Lengths of Time of Rice Husk Biochar Incorporation before Planting Affect Soil Properties and Rice Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Rice Husk Biochar
2.2. Greenhouse Experiment
2.3. Laboratory Analyses of Rice Husk Biochar, Soil, and Plant Tissue
2.3.1. Rice Husk Biochar
2.3.2. Soil
2.3.3. Plant Tissue
2.4. Statistical Analysis
3. Results and Discussion
3.1. Elemental Concentrations of Soil and Plant Tissue in Relation to Soil pH as Affected by Various Lengths of Time of Rice Husk Biochar Incorporation
3.1.1. Elements of Macronutrients in Rice
3.1.2. Elements of Micronutrients of Rice, Fe, and Mn, and Non-Nutrient Al
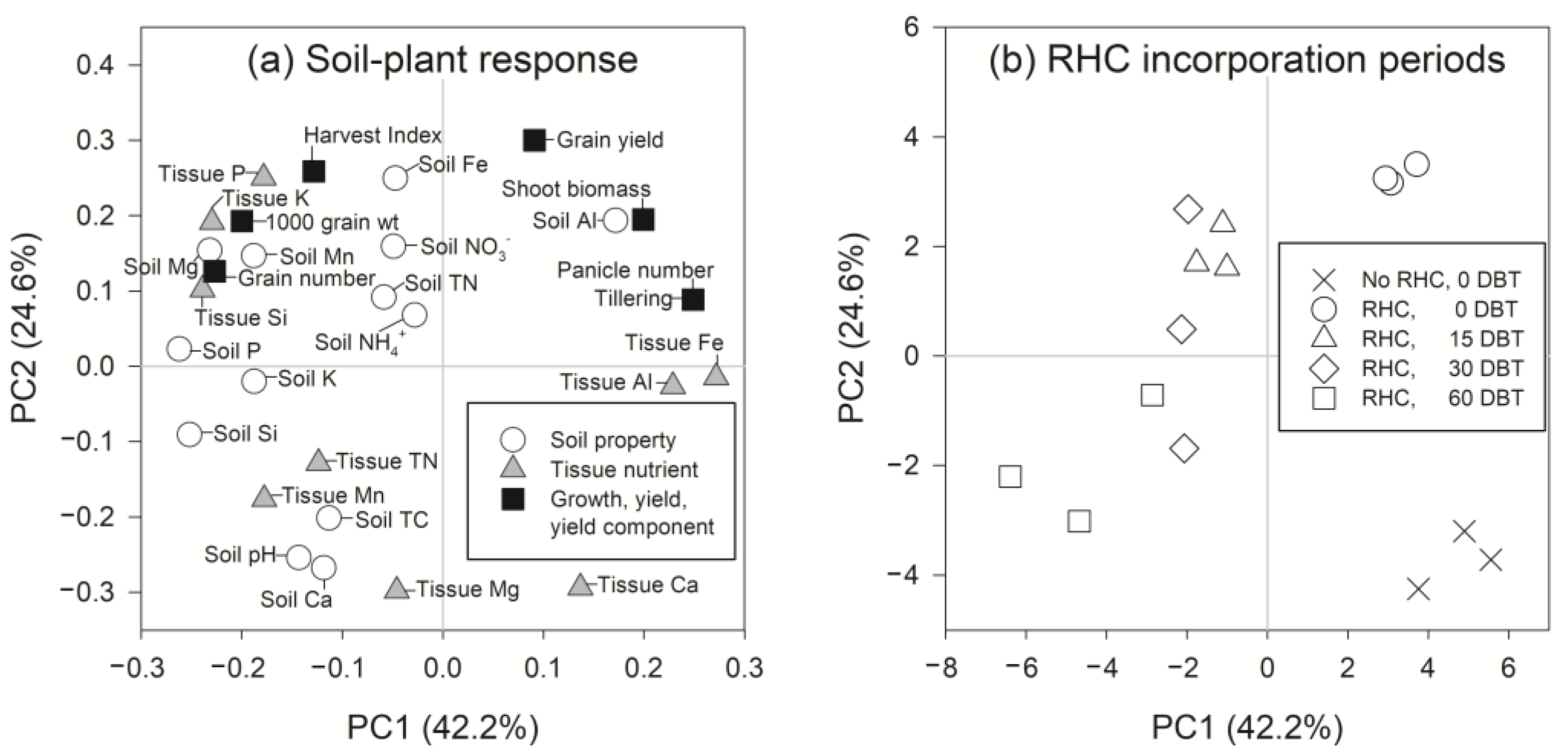
3.2. Rice Yield Decreases with Increasing Durations of Rice Husk Incorporation into Soil as Influenced by Soil and Tissue Elemental Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ly, P.; Duong Vu, Q.; Jensen, L.S.; Pandey, A.; de Neergaard, A. Effects of rice straw, biochar and mineral fertiliser on methane (CH4) and nitrous oxide (N2O) emissions from rice (Oryza sativa L.) grown in a rain-fed lowland rice soil of Cambodia: A pot experiment. Paddy Water Environ. 2015, 13, 465–475. [Google Scholar] [CrossRef]
- Si, L.; Xie, Y.; Ma, Q.; Wu, L. The short-term effects of rice straw biochar, nitrogen and phosphorus fertilizer on rice yield and soil properties in a cold waterlogged paddy field. Sustainability 2018, 10, 537. [Google Scholar] [CrossRef]
- Kumputa, S.; Vityakon, P.; Saenjan, P.; Lawongsa, P. Carbonaceous greenhouse gases and microbial abundance in paddy soil under combined biochar and rice straw amendment. Agronomy 2019, 9, 228. [Google Scholar] [CrossRef]
- Latawiec, A.E.; Królczyk, J.B.; Kuboń, M.; Szwedziak, K.; Drosik, A.; Polańczyk, E.; Grotkiewicz, K.; Strassburg, B.B.N. Willingness to adopt biochar in agriculture: The producer’s perspective. Sustainability 2017, 9, 655. [Google Scholar] [CrossRef]
- Thambhitaks, K.; Kitchaicharoen, J. Valuation of external costs of wet-season lowland rice production systems in Northern Thailand. CMUJ Nat. Sci. 2021, 20, e2021057. [Google Scholar] [CrossRef]
- Zaman, A.U. A strategic framework for working toward zero waste societies based on perceptions surveys. Recycling 2017, 2, 1. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, C.; Sardans, J.; Vancov, T.; Fang, Y.; Wu, L.; Huang, X.; Gargallo-Garriga, A.; Peñuelas, J.; Wang, W. Effect of soil degradation on the carbon concentration and retention of nitrogen and phosphorus across Chinese rice paddy fields. CATENA 2022, 209, 105810. [Google Scholar] [CrossRef]
