Soil Bacterial Community Structure and Function under the Substitution of Chemical Fertilizer with Maize Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Sampling and Analysis Methods
2.3. Statistical Analysis
3. Results
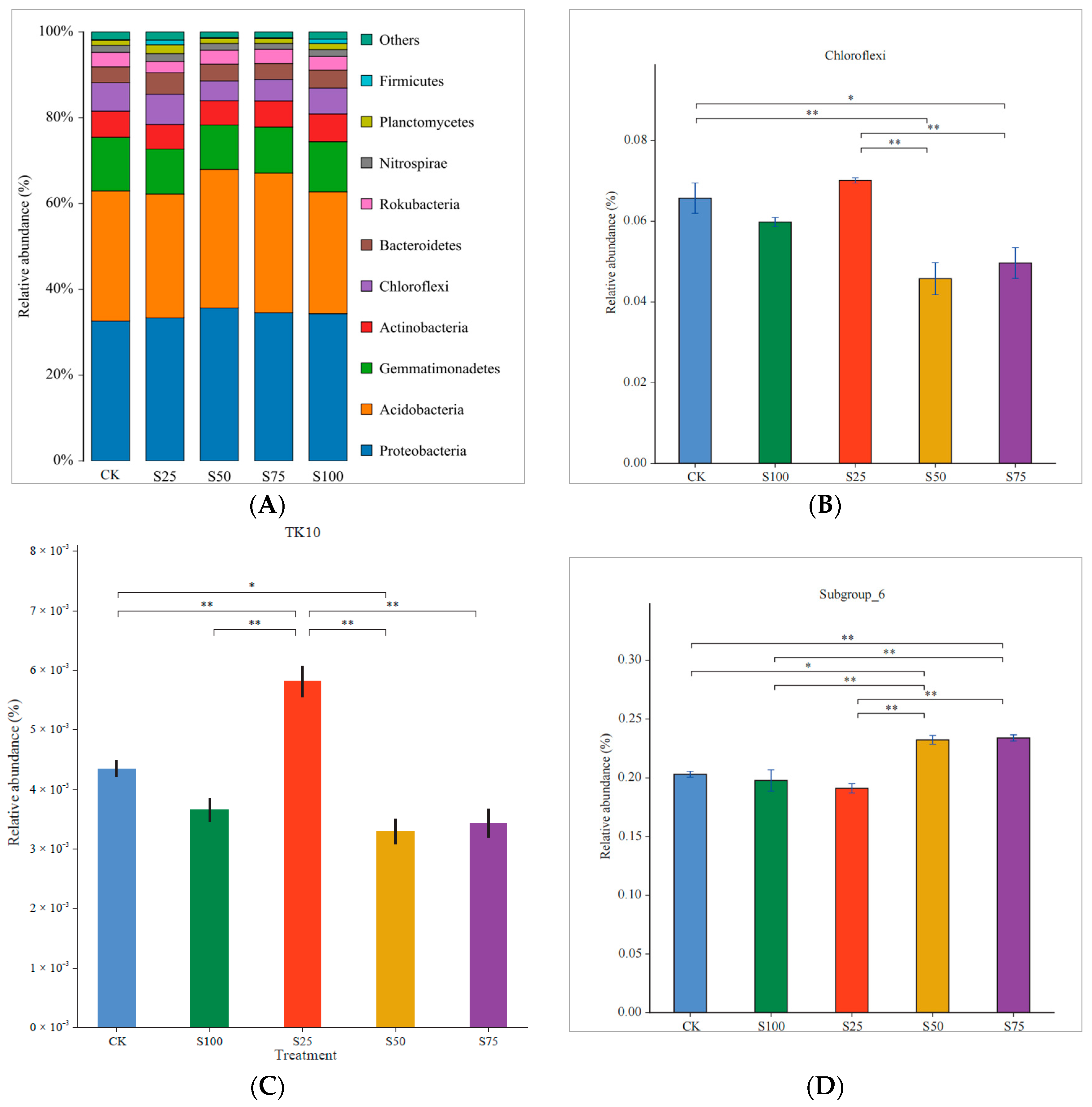
3.1. Relative Abundance of Bacteria
3.2. Ace and Shannon Index
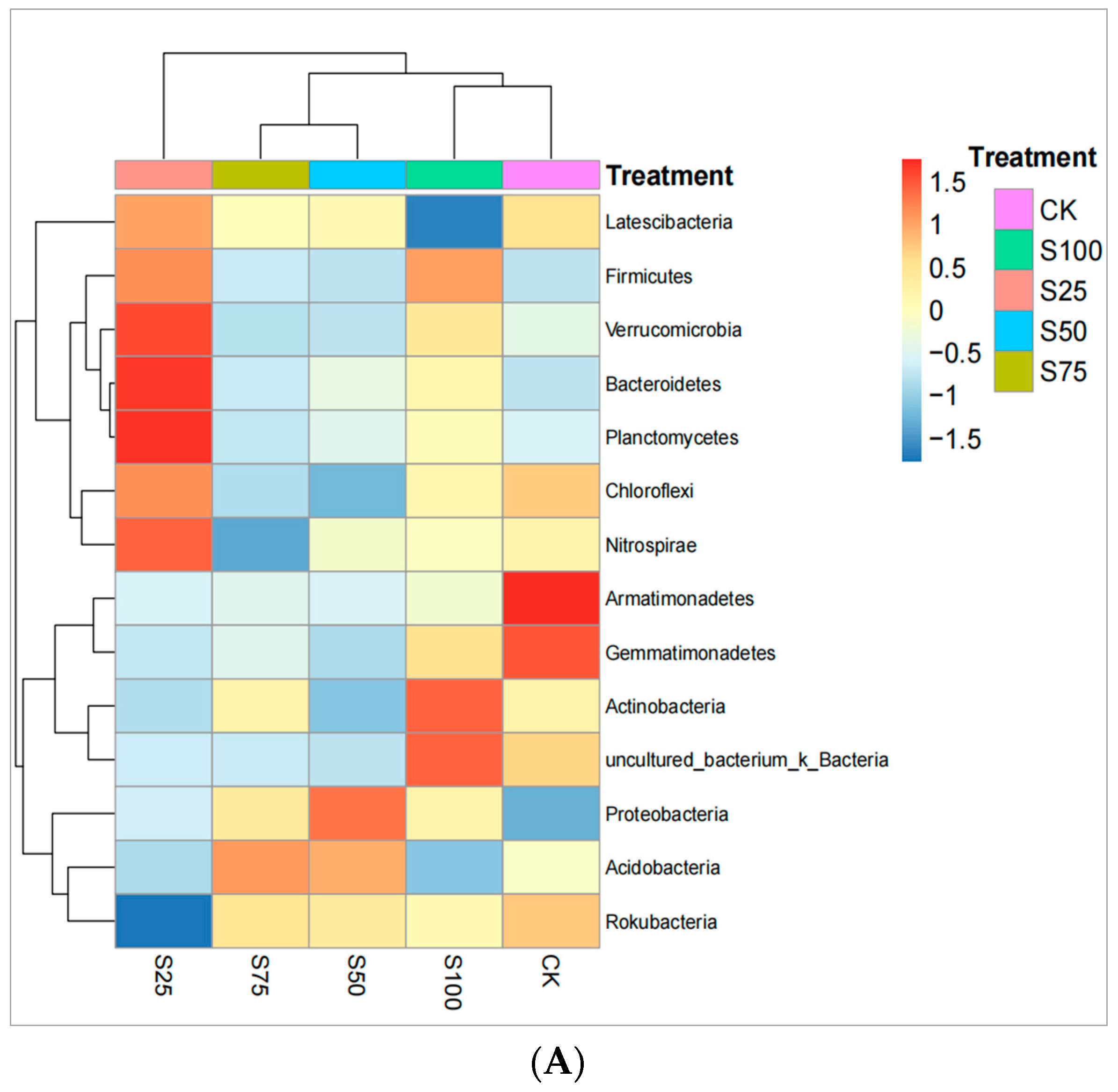
3.3. Clustered Heat Maps
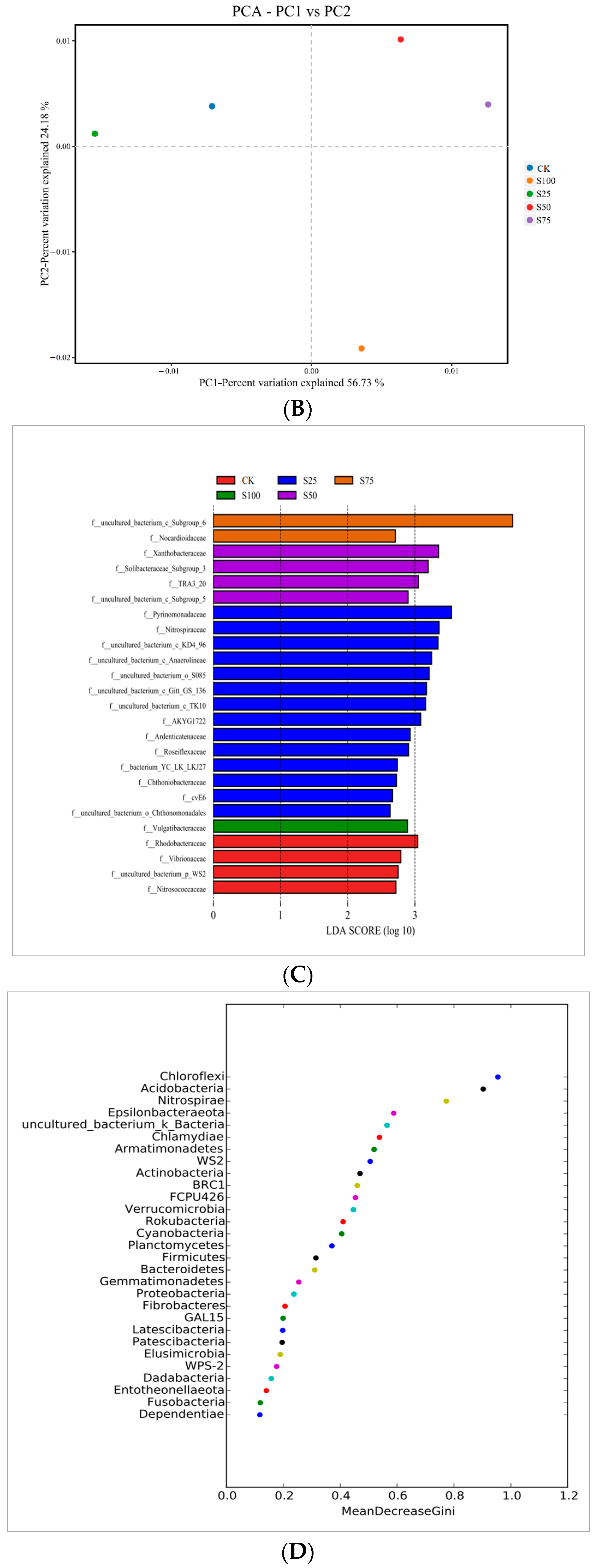
3.4. PCA
3.5. LEfSe
3.6. Random Forests
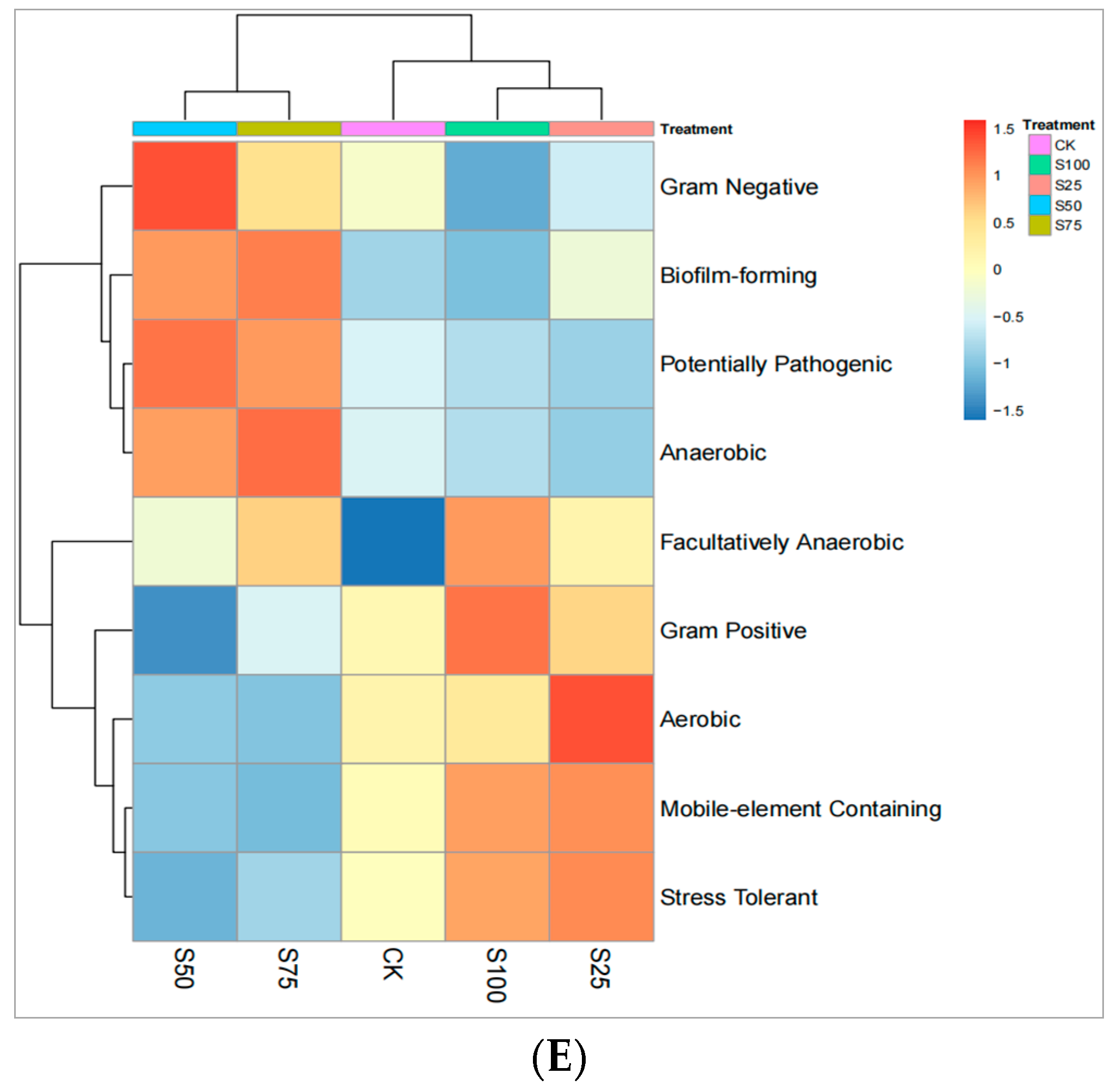
3.7. Bacterial Phenotypes
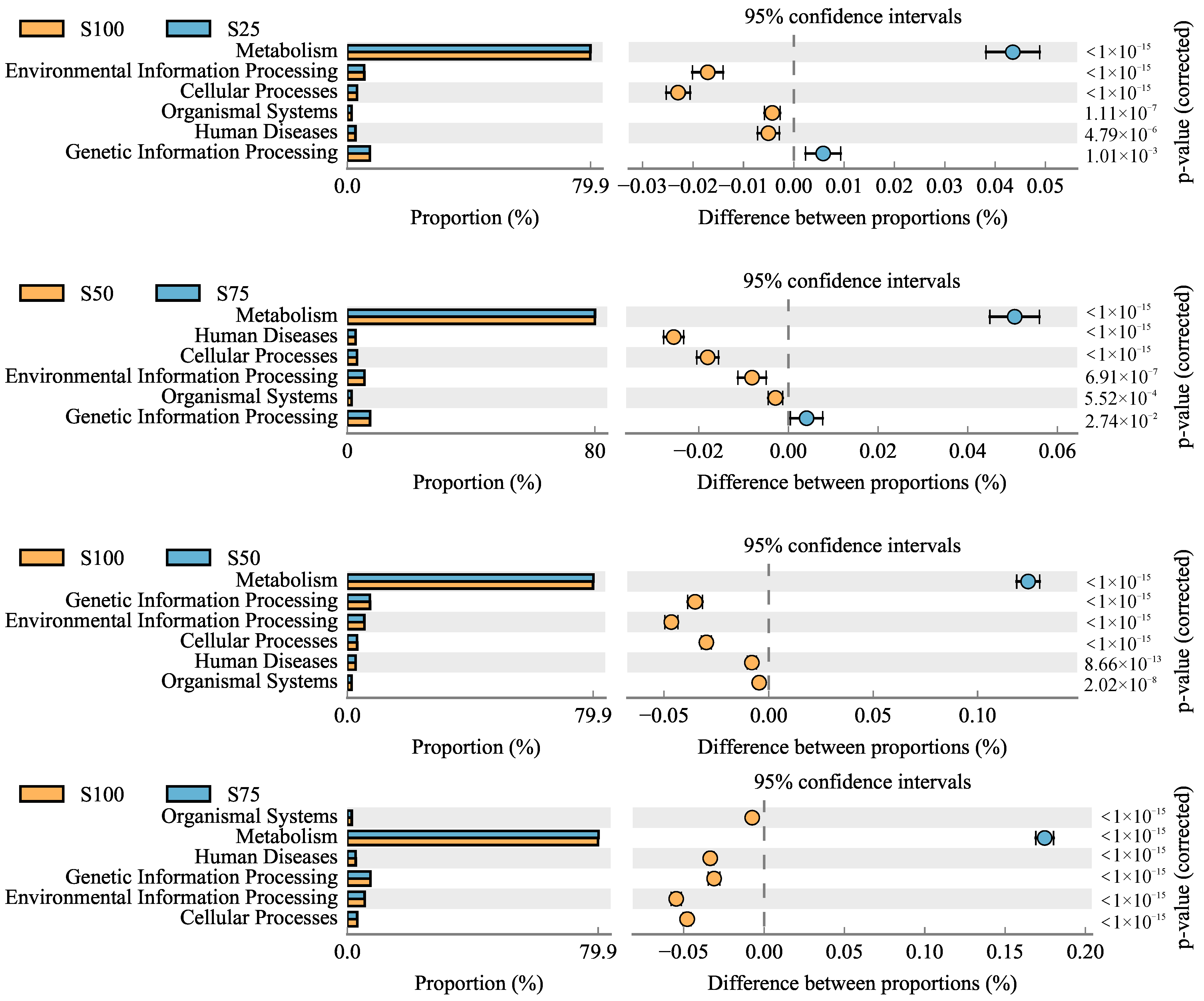
3.8. Functions Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mapelli, F.; Marasco, R.; Rizzi, A.; Baldi, F.; Ventura, S.; Borin, D.S. Bacterial Communities Involved in Soil Formation and Plant Establishment Triggered by Pyrite Bioweathering on Arctic Moraines. Microb. Ecol. 2011, 61, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Huang, X.; Zhang, Y.; Zhao, Y.; Shi, X. Effects of long-term application of chloride containing fertilizers on the biological fertility of purple soil under a rice-wheat rotation system. Sci. Agric. Sin. 2016, 49, 686–694. [Google Scholar]
