Acid Hydrothermal Amendment of Grape Wine Pomace: Enhancement of Phenol and Carbohydrate Co-Solubilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Hydrothermal Treatment
2.3. Chemical Analysis
3. Results and Discussion
3.1. Solubilization of Organic Matter
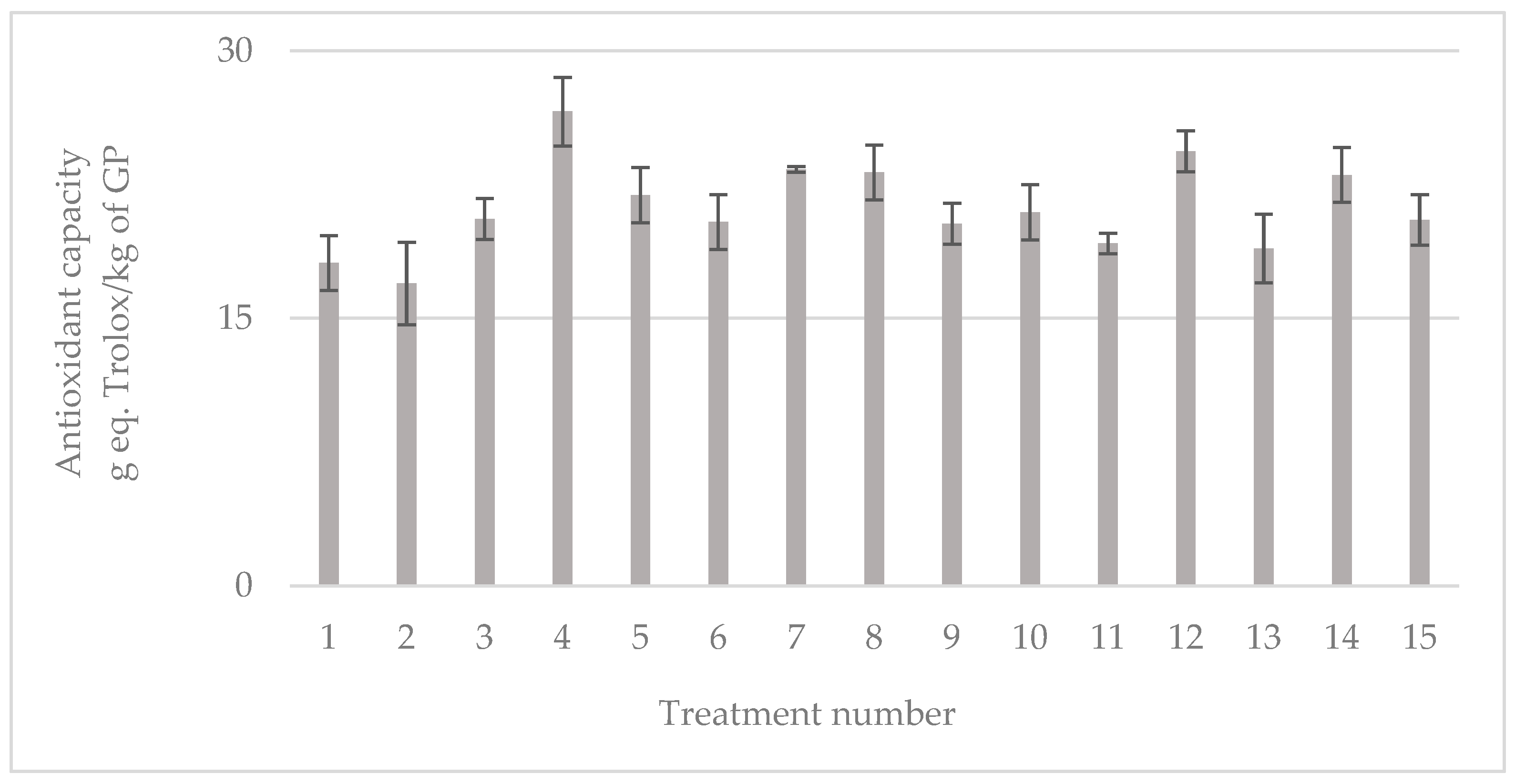
3.2. Bioactive Compound Characterization
3.3. Characterization of Individual Bioactive Compounds
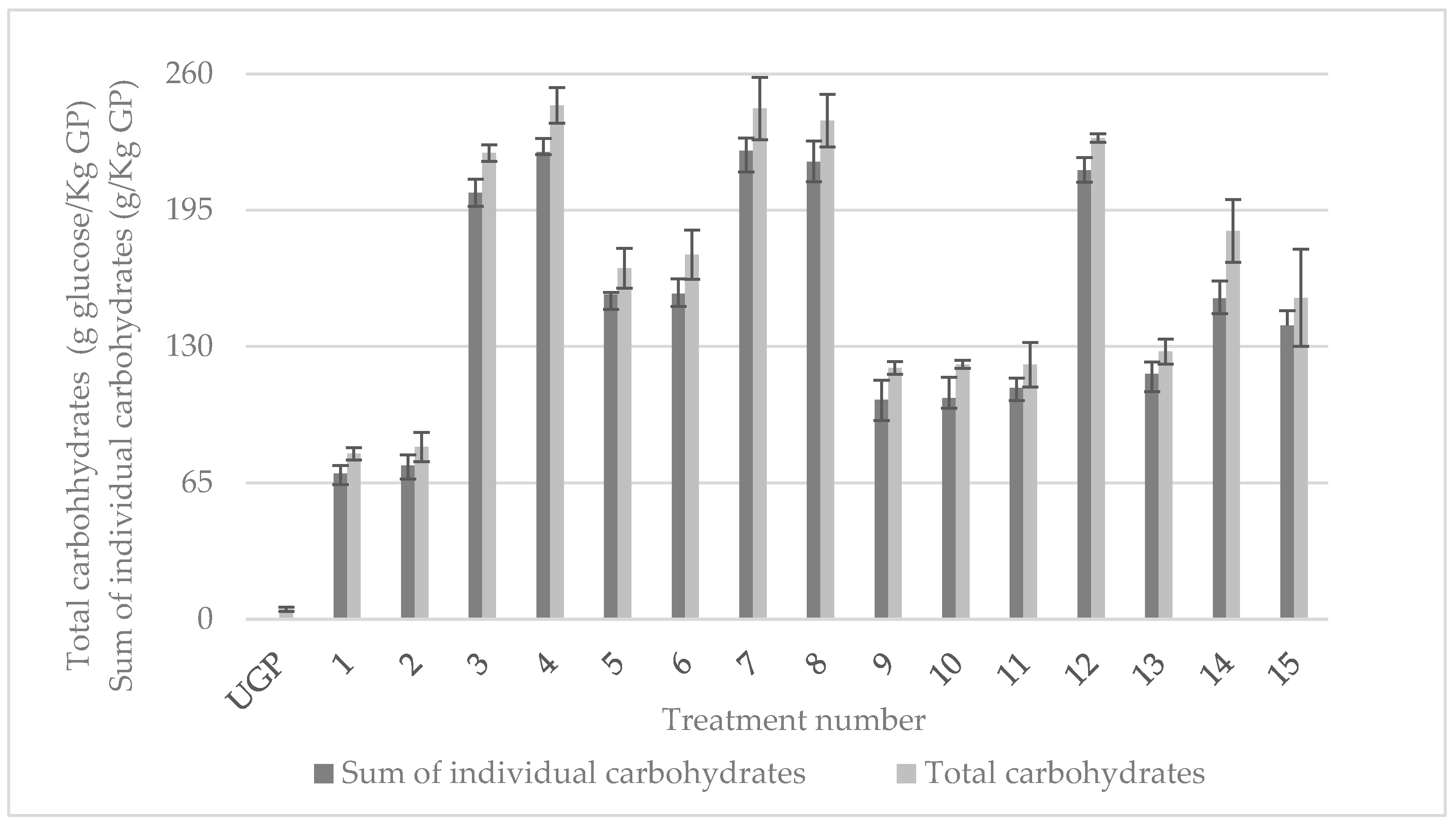
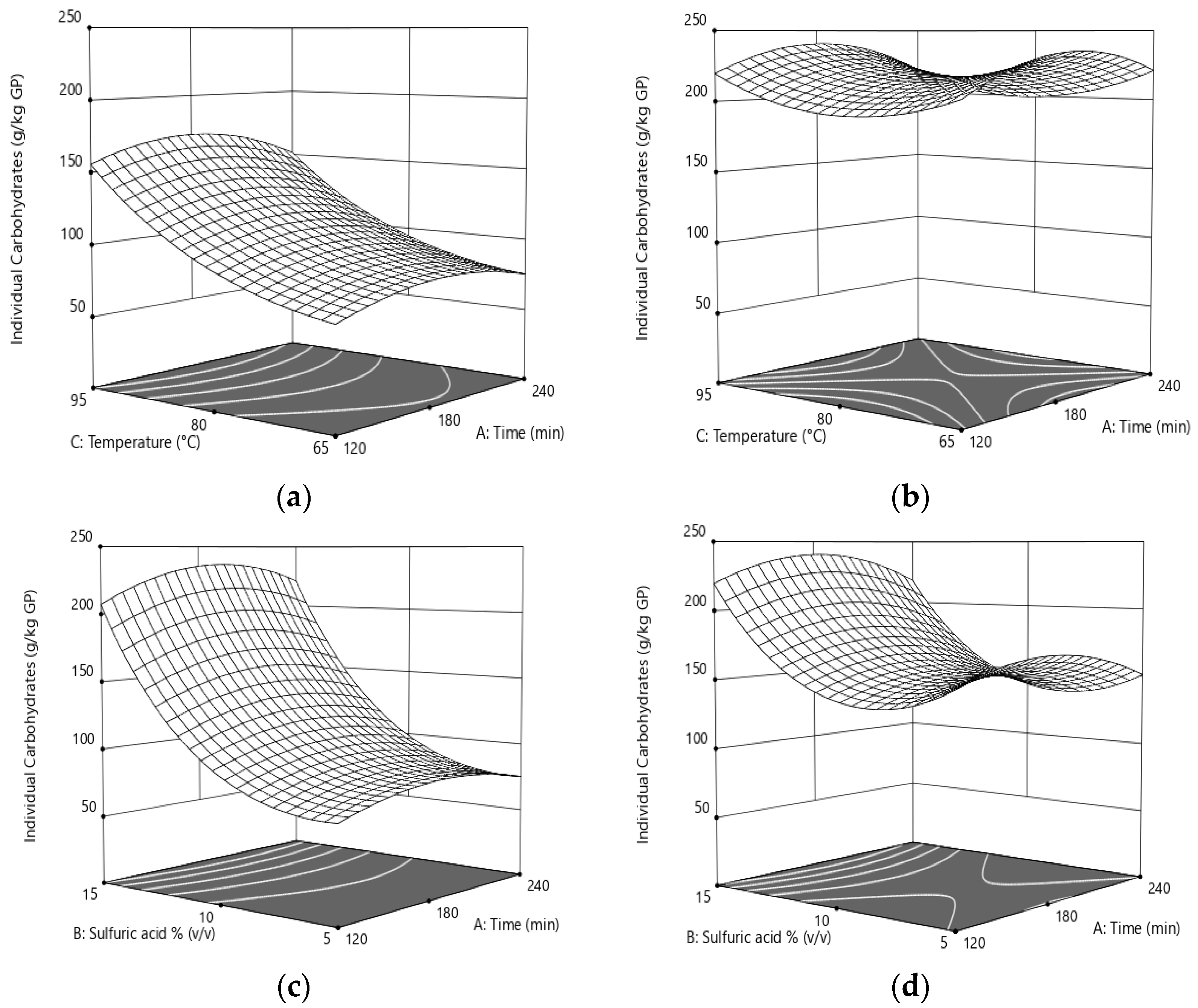
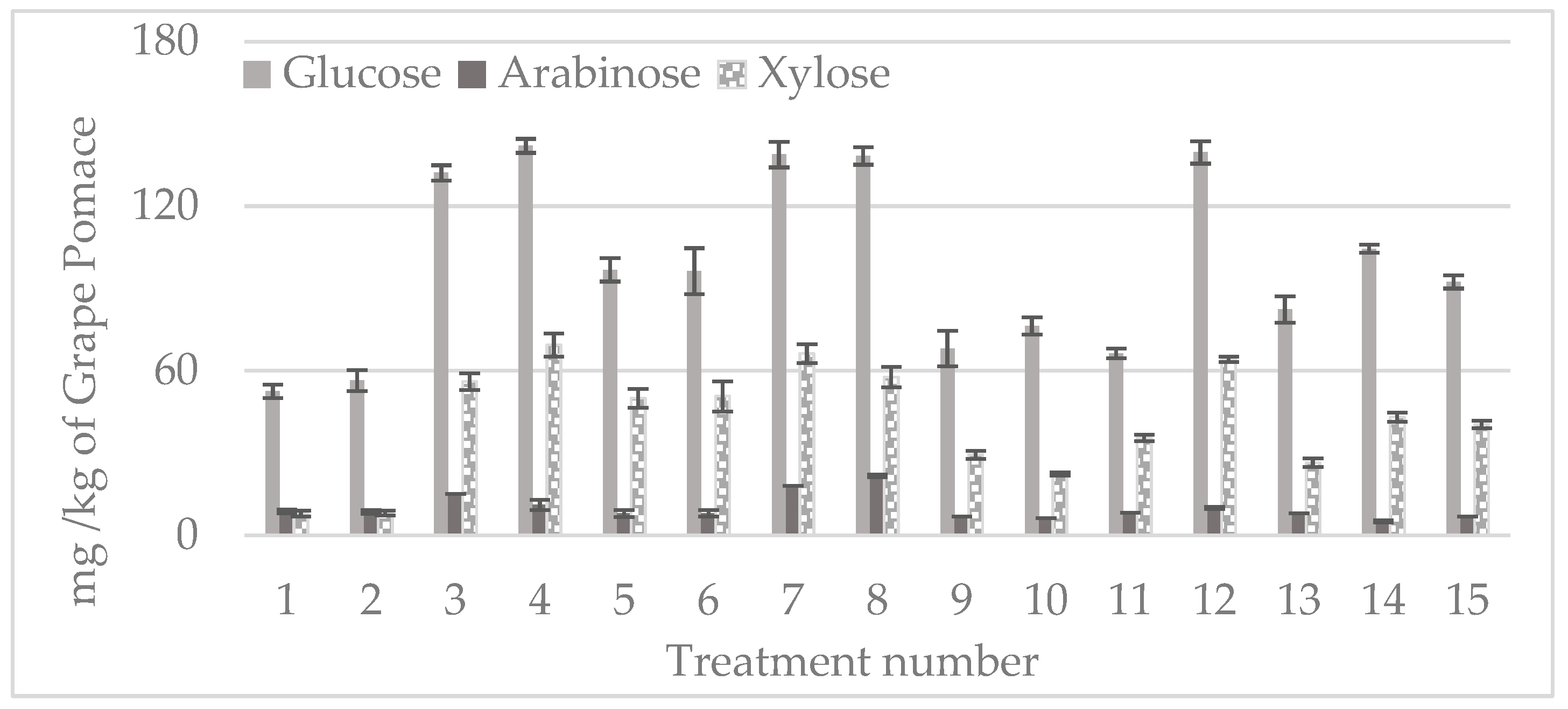
3.4. Carbohydrates’ Solubilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Achkar, J.H.; Lendormi, T.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.-L.; Hobaika, Z. Influence of pretreatment conditions on lignocellulosic fractions and methane production from grape pomace. Bioresour. Technol. 2018, 247, 881–889. [Google Scholar] [CrossRef]
- Arboleda Mejia, J.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Ionela-daniela, S.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Letizia, M.; Manconi, M. Biomedicine & Pharmacotherapy Extraction of the antioxidant phytocomplex from wine-making by-products and sustainable loading in phospholipid vesicles specifically tailored for skin protection. Biomed. Pharmacother. 