Arbuscular Mycorrhizae Fungi Diversity in the Root–Rhizosphere–Soil of Tetraena mongolica, Sarcozygium xanthoxylon, and Nitraria tangutorum Bobr in Western Ordos, China
Abstract
:1. Introduction
2. Materials and Methods
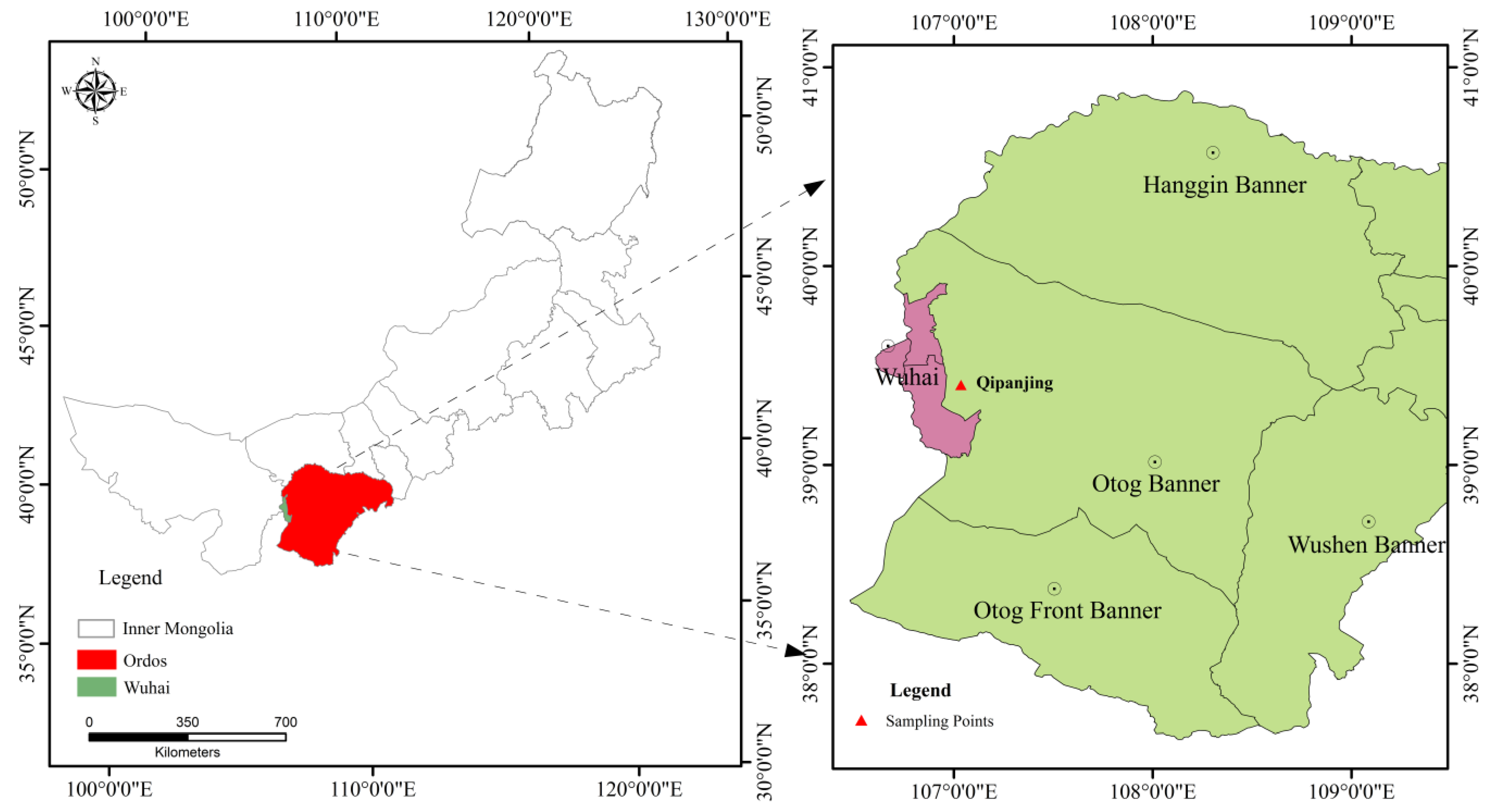
2.1. Study Region
2.2. Analysis and Sampling of Soil and Plants
2.3. DNA Extraction and PCR Amplification
2.4. Illumina Sequencing and Bioinformatics Analysis
2.5. Soil Physicochemical Properties
2.6. Statistical Analysis
3. Results
3.1. An Analysis of the Sequencing Results and the Abundance of the AMF Community
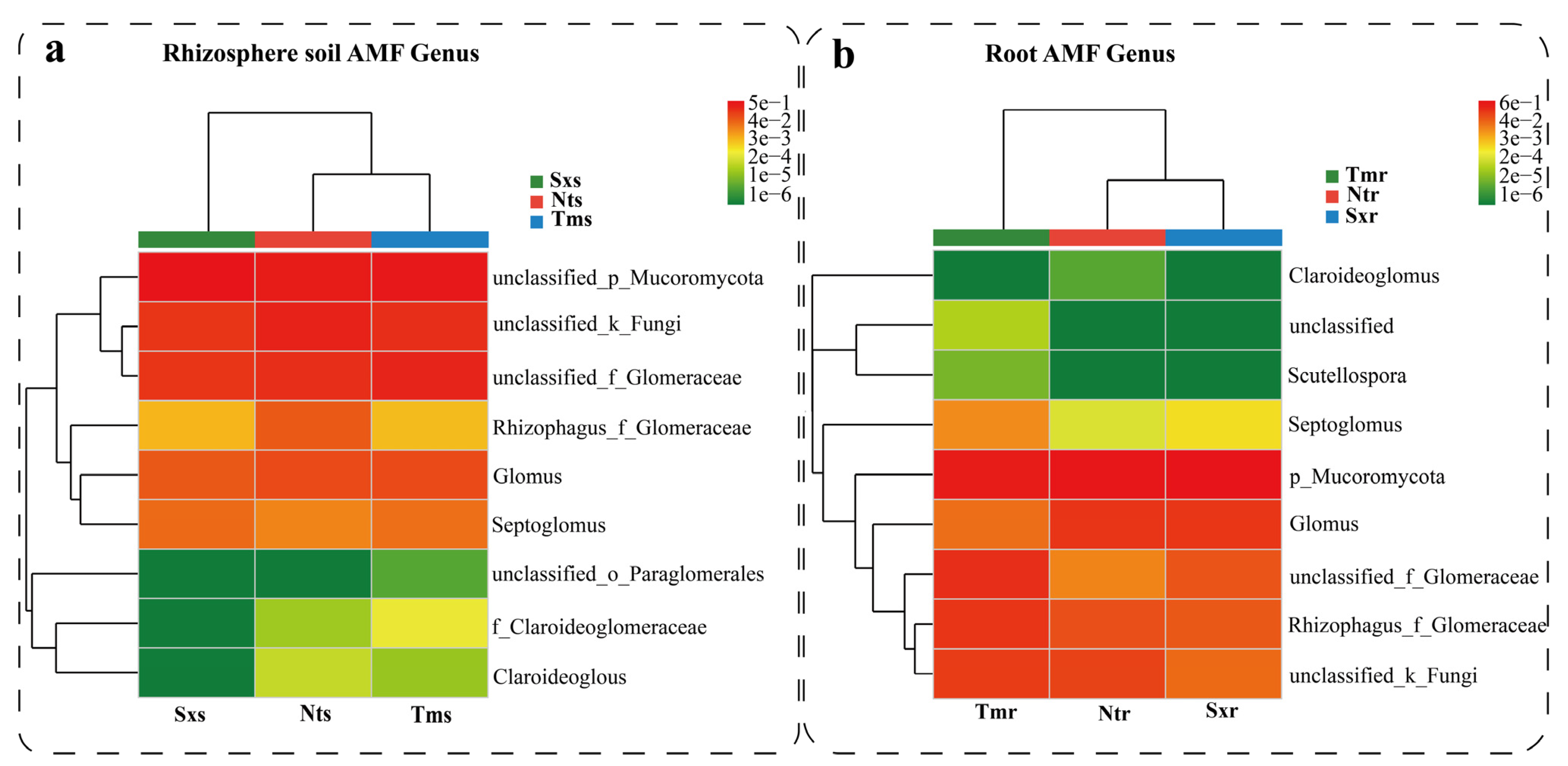
3.2. Analysis of Three Endangered Plants’ Root–Rhizosphere–Soil AM Fungi Compositions
3.3. LEfSe Analysis and Functional Characteristics
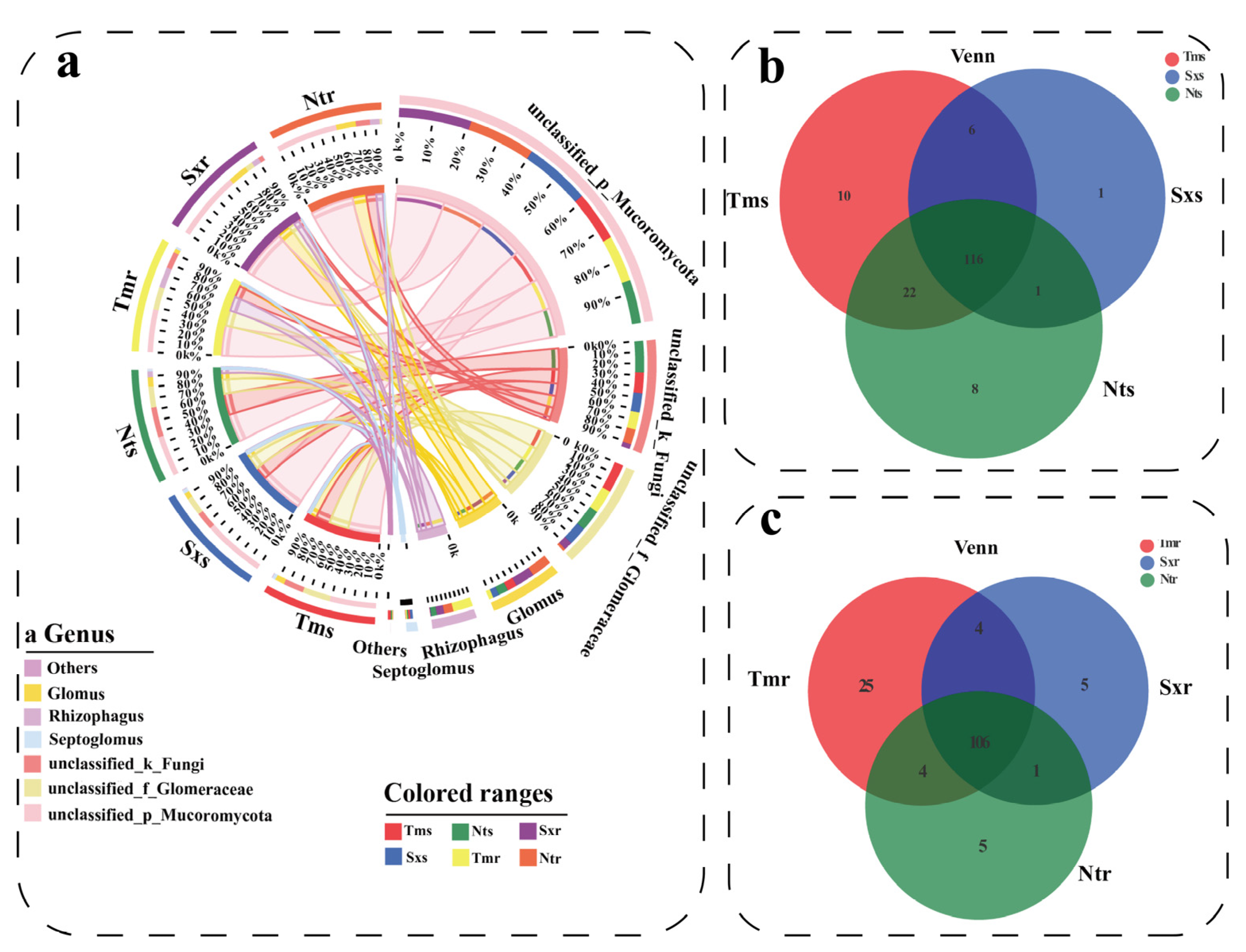
3.4. β-Diversity and Composition of Soil and Root AM Fungi Communities
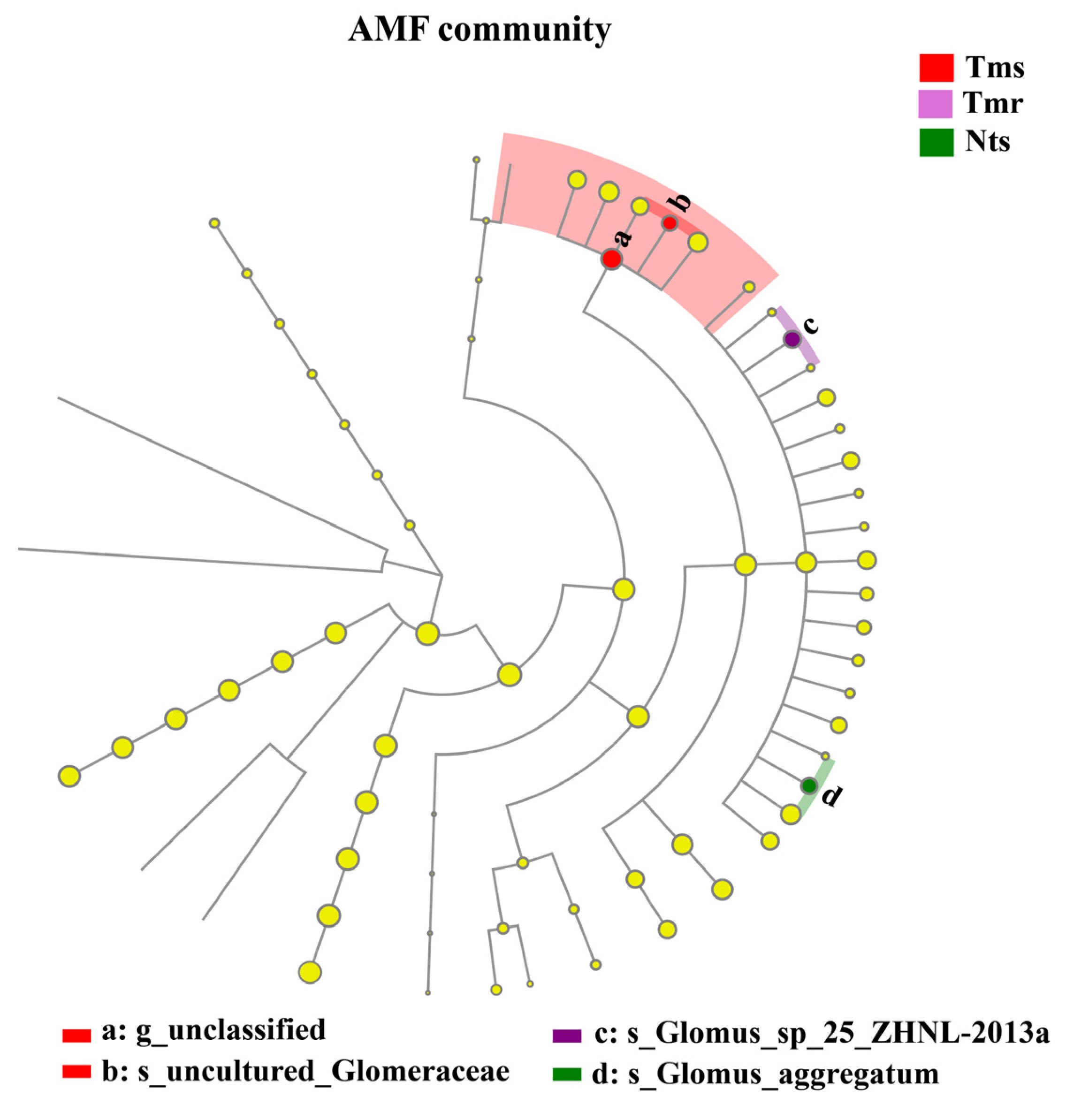
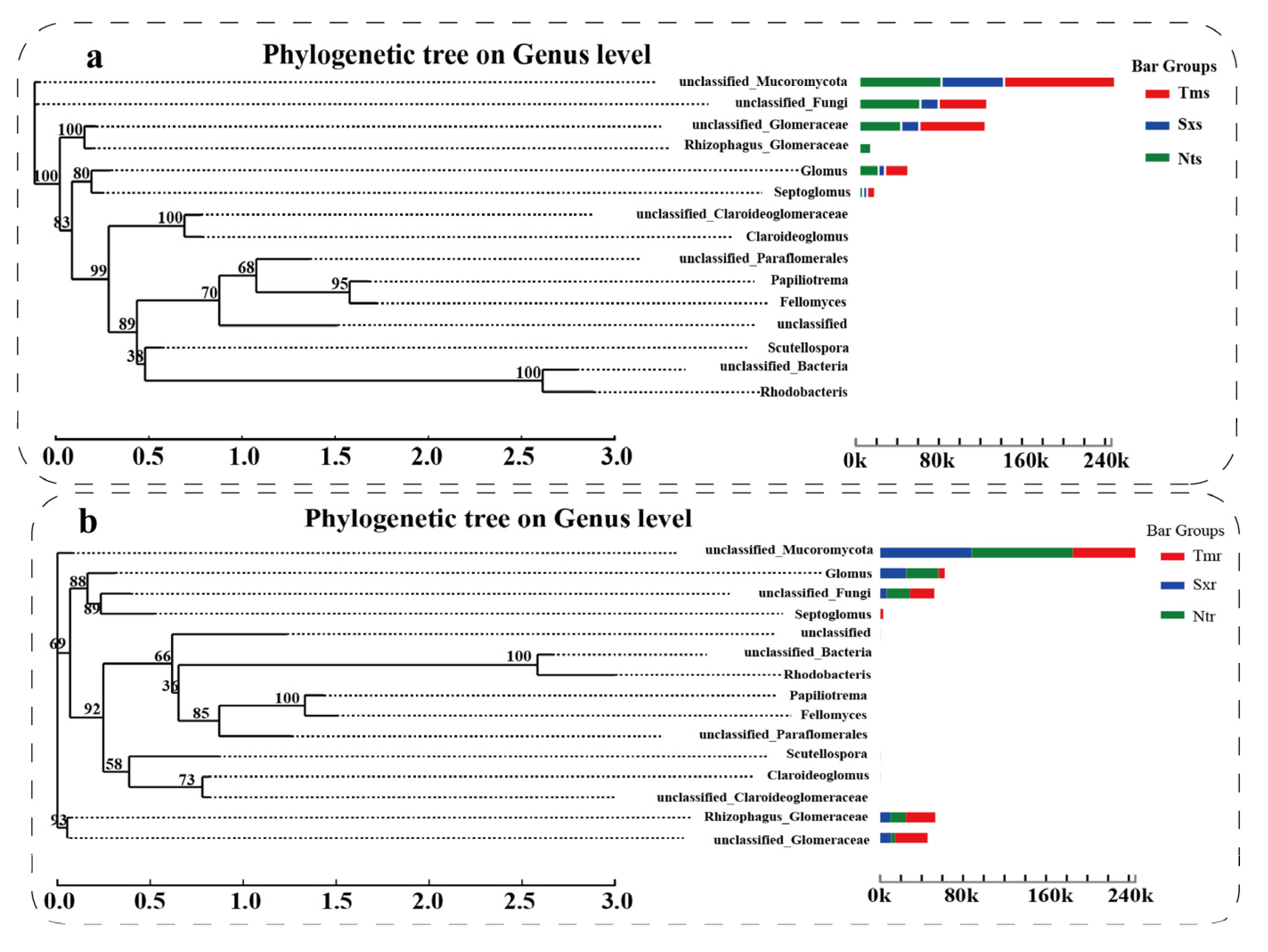
3.5. Phylogenetic Analysis
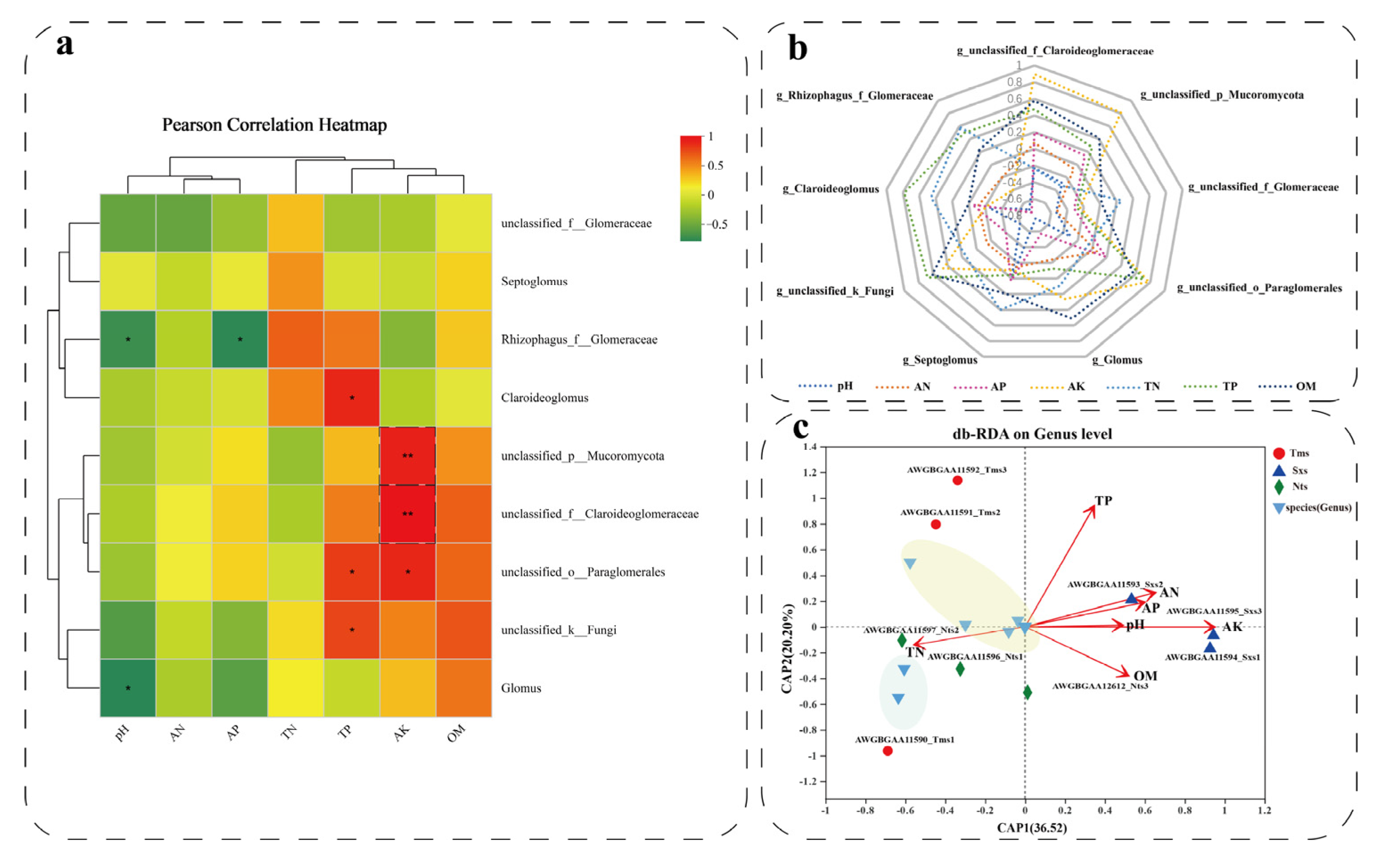
3.6. An Analysis of the Relationship between AMF Abundance and Environmental Factors
4. Discussion
4.1. Community Diversity of AM Fungi in Root and Rhizosphere Soil
4.2. Patterns of AM Fungal Keystone Taxa
4.3. Diverse AM Fungi Communities and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.J.; Chen, N.M.; Feng, J.C.; Zhou, M.Q.; He, J.Q.; Zou, Y.L.; Shi, S.; Zhou, Y.J.; Jenks, M.A. Comparative analyses of leaf cuticular lipids of two succulent xerophytes of the Ordos Plateau (Gobi Desert), Tetraena mongolica maxim and Zygophyllum xanthoxylum (Bunge) Engl. Environ. Exp. Bot. 2020, 177, 104129. [Google Scholar] [CrossRef]
- Zhu, G.P.; Li, H.Q.; Zhao, L.; Man, L.; Liu, Q. Mapping the ecological dimensions and potential distributions of endangered relic shrubs in western Ordos biodiversity center. Sci. Rep. 2016, 6, 26268. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Ma, X.K.; Lai, X.L. Petrography, geochemistry and genesis of dolomites in the upper Cambrian Sanshanzi Formation of the western Ordos Basin, northern China. J. Asian Earth Sci. 2022, 223, 104980. [Google Scholar] [CrossRef]
- Yang, Y.C.; Jia, Y.; Zhao, Y.L.; Wang, Y.L.; Zhou, T. Comparative chloroplast genomics provides insights into the genealogical relationships of endangered Tetraena mongolica and the chloroplast genome evolution of related Zygophyllaceae species. Front. Genet. 2023, 13, 1026919. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Xu, Y.; Maitra, P.; Babalola, B.J.; Zhao, Y.L. Temporal variations in root-associated fungal communities of Potaninia mongolica, an endangered relict shrub species in the semi-arid desert of Northwest China. Front. Plant Sci. 2022, 13, 975369. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Fang, X.; Hu, J.P.; Zhang, Y.Q.; Ji, Q.B.; Liu, J.L.; Pan, Y.Q.; Zhang, J.L. Branch Lignification of the Desert Plant Nitraria tangutorum Altered the Structure and Function of Endophytic Microorganisms. Agronomy 2023, 13, 90. [Google Scholar] [CrossRef]