- Fageria, N.K. Mineral Nutrition of Rice; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Antal, M.J.; Gronli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Phuong, H.T.; Uddin, M.A.; Kato, Y. Characterization of biochar from pyrolysis of rice husk and rice straw. J. Biobased Mater. Bioenergy 2015, 9, 439–446. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237–238, 105–116. [Google Scholar] [CrossRef]
- Deenik, J.L.; Diarra, A.; Uehara, G.; Campbell, S.; Sumiyoshi, Y.; Antal, M.J. Charcoal ash and volatile matter effects on soil properties and plant growth in an acid Ultisol. Soil Sci. 2011, 176, 336–345. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Amrhein, C. Wood–ash composition and soil pH following intense burning. Soil Sci. 1993, 156, 358–364. [Google Scholar] [CrossRef]
- Pels, J.R.; Nie, D.S.; Kiel, J.H.A. Utilization of ashes from biomass combustion and gasification. In Proceedings of the 13th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Berek, A.K.; Hue, N.V. Characterization of biochars and their use as an amendment to acid soils. Soil Sci. 2016, 181, 412–426. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Dual role of biochars as adsorbents for aluminum: The effects of oxygen–containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Buss, W.; Jansson, S.; Mašek, O. Unexplored potential of novel biochar–ash composites for use as organo–mineral fertilizers. J. Clean. Prod. 2019, 208, 960–967. [Google Scholar] [CrossRef]
- Bieser, J.M.H.; Thomas, S.C. Biochar and high–carbon wood ash effects on soil and vegetation in a boreal clearcut. Can. J. For. Res. 2019, 49, 1124–1134. [Google Scholar] [CrossRef]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.A.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Thammasom, N.; Vityakon, P.; Lawongsa, P.; Saenjan, P. Biochar and rice straw have different effects on soil productivity, greenhouse gas emission and carbon sequestration in Northeast Thailand paddy soil. Agric. Nat. Resour. 2016, 50, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Chen, B. Biochar impacts on soil silicon dissolution kinetics and their interaction mechanisms. Sci. Rep. 2018, 8, 8040. [Google Scholar] [CrossRef] [PubMed]
- Land Development Department. Thailand Soil Map Scale 1:25000. Available online: http://eis.ldd.go.th/lddeis/SoilView.aspx (accessed on 15 May 2019).