- Dhaliwal, S.S.; Naresh, R.K.; Gupta, R.K.; Panwar, A.S.; Mahajan, N.C.; Singh, R.; Mandal, A. Effect of tillage and straw return on carbon footprints, soil organic carbon fractions and soil microbial community in different textured soils under rice–wheat rotation: A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 103–115. [Google Scholar] [CrossRef]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L.; He, Z.C.; Shen, A.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wu, J.; Ahmed, S.; Hu, U.; Chen, X.; Li, X. Short-term effects of different straw returning methods on the soil physicochemical properties and quality index in dryland farming in NE China. Sustainability 2020, 12, 2631. [Google Scholar] [CrossRef]
- Tian, P.; Lian, H.; Wang, Z.; Jiang, Y.; Li, C.; Sui, P.; Qi, H. Effects of deep and shallow tillage with straw incorporation on soil organic carbon, total nitrogen and enzyme activities in Northeast China. Sustainability 2020, 12, 8679. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Zhou, W.; Qiu, S.; Huang, S.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Yuan, H. Development of Ignition-Assisting Agents for Densified Corn Stover Briquetting Fuel and Experimental Study and Simulation of Combustion Characteristics. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, 2010. [Google Scholar]
- Yang, H.; Ma, J.; Rong, Z.; Zeng, D.; Wang, Y.; Hu, S.; Ye, W.; Zheng, X. Wheat Straw Return Influences Nitrogen-Cycling and Pathogen Associated Soil Microbiota in a Wheat–Soybean Rotation System. Front. Microbiol. 2019, 10, 1811. [Google Scholar] [CrossRef]
- Yang, H.; Meng, Y.; Feng, J.; Li, Y.; Zhai, S.; Liu, J. Direct and indirect effects of long-term ditch-buried straw return on soil bacterial community in a rice–wheat rotation system. Land Degrad. Dev. 2020, 31, 851–867. [Google Scholar] [CrossRef]
- Qiang, X.; Yuan, H.; Gao, W. The influence of straw returning volume on soil CO2 release and soil microbial content. J. Appl. Ecol. 2004, 15, 469–472. [Google Scholar]
- Samoura, M. Integrative Effects of Tillage and Straw Incorporation on Crop Yield and Greenhouse Gas Emission in a Double Rice Cropping System. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Gosal, S.K.; Gill, G.K.; Sharma, S.; Walia, S.S. Soil nutrient status and yield of rice as affected by long-term integrated use of organic and inorganic fertilizers. J. Plant. Nutr. 2018, 41, 539–544. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Di, L.; Yang, Y.; Xu, F.; Qian, X. Microbial community structure and abundance of iron oxidizing bacteria as a result of straw retuning in paddy soil. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2022, 43, 97–104. [Google Scholar]