- Xu, D.L.; Yu, X.W.; Yang, J.B.; Zhao, X.P.; Bao, Y.Y. High-Throughput Sequencing Reveals the Diversity and Community Structure in Rhizosphere Soils of Three Endangered Plants in Western Ordos, China. Curr. Microbiol. 2020, 77, 2713–2723. [Google Scholar] [CrossRef]
- Zhai, B.; Dang, X.H.; Liu, X.J.; Wang, J. Fertile island effect in the sedimentary process of Tetraena mongolica Maxim nebkhas in steppe-desert ecotones on the Inner Mongolia Plateau, China. J. Mt. Sci. 2022, 19, 2791–2805. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Pan, B.R.; Zhang, M.L. Phylogeography and conservation genetics of the endangered Tugarinovia mongolica (Asteraceae) from Inner Mongolia, Northwest China. PLoS ONE 2019, 14, e0211696. [Google Scholar] [CrossRef]
- Frew, A. Aboveground herbivory suppresses the arbuscular mycorrhizal symbiosis, reducing plant phosphorus uptake. Appl. Soil Ecol. 2021, 168, 104133. [Google Scholar] [CrossRef]
- Kalamulla, R.; Karunarathna, S.C.; Tibpromma, S.; Galappaththi, M.C.A.; Suwannarach, N.; Stephenson, S.L.; Asad, S.; Salem, Z.S.; Yapa, N. Arbuscular Mycorrhizal Fungi in Sustainable Agriculture. Sustainability 2022, 14, 12250. [Google Scholar] [CrossRef]
- Rui, W.J.; Mao, Z.P.; Li, Z.F. The Roles of Phosphorus and Nitrogen Nutrient Transporters in the Arbuscular Mycorrhizal Symbiosis. Int. J. Mol. Sci. 2022, 23, 11027. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Ridwan, M. Arbuscular mycorrhizal fungi species differentially regulate plant growth, phosphorus uptake and stress tolerance of soybean in lead contaminated soil. J. Plant Nutr. 2021, 44, 1633–1648. [Google Scholar] [CrossRef]
- Hu, Y.N.; Pandey, A.K.; Wu, X.Y.; Fang, P.P.; Xu, P. The Role of Arbuscular Mycorrhiza Fungi in Drought Tolerance in Legume Crops: A Review. Legume Res. 2022, 45, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.X.; Lu, J.H.; Cui, L.; Tang, Z.H.; Ci, D.W.; Zou, X.X.; Zhang, X.J.; Yu, X.A.; Wang, Y.F.; Si, T. The multifaceted roles of Arbuscular Mycorrhizal Fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.Q.; Cao, H.Y.; Yang, G.L.; Deng, L.L.; Wang, Y.J.; Zhou, Y.Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2020, 402, 123919. [Google Scholar] [CrossRef]
- Quan, L.T.; Zhang, J.H.; Wei, Q.P.; Wang, Y.; Qin, C.; Hu, F.; Chen, Y.H.; Shen, Z.G.; Xia, Y. Promotion of Zinc Tolerance, Acquisition and Translocation of Phosphorus in Mimosa pudica L. Mediated by Arbuscular Mycorrhizal Fungi. Bull. Environ. Contam. Toxicol. 2021, 106, 507–515. [Google Scholar] [CrossRef]