- Pratiwi, E.P.A.; Shinogi, Y. Rice husk biochar application to paddy soil and its effects on soil physical properties, plant growth, and methane emission. Paddy Water Environ. 2016, 14, 521–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Peng, S.; Xing, D.; Qin, J.; Laza, R.C.; Punzalan, B.R. Water use efficiency and physiological response of rice cultivars under alternate wetting and drying conditions. Sci. World J. 2012, 2012, 287907. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, M.C.S.; Kropff, M.J.; Maligaya, A.R.; Tuong, T.P. Drought–stress responses of two lowland rice cultivars to soil water status. Field Crops Res. 1996, 46, 21–39. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Powlson, D.; Min, J.; Shi, W. Rice production, nitrous oxide emission and ammonia volatilization as impacted by the nitrification inhibitor 2–chloro–6–(trichloromethyl)–pyridine. Field Crops Res. 2015, 173, 1–7. [Google Scholar] [CrossRef]
- American Standard of Testing Material. Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis; American Standard of Testing Material International: West Conshohocken, PA, USA, 2015.
- Miller, R.O. Nitric–perchloric acid wet digestion in an open vessel. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 57–61. [Google Scholar]
- Novozamsky, I.; van Eck, R.; Houba, V.J.G.; van der Lee, J.J. Solubilization of plant tissue with nitric acid–hydrofluoric acid–hydrogen peroxide in a closed–system microwave digestor. Commun. Soil Sci. Plant Anal. 1996, 27, 867–875. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propterties; Spark, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen–Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propterties; Spark, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Stevenson, F.J. Nitrogen–Inorganic forms. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propterties; Spark, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Fixen, P.E.; Grove, J.H. Testing soils for phosphorus. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 141–181. [Google Scholar]
- Bertsch, P.M.; Bloom, P.R. Aluminum. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; SSSA Book Ser. 5.; SSSA: Madison, WI, USA, 1996; pp. 517–550. [Google Scholar]
- Korndörfer, G.H.; Snyder, G.H.; Ulloa, M.; Powell, G.; Datnoff, L.E. Calibration of soil and plant silicon analysis for rice production. J. Plant Nutr. 2001, 24, 1071–1084. [Google Scholar] [CrossRef]
- Horneck, D.A.; Miller, R. Determination of total nitrogen in plant tissue. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.1: User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 17–39. [Google Scholar]
- Qian, L.; Chen, B. Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J. Agric. Food Chem. 2013, 62, 373–380. [Google Scholar] [CrossRef]
- Yao, F.X.; Arbestain, M.C.; Virgel, S.; Blanco, F.; Arostegui, J.; Maciá-Agulló, J.A.; Macías, F. Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 2010, 80, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Unzué-Belmonte, D.; Cornelis, J.-T.; Linden, C.V.; Struyf, E.; Ronsse, F.; Delvaux, B. Effects of phytolithic rice–straw biochar, soil buffering capacity and pH on silicon bioavailability. Plant Soil 2019, 438, 187–203. [Google Scholar] [CrossRef]