- Zheng, J.; Cheng, M.; Luan, L.; Kong, P.; Sun, B.; Jiang, Y. Effects of straw returning on the ammonia-oxidizers and nitrification in the rhizosphere of maize in a red soil. Acta Ecol. Sin. 2022, 42, 1–12. [Google Scholar]
- Yu, M.; Gao, X.; Liu, X.; Wang, Z. Effects of different straw returning amount on soil nutrients and bacterial community structure in dryland. J. Arid. Land. Resour. Environ. 2022, 36, 171–177. [Google Scholar]
- Wang, J.; Hu, J.; Di, L.; Liu, L.; Wang, G.; Qian, X.; Zhang, Z. Effects of straw returning and nitrogen management on soil microbial community structure at different rice growth stages. Jiangsu J. Agric. Sci. 2021, 37, 1460–1470. [Google Scholar]
- Liu, S.; Zheng, X.; Chen, J.; Wu, B. Effect of irrigation and nitrogen treatments on nitrogen migration and accumulation in soil phase transition during freezing-thawing period. Agric. Res. Arid. Areas 2017, 035, 166–172. [Google Scholar]
- Molina-Montenegro, M.A.; Oses, R.; Atala, C.; Torres-Díaz, C.; Bolados, G.; León-Lobos, P. Nurse effect and soil microorganisms are key to improve the establishment of native plants in a semiarid community. J. Arid. Environ. 2016, 126, 54–61. [Google Scholar] [CrossRef]