- Eagar, A.C.; Mushinski, R.M.; Horning, A.L.; Smemo, K.A.; Phillips, R.P.; Blackwood, C.B. Arbuscular Mycorrhizal Tree Communities Have Greater Soil Fungal Diversity and Relative Abundances of Saprotrophs and Pathogens than Ectomycorrhizal Tree Communities. Appl. Environ. Microbiol. 2022, 88, e01782-21. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, S.S.; Liu, N.; He, H.; Cao, X.Y.; Lv, C.; Zhang, K.; Dai, J.L. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. Res. 2021, 28, 23036–23047. [Google Scholar] [CrossRef]
- Marinho, F.; Oehl, F.; Silva, I.; Coyne, D.; Veras, J.S.D.; Maia, L.C. High diversity of arbuscular mycorrhizal fungi in natural and anthropized sites of a Brazilian tropical dry forest (Caatinga). Fungal Ecol. 2019, 40, 82–91. [Google Scholar] [CrossRef]
- Makaure, B.T.; Aremu, A.O.; Magadlela, A. Soil Nutritional Status Drives the Co-occurrence of Nodular Bacterial Species and Arbuscular Mycorrhizal Fungi Modulating Plant Nutrition and Growth of Vigna unguiculata L. (Walp) in Grassland and Savanna Ecosystems in KwaZulu-Natal, South Africa. J. Soil Sci. Plant Nutr. 2023, 23, 204–219. [Google Scholar] [CrossRef]
- Ruizlozano, J.M.; Azcon, R.; Gomez, M. Effects of arbuscular-mycorrhizae glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Song, S.L.; Ma, C.Y.; Zhang, W.; Sun, K.; Tang, M.J.; Xie, X.G.; Fan, K.K.; Dai, C.C. Endophytic fungus improves peanut drought resistance by reassembling the root-dwelling community of arbuscular mycorrhizal fungi. Fungal Ecol. 2020, 48, 100993. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z.H. How do arbuscular mycorrhizas affect reproductive functional fitness of host plants? Front. Plant Sci. 2022, 13, 975488. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Ferlian, O.; Huang, Y.Y.; Luo, S.; Quosh, J.; Eisenhauer, N. Tree diversity effects on productivity depend on mycorrhizae and life strategies in a temperate forest experiment. Ecology 2023, 104, e3896. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. Beneficial Features of Biochar and Arbuscular Mycorrhiza for Improving Spinach Plant Growth, Root Morphological Traits, Physiological Properties, and Soil Enzymatic Activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef]
- Moukarzel, R.; Ridgway, H.J.; Waller, L.; Guerin-Laguette, A.; Cripps-Guazzone, N.; Jones, E.E. Soil Arbuscular Mycorrhizal Fungal Communities Differentially Affect Growth and Nutrient Uptake by Grapevine Rootstocks. Microb. Ecol. 2023. [Google Scholar] [CrossRef]