- Costa, H.M.d.; Visconte, L.L.Y.; Nunes, R.C.R.; Furtado, C.R.G. Rice husk ash filled natural rubber. III. Role of metal oxides in kinetics of sulfur vulcanization. J. Appl. Polym. Sci. 2003, 90, 1519–1531. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Chen, M. Novel alleviation mechanisms of aluminum phytotoxicity via released biosilicon from rice straw–derived biochars. Sci. Rep. 2016, 6, 29346. [Google Scholar] [CrossRef]
- Li, Z.; Delvaux, B. Phytolith–rich biochar: A potential Si fertilizer in desilicated soils. GCB Bioenergy 2019, 11, 1264–1282. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M. The fate of phosphorus of ash–rich biochars in a soil–plant system. Plant Soil 2014, 375, 61–74. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the mechanism of the dissolution of quartz and silica in aqueous solutions. J. Am. Chem. Soc. 2017, 2, 1116–1127. [Google Scholar] [CrossRef]
- Semerci, N.; Ahadi, S.; Coşgun, S. Comparison of dried sludge and sludge ash for phosphorus recovery with acidic and alkaline leaching. Water Environ. J. 2021, 35, 359–370. [Google Scholar] [CrossRef]
- Lindsay, W.L. Soil and plant relationships associated with iron deficiency with emphasis on nutrient interactions. J. Plant Nutr. 1984, 7, 489–500. [Google Scholar] [CrossRef]
- Tanaka, A.; Navasero, S.A. Aluminum toxicity of the rice plant under water culture conditions. Soil Sci. Plant Nutr. 1966, 12, 9–14. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry; Academic Press: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Jugdaohsingh, R.; Brown, A.; Dietzel, M.; Powell, J.J. High–aluminum–affinity silica is a nanoparticle that seeds secondary aluminosilicate formation. PLoS ONE 2013, 8, e84397. [Google Scholar] [CrossRef] [PubMed]
- Hsu, O.H. Fixation of phosphate by aluminum and iron in acidic soils. Soil Sci. 1965, 99, 398–402. [Google Scholar] [CrossRef]
- Tubaña, B.S.; Heckman, J.R. Silicon in soils and plants. In Silicon and Plant Diseases; Rodrigues, F.A., Datnoff, L.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 7–51. [Google Scholar]
- Wu, C.; Zou, Q.; Xue, S.-G.; Pan, W.-S.; Huang, L.; Hartley, W.; Mo, J.-Y.; Wong, M.-H. The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL). Environ. Pollut. 2016, 212, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.-T. The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef]
- Sombroek, W.G. Amazon Soils: A Reconnaissance of the Soils of the Brazilian Amazon Region; Centre for Agricultural Publications and Documentation: Wageningen, The Netherlands, 1966. [Google Scholar]
- Glaser, B.; Guggenberger, L.H.G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Sci. Nat. 2001, 88, 37–41. [Google Scholar] [CrossRef]
| Soil Properties | Value |
|---|---|
| Soil particle size distribution | |
| Sand (%) | 48.4 |
| Silt (%) | 40.4 |
| Clay (%) | 11.2 |
| Soil texture | Loam |
| Bulk density (g cm−3) | 1.45 |
| pH (Soil:H2O = 1:1 w/v) | 4.54 |
| Electrical conductivity (mS cm−1) | 0.039 |
| Total C (g kg−1) | 5.53 |
| Total N (g kg−1) | 0.44 |
