in low-cost metal and carbon-based materials. Chemosphere 2019, 230, 84–91. [Google Scholar] [CrossRef]
- Adesemuyi, M.F.; Adebayo, M.A.; Akinola, A.O.; Olasehinde, E.F.; Adewole, K.A.; Lajife, L. Preparation and characterisation of biochars from elephant grass and their utilisation for aqueous nitrate removal: Effect of pyrolysis temperature. J. Environ. Chem. Eng. 2020, 8, 104507. [Google Scholar] [CrossRef]
- Banu, H.A.T.; Karthikeyan, P.; Meehakshi, S. Comparative studies on revival of nitrate and phosphate ions using quaternized corn husk and jackfruit peel. Bioresour. Technol. Rep. 2019, 8, 100331. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Daso, A.P.; Okonkwo, J.O. A review of the application of agricultural wastes as precursor materials for the adsorption of per and plyfluoroalkyl substances: A focus on current approaches and methodologies. Environ. Technol. Innov. 2018, 9, 100–114. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Masek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Asanjarani, N.; Shariatmanesh, T. Taguchi L16 orthogonal array optimization for Cd(II) removal using Carpinus betulus tree leaves. Adsorption characterization. Inter. Biodeter. Biodegrad. 2013, 85, 66–77. [Google Scholar] [CrossRef]
- Rahul, D.; Pretesh, J. Application of Taguchi-based design of experiments for industrial chemical processes, statistical approaches with emphasis on design of experiments applied to chemical processes. Valter Silva IntechOpen 2018, 69501, 139. [Google Scholar]
- Fernández-López, J.A.; Angosto, J.M.; Roca, M.J.; Doval Miñarro, M. Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci. Total Environ. 2018, 24, 11154–11161. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.Y.; Lin, C.P. Adsorption of Cd(II) from wastewater using spent coffee grounds by Taguchi optimization. Desalin. Wat. Treat. 2016, 24, 11154–11161. [Google Scholar] [CrossRef]
- Varala, S.; Kumari, A.; Dharanija, B.; Bhargava, S.K.; Parthasarathy, R.; Satyavathi, B. Removal of thorium (IV) from aqueous solutions by deoiled karanja seed cake: Optimization using Taguchi method, equilibrium, kinetic and thermodynamic studies. J. Environ. Chem. 2016, 4, 405–417. [Google Scholar] [CrossRef]
- Pundir, R.; Chary, C.H.V.C.; Dastidar, M.G. Application of Taguchi method for optimizing the process parameters for the removal of copper and nickel by growing Aspergillus sp. Water Res. Ind. 2018, 20, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Fan, Y.; Zhang, M.; Ming, Z.; Yang, S.; Arkin, A. Functionalized agricultural biomass as a low-cost adsorbent: Utilization of rice straw incorporated with amine groups for the adsorption of Cr(VI) and Ni(II) from single and binary systems. Biochem. Eng. J. 2016, 105, 27–35. [Google Scholar] [CrossRef]
- Hsieh, K.L.; Tong, L.I.; Chiu, H.P.; Yeh, H.Y. Optimization of a multi-response problem in Taguchi’s dynamic system. Comput. Ind. Eng. 2005, 4, 556–571. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. Comparison of linearization methods for modelling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Multicomponent adsorption study of metal ions onto bagasse fly ash using Taguchi´s design of experimental methodology. Ind. Eng. Chem. Res. 2007, 46, 5697–5706. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Boota, R.; Bhatti, H.N.; Hanif, M.A. Removal of Cu(II) and Zn(II) using lignocellulosic fiber derived from Citrus reticulata (kinnow) waste biomass. Sep. Sci. Technol. 2009, 44, 4000–4022. [Google Scholar] [CrossRef]