- Cera, A.; Duplat, E.; Montserrat-Marti, G.; Gomez-Bolea, A.; Rodriguez-Echeverria, S.; Palacio, S. Seasonal variation in AMF colonisation, soil and plant nutrient content in gypsum specialist and generalist species growing in P-poor soils. Plant Soil 2021, 468, 509–524. [Google Scholar] [CrossRef]
- Li, E.G.; Huang, Y.M.; Chen, H.Y.; Zhang, J.H. Floristic diversity analysis of the Ordos Plateau, a biodiversity hotspot in arid and semi-arid areas of China. Folia Geobot. 2019, 53, 405–416. [Google Scholar] [CrossRef]
- Liu, Z.K.; Wang, C.W.; Yang, X.J.; Liu, G.F.; Cui, Q.G.; Indree, T.; Ye, X.H.; Huang, Z.Y. The Relationship and Influencing Factors between Endangered Plant Tetraena mongolica and Soil Microorganisms in West Ordos Desert Ecosystem, Northern China. Plant-Basel 2023, 12, 1048. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Beckers, B.; De Beeck, M.O.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, L.Y.; Ma, K.M.; Yang, X.Y. The community composition of arbuscular mycorrhizal fungi in the rhizosphere of Bauhinia faberi var. microphylla in the dry valley of Minjiang River. Mycosystema 2016, 35, 39–51. [Google Scholar]
- Suzuki, K.; Takahashi, K.; Harada, N. Evaluation of primer pairs for studying arbuscular mycorrhizal fungal community compositions using a MiSeq platform. Biol. Fertil. Soils 2020, 56, 853–858. [Google Scholar] [CrossRef]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizae fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl. Sci. 2010, 51, 179–181. [Google Scholar] [CrossRef]
- Agnolucci, M.; Battini, F.; Cristani, C.; Giovannetti, M. Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizae fungal isolates. Biol. Fertil. Soil 2015, 51, 379–389. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Lu, X.L. Effect of Intercropping Soybean on the Diversity of the Rhizosphere Soil Arbuscular Mycorrhizal Fungi Communities in Wheat Fields. Clean-Soil Air Water 2022, 50, 6. [Google Scholar] [CrossRef]
- Nadimi, M.; Daubois, L.; Hijri, M. Mitochondrial comparative genomics and phylogenetic signal assessment of mtDNA among arbuscular mycorrhizal fungi. Mol. Phylogenetics Evol. 2016, 98, 74–83. [Google Scholar] [CrossRef]
- Chen, J.; Mo, L.; Zhang, Z.C.; Nan, J.; Xu, D.L.; Chao, L.M.; Zhang, X.D.; Bao, Y.Y. Evaluation of the ecological restoration of a coal mine dump by exploring the characteristics of microbial communities. Appl. Soil Ecol. 2020, 147, 103430. [Google Scholar] [CrossRef]
- Li, J.C.; Wang, Y.; Zhang, L.S.; Han, L.Y.; Hu, G.Y. Aeolian desertification in China’s northeastern Tibetan Plateau: Understanding the present through the past. Catena 2019, 172, 764–769. [Google Scholar] [CrossRef]
- Chen, J.; Nan, J.; Xu, D.L.; Mo, L.; Zheng, Y.X.; Chao, L.M.; Qu, H.T.; Guo, Y.Q.; Li, F.S.; Bao, Y.Y. Response differences between soil fungal and bacterial communities under opencast coal mining disturbance conditions. Catena 2020, 194, 104779. [Google Scholar] [CrossRef]