2021, 142, 111959. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.M.; Inês, M.; José, M.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Review article Potential uses and applications of treated wine waste: A review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-saiz, M.; Muñiz, P. From winery by-product to healthy product: Bioavailability, redox signaling and oxidative stress modulation by wine pomace product. Crit. Rev. Food Sci. Nutr. 2021, 62, 7427–7448. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-farin, B.; Ferna, J.; Borja, R.; Rodr, G. Valuable Compound Extraction, Anaerobic Digestion, and Composting: A Leading Biore fi nery Approach for Agricultural Wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Deng, J.; Li, H.; Lu, J. Phenolic Concentrations and Antioxidant Properties of Wines Made from North American Grapes Grown in China. Molecules 2012, 17, 3304–3323. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, L.; Toro, M.; Vazquez, F.; Correa-daneri, M.; Gouiric, S.; Vallejo, M. Bioethanol production from grape and sugar beet pomaces by solid-state fermentation. Int. J. Hydrogen Energy 2010, 35, 5914–5917. [Google Scholar] [CrossRef]
- Hungría, J.; Siles, J.A.; Chica, A.F.; Gil, A.; Martín, M.A. Anaerobic co-digestion of winery waste: Comparative assessment of grape marc waste and lees derived from organic crops. Environ. Technol. 2020, 42, 3618–3626. [Google Scholar] [CrossRef]
- Follonier, S.; Riesen, R.; Zinn, M. Pilot-scale Production of Functionalized mcl-PHA from Grape Pomace Supplemented with Fatty Acids. Chem. Biochem. Eng. Q. 2015, 29, 113–121. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Food and Bioproducts Processing Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process. 2019, 124, 1–10. [Google Scholar] [CrossRef]
- Arenas Sevillano, C.B.; Chiappero, M.; Gomez, X.; Fiore, S.; Martínez, J. Improving the Anaerobic Digestion of Wine-Industry Liquid Wastes: Treatment by Electro-Oxidation and Use of Biochar as an Additive. Energies 2020, 13, 5971. [Google Scholar] [CrossRef]