- Ali, D.; Agarwal, R.; Hanifa, M.; Rawat, P.; Paswan, R.; Rai, D.; Tyagi, I.; Naik, S.; Pippal, A. Thermo-physical properties and microstructural behaviour of biochar-incorporated cementitious material. J. Build. Eng. 2023, 64, 105695. [Google Scholar] [CrossRef]
- Aman, A.M.N.; Selvarajoo, A.; Lau, T.L.; Chen, W. Optimization via response surface methodology of palm kernel shell biochar for supplementary cementitious replacement. Chemophere 2023, 313, 137477. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Doval Miñarro, M.; Angosto, J.M.; Fernández-Lledó, J. Adsoptive and surface characterization of Mediterranean agrifood processing wastes: Prospection for pesticide removal. Agronomy 2021, 11, 561. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K.A. A correlation for calculating elemental composition from proximate analysis of biomass materials. Fuel 2007, 86, 1710–1719. [Google Scholar] [CrossRef]
- Barka, N.; Ouzaouit, K.; Abdennouri, M.; El Makhfouk, M. Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J. Taiwan Int. Chem. Eng. 2013, 44, 52–60. [Google Scholar] [CrossRef]
- Nouri, H.; Abdedayem, A.; Hamidi, I.; Najjar, S.; Ouederni, A. Biosorption of lead heavy metal of prckly pear cactus biomaterial; kinetic, thermodynamic and regeneration studies. Cellul. Chem. Technol. 2021, 55, 932. [Google Scholar] [CrossRef]
- Barrera, H.; Ureña-Núñez, F.; Bilyeu, B.; Barrera-Díaz, C. Removal of chromium and toxic ions present in mine drainage by Ectodermis of Opuntia. J. Hazard. Mat. 2006, 136, 846–853. [Google Scholar] [CrossRef]
- Petrosyan, A.M.; Sukiasyan, R.P. Vibrational spectra of l-arginine nitrates. J. Molec. Struct. 2008, 874, 51–56. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modelling. J. Molec. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mat. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Amirmahmoodi, S.; Ghafari-Nazari, A. Taguchi optimization approach for Pb(II) and Hg(II) removal from aqueous solutions using modified mesoporous carbon. J. Hazard. Mat. 2011, 192, 1046–1055. [Google Scholar] [CrossRef]
- Fotsing, P.N.; Bouazizi, N.; Woumfo, E.D.; Mofaddel, N.; Le Derf, F.; Vieillard, J. Investigation of chromate and nitrate removal by adsoption at the surface on an amine-modified cocoa shell adsorbent. J. Environ. Chem. Eng. 2021, 9, 104618. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Dovi, E.; Aryee, A.; Li, J.; Li, Z.; Qu, L.; Han, R. Amine-grafted walnut shell for efficient removal of phosphate and nitrate. Environ. Sci. Pollut. Res. 2021, 29, 20976–20995. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modelling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Karnitz, O., Jr.; Gurgel, L.V.A.; De Melo, J.C.P.; Botaro, V.R.; Melo, T.M.S.; de Freitas Gil, R.P.; Gil, L.F. Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresour. Technol. 2007, 98, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Bulut, E.; Ozacar, M.; Sengil, A. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microp. Mesop. Mat. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Obon, J.M.; Angosto, J.M.; González-Soto, F.; Ascua, A.; Fernández-López, J.A. prototyping a spinning adsorber submerged filter for continuous removal of wastewater contaminants. J. Water Proc. Eng. 2022, 45, 102515. [Google Scholar] [CrossRef]