| NH4+–N (mg kg−1) | 3.50 |
| NO3−–N (mg kg−1) | 2.80 |
| Extractable P (mg kg−1) | 21.8 |
| Extractable K (mg kg−1) | 85.8 |
| Extractable Ca (mg kg−1) | 226 |
| Extractable Mg (mg kg−1) | 39.3 |
| Extractable Fe (mg kg−1) | 243 |
| Extractable Al (mg kg−1) | 81.9 |
| Extractable Mn (mg kg−1) | 21 |
| Extractable Si (mg kg−1) | 2.67 |
| Biochar Characteristic | Value |
|---|---|
| Proximate analysis | |
| Fixed carbon (%) | 51.6 |
| Ash (%) | 30.3 |
| Volatile matter (%) | 18.1 |
| Total C (g kg−1) | 424 |
| Total N (g kg−1) | 4.22 |
| Total P (g kg−1) | 3.68 |
| Total K (g kg−1) | 5.05 |
| Total Ca (g kg−1) | 1.75 |
| Total Mg (g kg−1) | 1.15 |
| Total Si (g kg−1) | 125.9 |
| Treatment | pH (1:1) | TC † (g kg−1) | TN (g kg−1) | Mineral N (mg kg−1) | Extractable Elements (mg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NH4+–N | NO3−–N | P | K | Ca | Mg | Fe | Al | Mn | Si | ||||
| No RHB, 0 DBT ‡ | 4.68 ab § | 5.80 | 0.40 | 3.15 | 1.40 | 17.6 d | 51.9 c | 411 | 38.6 b | 235 | 70.4 a | 19.8 c | 2.79 d |
| RHB, 0 DBT | 4.45 b | 5.10 | 0.48 | 3.15 | 1.75 | 22.3 c | 49.0 c | 223 | 48.2 a | 279 | 77.5 a | 22.2 bc | 2.81 d |
| RHB, 15 DBT | 4.52 b | 5.80 | 0.44 | 3.50 | 1.87 | 27.8 b | 61.6 ab | 256 | 52.9 a | 290 | 77.5 a | 27.9 a | 3.66 c |
| RHB, 30 DBT | 4.73 ab | 5.80 | 0.43 | 3.27 | 1.87 | 29.5 ab | 55.1 bc | 418 | 52.1 a | 282 | 56.2 ab | 25.8 ab | 4.72 b |
| RHB, 60 DBT | 4.90 a | 5.80 | 0.46 | 3.15 | 1.63 | 33.3 a | 62.5 a | 514 | 53.5 a | 260 | 40.8 b | 24.4 ab | 6.23 a |
| p-value | 0.05 | 0.078 | 0.701 | 0.972 | 0.832 | <0.001 | 0.008 | 0.122 | <0.001 | 0.497 | 0.05 | 0.007 | <0.001 |
| F-test | * | ns | ns | ns | ns | *** | ** | ns | *** | ns | * | ** | *** |
| CV (%) | 3.88 | 5.85 | 17.19 | 23.31 | 33.13 | 8.1 | 7.2 | 37.58 | 5.91 | 14.92 | 24.88 | 8.76 | 4.58 |
| Treatment | N (g kg−1) | P (g kg−1) | K (g kg−1) | Ca (g kg−1) | Mg (g kg−1) | Fe (g kg−1) | Mn (g kg−1) | Al (g kg−1) | Si (g kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| No RHB, 0 DBT † | 3.20 | 1.17 b ‡ | 6.8 c | 3.83 a | 0.90 a | 0.44 a | 0.34 b | 7.00 a | 11.4 c |
| RHB, 0 DBT | 2.97 | 1.45 a | 14.4 b | 2.46 c | 0.51 b | 0.40 a | 0.20 c | 6.42 a | 27.9 b |
| RHB, 15 DBT | 3.27 | 1.50 a | 17.6 a | 2.48 c | 0.56 b | 0.27 b | 0.31 b | 1.43 b | 37.9 ab |
| RHB, 30 DBT | 3.10 | 1.50 a | 16.8 ab | 2.40 c | 0.82 a | 0.26 b | 0.34 b | 2.53 b | 35.9 ab |
| RHB, 60 DBT | 3.37 | 1.45 a | 18.0 a | 2.91 b | 0.87 a | 0.20 c | 0.83 a | 1.72 b | 47.2 a |
| p-value | 0.375 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.0149 | <0.001 |
| F-test | ns | *** | *** | *** | *** | *** | *** | * | *** |
| CV (%) | 7.7 | 4.28 | 10.46 | 3.66 | 6.91 | 8.58 | 14.73 | 52.73 | 20.48 |
| Treatment | Biomass Yield (g Hill−1) | Shoot/Root Ratio | |
|---|---|---|---|
| Root Dry Biomass | Shoot Dry Biomass | ||
| No RHB, 0 DBT † | 28.0 a ‡ | 70.0 b | 2.51 |
| RHB, 0 DBT | 29.9 a | 82.7 a | 2.76 |
| RHB, 15 DBT | 23.0 b | 67.7 bc | 2.95 |
| RHB, 30 DBT | 24.2 b | 67.6 bc | 2.81 |
| RHB, 60 DBT | 21.5 b | 62.5 c | 2.91 |
| p-value | <0.001 | <0.001 | 0.258 |
| F-test | *** | *** | ns |
| CV (%) | 6.2 | 4.97 | 8.77 |