- Siles, J.A.; García-García, I.; Martín, A.; Martín, M.A. Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing. J. Hazard. Mater. 2011, 188, 247–253. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Shi, T.; Gui, C.; Huang, Y.; Chen, Y.; Xie, M. Recovery of dietary fiber and polyphenols from grape juice pomace and evaluation of their functional properties and polyphenol composition. Food Funct. 2017, 8, 341–351. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Sun, B.S. Extraction Yields and Anti-oxidant Activity of Proanthocyanidins from Different Parts of Grape Pomace: Effect of Mechanical Treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Bioresour. Technol. 2022, 343, 125989. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Chemical Engineering & Processing: Process Intensi fi cation Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process. Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Mora-Garrido, A.B.; Cejudo-Bastante, M.J.; Heredia, F.J.; Escudero-Gilete, L.M. Revalorization of residues from the industrial exhaustion of grape. LWT Food Sci. Technol. 2022, 156, 113057. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Fermoso, F.G.; Rodríguez-gutierrez, G.; Fernandez-bolaños, J.; Borja, R. Biomethanization of olive mill solid waste after phenols recovery through low-temperature thermal pre-treatment. Waste Manag. 2017, 61, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubero-cardoso, J.; Trujillo-reyes, Á.; Marín-Ayllón, P.; Rodr, G.; Villa-gomez, D.; Serrano, A.; Borja, R.; Fermoso, F.G. Solubilization of Phenols and Sugars from Raspberry Extrudate by Hydrothermal Treatments. Processes 2020, 8, 842. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Phenols recovery after steam explosion of Olive Mill Solid Waste and its influence on a subsequent biomethanization process. Bioresour. Technol. 2017, 243, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Jain, S.; Wolf, T.I.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Rowell, R. The Chemistry of Solid Wood. Based on Short Course Andsymposium Sponsored by the Division of Cellulose, Paper and Textile Chemistry. In Proceedings of the 185th Meeting of the American Chemical Society, Seattle, Washington, DC, USA, 20–25 March 1983; pp. 70–72. [Google Scholar]
- Tappi Test Methodos Standard Methods for Pulp and Paper, Technical Association of the Pulp and Paper Industry; Tappi Press: Atlanta, GA, USA, 1997.