- Yang, L.; Yang, M.; Xu, P.; Zhao, X.; Bai, H.; Li, H. Characteristics of nitrate removal from aqueous solution by modified steel slag. Water 2017, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Fotsing, P.N.; Woumfo, E.D.; Mezghich, S.; Mignot, M.; Moffadel, N.; Le Derf, F.; Viellard, J. Surface modification of biomaterials bases on cocoa shell with improve nitrate and Cr (VI) remova. RSC Adv. 2020, 10, 20009–20019. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, X.; Wang, X.; Gao, B.; Yue, Q.; Song, W.; Zhang, L.; Wang, H. FITIR, Raman and XPS analysis during phosphate, nitrate and Cr (VI) removal by amine cross-linking biosorbent. J. Coll. Sci. 2016, 468, 313–323. [Google Scholar]
- Quiao, H.; Mei, L.; Chen, G.; Liu, H.; Peng, C.; Ke, F.; Hour, R.; Wan, X.; Cai, H. Adsorption of nitrate and phosphate aqueous solution using amne cross-linked tea wastes. Appl. Surf. Sci. 2019, 483, 114–122. [Google Scholar] [CrossRef]
- Keränen, A.; Leiviskä, T.; Hormi, O.; Tanskanen, J. Removal of nitrate by modified pine sawdust: Effects of temperature and co-existing anions. J. Environ. Manag. 2015, 147, 46–54. [Google Scholar] [CrossRef]


| Factor | Description | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| A | Sorbent dosage (g·L−1) | 0.5 | 1.0 | 2.0 |
| B | Initial nitrate concentration (mg·L−1) | 40 | 200 | 400 |
| C | Sorbent particle size (mm) | 0.2–0.5 | 0.5–0.8 | 0.8–1.0 |
| RUN | Sorbent Dosage (g·L−1) | [NO3−] (g·L−1) | Particle Size (mm) | qe (mg·g−1) | S/N Ratio |
|---|---|---|---|---|---|
| 1 | 0.5 | 40 | 0.2–0.5 | 34 ± 0.2 | 30.63 ± 0.3 |
| 2 | 0.5 | 200 | 0.5–0.8 | 40 ± 0.3 | 32.04 ± 0.2 |
| 3 | 0.5 | 400 | 0.8–1.0 | 40 ± 0.2 | 32.04 ± 0.2 |
| 4 | 1.0 | 40 | 0.5–0.8 | 24 ± 0.4 | 27.60 ± 0.2 |
| 5 | 1.0 | 200 | 0.8–1.0 | 60 ± 0.3 | 35.56 ± 0.3 |
| 6 | 1.0 | 400 | 0.2–0.5 | 110 ± 0.5 | 40.83 ± 0.4 |
| 7 | 2.0 | 40 | 0.8–0.1 | 13.5 ± 0.2 | 22.61 ± 0.2 |
| 8 | 2.0 | 200 | 0.2–0.5 | 57.5 ± 0.4 | 35.19 ± 0.3 |
| 9 | 2.0 | 400 | 0.5–0.8 | 45 ± 0.3 | 33.06 ± 0.3 |
| Level | Sorbent Dosage | [NO3−] (g·L−1) | Particle Size |
|---|---|---|---|
| L1 | 31.5 | 26.9 | 35.5 |
| L2 | 34.6 | 34.2 | 30.9 |
| L3 | 30.2 | 35.3 | 30.1 |
| Delta | 4.3 | 8.36 | 5.48 |
| Rank | 3 | 1 | 2 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qmax (mg·g−1) | kL (mg·L−1) | R2 | KF (mg·g−1) | n | R2 |
| 142.86 | 0.0153 | 0.979 | 12.86 | 2.57 | 0.982 |
| Nitrate Ion | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg·g−1) | k1 (h−1) | R2 | qe (mg·g−1) | k2 (g·mg−1·h−1) | R2 | |
| NO3− | 57.12 | 1.76 | 0.93 | 110.16 | 0.41 | 0.99 |
| Bioadsorbent | Adsorption Capacity (mg·g−1) | Reference |
|---|---|---|
| Modified steel slag | 16.40 | [62] |
| Modified cocoa shell | 31.65 | [63] |
| Modified cocoa shell | 56.95 | [54] |
| Amine cross-linked reed | 102.00 | [64] |
| Amine cross-linked tea wastes | 136.43 | [65] |
| Modified pine sawdust | 145.26 | [66] |
| Modified olive mill residues | 110.00 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angosto, J.M.; Obón, J.M.; Roca, M.J.; Alacid, M.; Fernández-López, J.A. A Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues. Agronomy 2023, 13, 1325. https://doi.org/10.3390/agronomy13051325
Angosto JM, Obón JM, Roca MJ, Alacid M, Fernández-López JA. A Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues. Agronomy. 2023; 13(5):1325. https://doi.org/10.3390/agronomy13051325
Chicago/Turabian StyleAngosto, José M., José M. Obón, María J. Roca, Mercedes Alacid, and José A. Fernández-López. 2023. "A Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues" Agronomy 13, no. 5: 1325. https://doi.org/10.3390/agronomy13051325
APA StyleAngosto, J. M., Obón, J. M., Roca, M. J., Alacid, M., & Fernández-López, J. A. (2023). A Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues. Agronomy, 13(5), 1325. https://doi.org/10.3390/agronomy13051325