| Treatment | Tillering (Plant Hill−1) | Grain No. (Grain Panicle−1) | Panicle No. (Panicle Hill−1) | Grain Yield (g Hill−1) | 1000-Grain wt. (g) | Harvest Index (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Filled | Unfilled | Total | % Filled | Filled | Unfilled | Total | |||||
| No RHB, 0 DBT † | 18.3 a | 116 b ‡ | 8.1 | 124 b | 93.5 | 18.3 a | 53.6 c | 0.87 | 54.5 c | 22.3 b | 40.3 b |
| RHB, 0 DBT | 20.0 a | 139 a | 8.6 | 147 a | 94.2 | 20.0 a | 74.9 a | 1.04 | 76.0 a | 23.8 a | 44.4 a |
| RHB, 15 DBT | 15.7 b | 143 a | 11.1 | 154 a | 93.0 | 15.7 b | 60.3 b | 1.08 | 61.4 b | 23.9 a | 44.1 a |
| RHB, 30 DBT | 14.7 bc | 154 a | 10.6 | 165 a | 93.6 | 14.7 bc | 61.6 b | 0.92 | 62.5 b | 23.9 a | 44.6 a |
| RHB, 60 DBT | 12.7 c | 156 a | 9.3 | 165 a | 94.3 | 12.7 c | 53.7 c | 0.69 | 54.4 c | 24.2 a | 43.2 a |
| p-value | <0.001 | 0.004 | 0.668 | 0.009 | 0.811 | <0.001 | <0.001 | 0.442 | <0.001 | 0.004 | 0.014 |
| F-test | *** | ** | ns | ** | ns | *** | *** | ns | *** | ** | * |
| CV (%) | 8.40 | 6.97 | 29.72 | 7.78 | 1.61 | 8.40 | 5.28 | 28.6 | 5.31 | 2.03 | 3.03 |
| Plant Response | Elements in Soil | |||||
|---|---|---|---|---|---|---|
| a † | bP ‡ | bMg | bSi | p-Value | R2 | |
| Tillering (Plant Hill−1) | 25.8 *** | 0.0016 ns | −0.06 ns | −1.63 * | < 0.001 | 0.723 |
| Panicle Number (Panicle Hill−1) | 25.8 *** | 0.002 ns | −0.06 ns | −1.63 * | < 0.001 | 0.723 |
| Grain wt. (g Hill−1) | 122.1 *** | −0.29 ns | −0.93 *** | −2.61 * | 0.165 | 0.685 |
| Elements in shoot tissue | ||||||
| a | bK | bFe | bSi | p-value | R2 | |
| Tillering (Plant Hill−1) | 9.1 * | 0.24 ns | 19.99 ** | −0.09 ns | 0.002 | 0.862 |
| Panicle Number (Panicle Hill−1) | 9.1 * | 0.24 ns | 19.99 ** | −0.09 ns | 0.002 | 0.862 |
| Grain wt. (g Hill−1) | 67.5 *** | −1.73 *** | 56.36 *** | 0.002 ns | 0.006 | 0.865 |
| Treatment | Ca/K † | Mg/K | Ca/Mg | Si/Fe |
|---|---|---|---|---|
| No RHB, 0 DBT ‡ | 0.58 a § | 0.139 a | 4.28 b | 26.0 c |
| RHB, 0 DBT | 0.17 b | 0.036 b | 4.82 a | 69.4 c |
| RHB, 15 DBT | 0.14 b | 0.032 b | 4.47 ab | 141.4 b |
| RHB, 30 DBT | 0.14 b | 0.049 b | 2.94 c | 136.6 b |
| RHB, 60 DBT | 0.16 b | 0.048 b | 3.38 c | 244.6 a |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| F-test | *** | *** | *** | *** |
| CV (%) | 22.58 | 32.37 | 6.45 | 24.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butnan, S.; Vityakon, P. Lengths of Time of Rice Husk Biochar Incorporation before Planting Affect Soil Properties and Rice Yield. Agronomy 2023, 13, 1445. https://doi.org/10.3390/agronomy13061445
Butnan S, Vityakon P. Lengths of Time of Rice Husk Biochar Incorporation before Planting Affect Soil Properties and Rice Yield. Agronomy. 2023; 13(6):1445. https://doi.org/10.3390/agronomy13061445
Chicago/Turabian StyleButnan, Somchai, and Patma Vityakon. 2023. "Lengths of Time of Rice Husk Biochar Incorporation before Planting Affect Soil Properties and Rice Yield" Agronomy 13, no. 6: 1445. https://doi.org/10.3390/agronomy13061445
APA StyleButnan, S., & Vityakon, P. (2023). Lengths of Time of Rice Husk Biochar Incorporation before Planting Affect Soil Properties and Rice Yield. Agronomy, 13(6), 1445. https://doi.org/10.3390/agronomy13061445