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Rocha, S.; Marzialetti, T.; Kopp, M.; Cea, M. Reaction Mechanism of the Microwave-Assisted Synthesis of 5-Hydroxymethylfurfural from Sucrose in Sugar Beet Molasses. Catalysts 2021, 11, 1458. [Google Scholar] [CrossRef]
- Benavides, V.; Pinto-Ibieta, F.; Serrano, A.; Rubilar, O.; Ciudad, G. Use of Anthracophyllum discolor and Stereum hirsutum as a Suitable Strategy for Delignification and Phenolic Removal of Olive Mill Solid Waste. Foods 2022, 11, 1587. [Google Scholar] [CrossRef]
- Romero-Román, M.E.; Schoebitz, M.; Fuentealba, J.; García-Viguera, C.; López Belchí, M.D.; Voilley, A.; Kurek, M. Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient. Antioxidants 2021, 10, 1830. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Ghasimi, D.S.M.; Aboudi, K.; De Kreuk, M.; Zandvoort, M.H.; Lier, J.B. Van Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem. Eng. J. 2016, 295, 181–191. [Google Scholar] [CrossRef]
- Rodríguez-gutiérrez, G.; Cardoso, J.C.; Rubio-senent, F.; Serrano, A.; Borja, R.; Fernández-bolaños, J.; Fermoso, F.G. Thermally-treated strawberry extrudate: A rich source of antioxidant phenols and sugars. Innov. Food Sci. Emerg. Technol. 2019, 51, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, R.; Bacchi, E.M. Safety and Photoprotective E ffi cacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, M.; Huang, Y.; Li, C.; Pan, X.; Zhu, W.; Huang, Y. Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum-fermented loose tea. LWT Food Sci. Technol. 2017, 82, 248–254. [Google Scholar] [CrossRef]
- Nobre, D.; Macedo, W.; Silva, G.; Lopes, L.; Jaimes, E. Aplicación y efecto antioxidante del ácido gálico sobre la calidad de semillas de trigo—Application and effect antioxidant of gallic acid on the quality of wheat seeds. Rev. Ciencias Agrar. 2019, 42, 22–29. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Fernández, M.; Gonzalez-Ramirez, M.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses. Foods 2022, 11, 2355. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Review Article Extraction, bene fi ts and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016, 118, 503–511. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariña, B.; Rodríguez-gutierrez, G.; Fernandez-bolaños, J.; Borja, R. Olive mill solid waste biorefinery: High-temperature thermal pre- treatment for phenol recovery and biomethanization. J. Clean. Prod. 2017, 148, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J. Hydrothermal processing of lignocellulosic materials. Holz Roh Und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Jin, Q.; Hair, J.O.; Stewart, A.C.; Keefe, S.F.O.; Neilson, A.P.; Kim, Y.; Mcguire, M.; Lee, A.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef] [Green Version]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Stork, J.; Debolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Dussan, K.; Girisuta, B.; Lopes, M.; Leahy, J.J. Conversion of hemicellulose sugars catalyzed by formic acid: Kinetics of the dehydration of D-xylose, L-arabinose, and D-glucose. ChemSusChem 2015, 8, 1411–1428. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Ibieta, F.; Serrano, A.; Cea, M.; Ciudad, G.; Fermoso, F.G. Beyond PHA: Stimulating intracellular accumulation of added-value compounds in mixed microbial cultures. Bioresour. Technol. 2021, 337, 125381. [Google Scholar] [CrossRef]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [Green Version]