- Shi, M.Z.; Zhao, X.Y.; Zhu, L.J.; Wu, J.Q.; Mohamed, T.A.; Zhang, X.; Chen, X.M.; Zhao, Y.; Wei, Z.M. Elucidating the negative effect of denitrification on aromatic humic substance formation during sludge aerobic fermentation. J. Hazard. Mater. 2020, 388, 122086. [Google Scholar] [CrossRef]
- Chen, J.; Xu, D.L.; Chao, L.M.; Liu, H.J.; Bao, Y.Y. Microbial assemblages associated with the rhizosphere and endosphere of an herbage, Leymus chinensis. Microb. Biotechnol. 2020, 13, 1390–1402. [Google Scholar] [CrossRef]
- Zou, Y.N.; Zhang, F.; Srivastava, A.K.; Wu, Q.S.; Kuca, K. Arbuscular Mycorrhizal Fungi Regulate Polyamine Homeostasis in Roots of Trifoliate Orange for Improved Adaptation to Soil Moisture Deficit Stress. Front. Plant Sci. 2021, 11, 600792. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Sexton, W.K.; Fidero, M.; Spain, J.C.; Jiang, L.; Bucalo, K.; Cruse-Sanders, J.M.; Pullman, G.S. Characterization of endophytic bacterial communities within greenhouse and field-grown rhizomes of three rare pitcher plant species (Sarracenia oreophila, S. leucophylla, and S. purpurea spp. venosa) with an emphasis on nitrogen-fixing bacteria. Plant Soil 2020, 447, 257–279. [Google Scholar] [CrossRef]
- David, A.S.; Quintana-Ascencio, P.F.; Menges, E.S.; Thapa-Magar, K.B.; Searcy, C.A. Soil Microbiomes Underlie Population Persistence of an Endangered Plant Species. Am. Naruralist 2019, 194, 488–494. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Cruz, C.; Mahdhi, M.; Mars, M.; Caeiro, M.F. Arbuscular mycorrhizal fungi in soil, roots and rhizosphere of Medicago truncatula: Diversity and heterogeneity under semi-arid conditions. PeerJ 2019, 7, e6401. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiwa, Y.; Ishige, T.; Sakamoto, H.; Tanaka, K.; Uchino, M.; Tanaka, N.; Oguri, S.; Saitoh, H.; Tsushima, S. Bacterial diversity associated with the rhizosphere and endosphere of two halophytes: Glaux maritima and Salicornia europaea. Front. Microbiol. 2018, 9, 2878. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.M.; Rillig, M.C.; Nowak, R.S. Arbuscular mycorrhizal fungal abundance in the Mojave Desert: Seasonal dynamics and impacts of elevated CO2. J. Arid Environ. 2009, 73, 834–843. [Google Scholar] [CrossRef]
- Betancur-Agudelo, M.; Meyer, E.; Lovato, P.E. Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere 2021, 17, 100307. [Google Scholar] [CrossRef]
- Akatov, V.V.; Akatova, T.V.; Chefranov, S.G. Plant communities in harsh and favorable environments: Characteristics of their organization, their dominant structure and its relationship to species richness. Biol. Bull. Rev. 2020, 10, 215–229. [Google Scholar] [CrossRef]