- Fierer, N.; Wood, S.A.; Bueno de Mesquita, C.P. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Mu, X.; Zhao, X.; Tang, B.; Xia, L.; Zhao, Y.; Liu, T. Effects of Straw Microorganisms Returning on Soil Nutrients, Rhizosphere Soil Bacterial Community Diversity and Yield of Winter Wheat. J. Henan Agric. Sci. 2022, 50, 201–206. [Google Scholar]
- Dedysh, S.N.; Yilmaz, P. Refining the taxonomic structure of the phylum Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 3796–3806. [Google Scholar] [CrossRef]
- Fu, X.; Zheng, S.; Lv, X.; Fu, L.; Liu, Z.; Quan, G.; Ji, W.; Liu, L.; Zhang, J. Effects of combination of organic compound biological fertilizer and readily available fertilizer on rice yield and soil fertility. North Rice 2021, 51, 14–18. [Google Scholar]
- Zhang, T. Effects of Warming and StrawApplication on Soil Microbial Community in a Soybean—Winter Wheat Rotation Cropland. Master’s Thesis, Nanjing University of Information Science & Technology, Nanjing, China, 2020; pp. 32–37. [Google Scholar]
- Wang, Z.; Gao, M.; Wang, Z.; She, Z.; Hu, B.; Wang, Y.; Zhao, C. Comparison of physicochemical parameters during the forced-aeration composting of sewage sludge and maize straw at different initial C/N ratios. J. Air Waste Manag. Assoc. 2013, 63, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Jennings, V.L.K.; Rayner-Brandes, M.H.; Bird, D.J. Assessing chemical toxicity with the bioluminescent photobacterium (vibrio fischeri): A comparison of three commercial systems. Water Res. 2001, 35, 3448–3456. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xie, L.; Xu, L. Soil Bacterial Community Structure and Function under the Substitution of Chemical Fertilizer with Maize Straw. Agronomy 2023, 13, 1404. https://doi.org/10.3390/agronomy13051404
Wang X, Xie L, Xu L. Soil Bacterial Community Structure and Function under the Substitution of Chemical Fertilizer with Maize Straw. Agronomy. 2023; 13(5):1404. https://doi.org/10.3390/agronomy13051404
Chicago/Turabian StyleWang, Xiaojuan, Ling Xie, and Lulu Xu. 2023. "Soil Bacterial Community Structure and Function under the Substitution of Chemical Fertilizer with Maize Straw" Agronomy 13, no. 5: 1404. https://doi.org/10.3390/agronomy13051404
APA StyleWang, X., Xie, L., & Xu, L. (2023). Soil Bacterial Community Structure and Function under the Substitution of Chemical Fertilizer with Maize Straw. Agronomy, 13(5), 1404. https://doi.org/10.3390/agronomy13051404