- Bainard, L.D.; Dai, M.; Gomez, E.F.; Torres-Arias, Y.; Bainard, J.D.; Sheng, M.; Eilers, W.; Hamel, C. Arbuscular mycorrhizal fungal communities are influenced by agricultural land use and not soil type among the Chernozem great groups of the Canadian Prairies. Plant Soil 2015, 387, 351–362. [Google Scholar] [CrossRef]
- Tahiri, A.I.; Raklami, A.; Bechtaoui, N.; Anli, M.; Boutasknit, A.; Oufdou, K.; Meddich, A. Beneficial Effects of Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Compost on Lettuce (Lactuca sativa) Growth Under Field Conditions. Gesunde Pflanz 2022, 74, 219–235. [Google Scholar] [CrossRef]
- Gu, T.Y.; Mao, Y.Y.; Chen, C.; Wang, Y.; Lu, Q.; Wang, H.Q.; Cheng, W. Diversity of arbuscular mycorrhiza fungi in rhizosphere soil and roots in Vetiveria zizanioides plantation chronosequence in coal gangue heaps. Symbiosis 2022, 86, 111–122. [Google Scholar] [CrossRef]
- Santander, C.; Garcia, S.; Moreira, J.; Aponte, H.; Araneda, P.; Olave, J.; Vidal, G.; Cornejo, P. Arbuscular mycorrhizal fungal abundance in elevation belts of the hyperarid Atacama Desert. Fungal Ecol. 2021, 51, 101060. [Google Scholar] [CrossRef]
- Garo, G.; Van Geel, M.; Eshetu, F.; Swennen, R.; Honnay, O. Arbuscular mycorrhizal fungi community composition, richness and diversity on enset (Ensete ventricosum (Welw.) Cheesman) in Ethiopia is influenced by manure application intensity in low-input farming systems. Plant Soil 2020, 478, 409–425. [Google Scholar] [CrossRef]
- Qiu, L.; Bi, Y.L.; Jiang, B.; Wang, Z.G.; Zhang, Y.X.; Zhakypbek, Y. Arbuscular mycorrhizal fungi ameliorate the chemical properties and enzyme activities of rhizosphere soil in reclaimed mining subsidence in northwestern China. J. Arid Land 2019, 11, 135–147. [Google Scholar] [CrossRef]
- Nouri, E.; Matinizadeh, M.; Moshki, A.; Zolfaghari, A.; Rajaei, S.; Janouskova, M. Arbuscular mycorrhizal fungi benefit drought-stressed Salsola laricina. Plant Ecol. 2020, 221, 683–694. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, X.; Guo, Y.; Ren, C.J.; Wang, J.; Doughty, R. Elevation gradients affect the differences of arbuscular mycorrhizal fungi diversity between root and rhizosphere soil. Agric. For. Meteorol. 2020, 284, 107894. [Google Scholar] [CrossRef]
- Bonfim, J.A.; Vasconcellos, R.L.F.; Gumiere, T.; Mescolotti, D.D.C.; Oehl, F.; Cardoso, E.J.B. Diversity of arbuscular mycorrhizal fungi in a Brazilian Atlantic forest toposequence. Microb. Ecol. 2016, 71, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yue, Y.J.; Wang, Z.H.; Li, L.; Duan, G.Z.; Bai, S.L.; Li, T. Composition of the arbuscular mycorrhizal fungal community and changes in diversity of the rhizosphere of Clematis fruticosa over three seasons across different elevations. Eur. J. Soil Sci. 2020, 71, 511–523. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.L.; Ji, W.H.; Hai, Z.X.; Hu, N.C.; Li, X.T.; Liu, G.Y.; Yu, C.M.; Chen, Y.H.; Lian, B.L.; et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. J. Fungi 2022, 8, 124. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, H.C.; Wang, D.Z.; Guo, X.S.; Yang, T.; Xiang, X.J.; Walder, F.; Chu, H.Y. Differential Responses of Arbuscular Mycorrhizal Fungal Communities to Long-Term Fertilization in the Wheat: Rhizosphere and Root Endosphere. Appl. Environ. Microbiol. 2021, 87, e00349-21. [Google Scholar] [CrossRef]
- Gottel, N.R.; Castro, H.F.; Kerley, M.; Ya, Z.; Pelletier, D.A.; Podar, M.; Karpinets, T.; Uberbacher, E.; Tuskan, G.A.; Vilgalys, R. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoidesroots across contrasting soil types. Appl Environ. Microbiol. 2011, 77, 5934–5944. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Williams, M. Mycorrhizal mixtures affect the growth, nutrition, and physiological responses of soybean to water deficit. Acta Physiol. Plantaruam 2021, 43, 75. [Google Scholar] [CrossRef]
- Musyoka, D.M.; Njeru, E.M.; Mang’Erere, M.; Maingi, J.M. Arbuscular mycorrhizal fungi and Bradyrhizobium co-inoculation enhances nitrogen fixation and growth of green grams (Vigna radiata L.) under water stress. J. Plant Nutr. 2020, 43, 1036–1047. [Google Scholar] [CrossRef]
- Huang, X.Q.; Liu, X.Z.; Liu, X.; Zhang, S.R.; Li, L.; Zhao, H.T.; Zhao, J.; Zhang, J.B.; Cai, Z.C. Plant pathological condition is associated with fungal community succession triggered by root exudates in the plant-soil system. Soil Biol. Biochem. 2020, 151, 108046. [Google Scholar] [CrossRef]
- Sheldrake, M.; Rosenstock, N.P.; Mangan, S.; Revillini, D.; Sayer, E.J.; Olsson, P.A.; Verbruggen, E.; Tanner, E.V.J.; Turner, B.L.; Wright, S.J. Responses of arbuscular mycorrhizal fungi to long-term inorganic and organic nutrient addition in a lowland tropical forest. ISME J. 2018, 12, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Srivastava, A.K.; Wu, Q.S. Mycorrhizal fungi regulate root responses and leaf physiological activities in trifoliate orange. Not. Bot. Horti Agrobot. ClujNapoca 2017, 45, 17–21. [Google Scholar] [CrossRef]
- Kim, K.; Neuberger, P.; Daly, E.J.; Gorzelak, M.; Hernandez-Ramirez, G. Arbuscular mycorrhizal fungi community linkages to soil nutrient availability across contrasting agroecosystems. Appl. Soil Ecol. 2022, 176, 104464. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bosch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Shao, Q.; Lin, Z.; Zhou, C.; Zhu, P.; Yan, X.J. Succession of bacterioplankton communities over complete Gymnodinium-diatom bloom cycles. Sci. Total Environ. 2020, 709, 135951. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, W.; Li, X.; Gao, G.; Jiang, J.H. Linking bacterial community shifts with changes in the dissolved organic matter pool in a eutrophic lake. Sci. Total Environ. 2020, 719, 137387. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, Y.X.; Liu, M.H.; Li, Q.; Xiao, W.F.; Song, X.Z. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci. Rep. 2022, 10, e02293-21. [Google Scholar] [CrossRef]
- Van Geel, M.; Jacquemyn, H.; Plue, J.; Saar, L.; Kasari, L.; Peeters, G.; van Acker, K.; Honnay, O.; Ceulemans, T. Abiotic rather than biotic filtering shapes the arbuscular mycorrhizal fungal communities of European seminatural grasslands. New Phytol 2018, 220, 1262–1272. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liang, J.W.; Liu, Z.Y.; Kuang, Y.X.; Han, L.N.; Chen, H.; Xie, X.A.; Hu, W.T.; Tang, M. Transcriptional regulation of metal metabolism- and nutrient absorption-related genes in Eucalyptus grandis by arbuscular mycorrhizal fungi at different zinc concentrations. BMC Plant Biol. 2022, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Rincon, C.; Droh, G.; Villard, L.; Masclaux, F.G.; N’guetta, A.; Zeze, A.; Sanders, I.R. Hierarchical spatial sampling reveals factors influencing arbuscular mycorrhizal fungus diversity in Cote d’Ivoire cocoa plantations. Mycorrhiza 2021, 31, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Huang, G.; Li, Y.K.; Zhang, X.R.; Lei, Y.H.; Li, Y.; Xiong, J.; Sun, Y.F. Illumina MiSeq Sequencing Reveals Correlations among Fruit Ingredients, Environmental Factors, and AMF Communities in Three Lycium Barbarum Producing Regions of China. Microbiol. Spectrum. 2022, 10, e02293-21. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yu, X.; Chen, J.; Liu, H.; Zheng, Y.; Qu, H.; Bao, Y. Arbuscular Mycorrhizae Fungi Diversity in the Root–Rhizosphere–Soil of Tetraena mongolica, Sarcozygium xanthoxylon, and Nitraria tangutorum Bobr in Western Ordos, China. Agronomy 2023, 13, 1485. https://doi.org/10.3390/agronomy13061485
Xu D, Yu X, Chen J, Liu H, Zheng Y, Qu H, Bao Y. Arbuscular Mycorrhizae Fungi Diversity in the Root–Rhizosphere–Soil of Tetraena mongolica, Sarcozygium xanthoxylon, and Nitraria tangutorum Bobr in Western Ordos, China. Agronomy. 2023; 13(6):1485. https://doi.org/10.3390/agronomy13061485
Chicago/Turabian StyleXu, Daolong, Xiaowen Yu, Jin Chen, Haijing Liu, Yaxin Zheng, Hanting Qu, and Yuying Bao. 2023. "Arbuscular Mycorrhizae Fungi Diversity in the Root–Rhizosphere–Soil of Tetraena mongolica, Sarcozygium xanthoxylon, and Nitraria tangutorum Bobr in Western Ordos, China" Agronomy 13, no. 6: 1485. https://doi.org/10.3390/agronomy13061485