| Treatment Number | Duration (min) | H2SO4 (v/v) | Temperature (°C) |
|---|---|---|---|
| 1 | 120 | 5 | 65 |
| 2 | 240 | 5 | 65 |
| 3 | 120 | 15 | 65 |
| 4 | 240 | 15 | 65 |
| 5 | 120 | 5 | 95 |
| 6 | 240 | 5 | 95 |
| 7 | 120 | 15 | 95 |
| 8 | 240 | 15 | 95 |
| 9 | 120 | 10 | 80 |
| 10 | 240 | 10 | 80 |
| 11 | 180 | 5 | 80 |
| 12 | 180 | 15 | 80 |
| 13 | 180 | 10 | 65 |
| 14 | 180 | 10 | 95 |
| 15 | 180 | 10 | 80 |
| Treatment Number | Lignin Residue (%w/w) | Holocellulose Residue (%w/w) | Lignin Solubilization (%w/w) | Holocellulose Solubilization (%w/w) | Total Solids (g/kg) | Volatile Solids (g/kg) | VS/TS |
|---|---|---|---|---|---|---|---|
| UGP | 35.2 ± 5 | 59.6 ± 5.7 | - | - | 10.0 ± 1.2 | 8.9 ± 2.1 | 0.90 |
| 1 | 32.1 ± 2.7 | 21.9 ± 0.7 | 8.9 | 63.2 | 60.9 ± 3.1 | 59.8 ± 4.3 | 0.98 |
| 2 | 30.0 ± 2.9 | 22.6 ± 3.2 | 14.8 | 62.0 | 63.3 ± 7.1 | 62.2 ± 7.3 | 0.98 |
| 3 | 30.6 ± 3.1 | 14.2 ± 2.1 | 13.0 | 76.1 | 126.2 ± 7.6 | 124.9 ± 10.3 | 0.99 |
| 4 | 30.8 ± 5.2 | 11.7 ± 1.6 | 12.5 | 80.3 | 128.5 ± 5.2 | 126.7 ± 7.4 | 0.99 |
| 5 | 30.8 ± 1.2 | 18.2 ± 0.6 | 12.6 | 69.4 | 126.0 ± 8.1 | 124.0 ± 8.3 | 0.98 |
| 6 | 29.5 ± 4.0 | 19.1 ± 0.8 | 16.1 | 68.0 | 82.2 ± 5.4 | 80.5 ± 6.1 | 0.98 |
| 7 | 31.5 ± 2.3 | 9.9 ± 1.6 | 10.5 | 83.4 | 126.7 ± 3.2 | 124.9 ± 6.2 | 0.99 |
| 8 | 33.0 ± 2.7 | 4.7 ± 0.6 | 6.3 | 92.1 | 125.1 ± 5.1 | 123.4 ± 9.3 | 0.99 |
| 9 | 32.3 ± 2.6 | 17.2 ± 1.6 | 8.1 | 71.2 | 128.7 ± 4.2 | 127.7 ± 8.2 | 0.98 |
| 10 | 31.6 ± 2.7 | 16.9 ± 1.6 | 10.3 | 71.6 | 102.9 ± 2.4 | 101.3 ± 6.4 | 0.98 |
| 11 | 30.6 ± 4.1 | 18.5 ± 0.9 | 13.0 | 68.9 | 62.8 ± 7.8 | 61.3 ± 3.4 | 0.97 |
| 12 | 31.0 ± 2.4 | 9.5 ± 1.6 | 11.9 | 84.1 | 129.4 ± 12.2 | 127.5 ± 8.3 | 0.99 |
| 13 | 31.8 ± 2.1 | 15.1 ± 1.6 | 9.5 | 74.7 | 128.4 ± 6.2 | 125.8 ± 9.2 | 0.99 |
| 14 | 32.3 ± 3.1 | 17.2 ± 0.6 | 8.4 | 71.2 | 107.5 ± 4.3 | 105.4 ± 3.5 | 0.98 |
| 15 | 33.4 ± 1.9 | 18.6 ± 2.8 | 5.0 | 68.8 | 101.1 ± 4.6 | 99.4 ± 3.4 | 0.98 |
| Treatment Number | sCOD (mg/kg) | Furfural (g/L) | HMF (g/L) | Acetic Acid (mg/kg) |
|---|---|---|---|---|
| UGP | 594 ± 5 | n.d. | n.d. | n.d. |
| 1 | 754 ± 18 | n.d. | n.d. | 4.6 ± 0.3 |
| 2 | 715 ± 21 | n.d. | n.d. | 4.6 ± 0.4 |
| 3 | 955 ± 12 | n.d. | n.d. | 9.3 ± 0.8 |
| 4 | 940 ± 5 | 0.43 ± 0.01 | <0.005 | 9.4 ± 0.9 |
| 5 | 850 ± 17 | n.d. | <0.005 | 8.6 ± 1.1 |
| 6 | 740 ± 19 | 0.008 ± 0.01 | <0.005 | 8.4 ± 0.1 |
| 7 | 944 ± 8 | n.d. | 0.05 ± 0.005 | 9.3 ± 1.1 |
| 8 | 929 ± 29 | 0.29 ± 0.03 | <0.005 | 9.1 ± 1.4 |
| 9 | 748 ± 19 | n.d. | <0.005 | 8.6 ± 0.9 |
| 10 | 753 ± 5 | n.d. | <0.005 | 8.3 ± 0.9 |
| 11 | 748 ± 5 | n.d. | n.d. | 7.7 ± 0.6 |
| 12 | 959 ± 9 | 0.38 ± 0.02 | <0.005 | 9.3 ± 0.9 |
| 13 | 732 ± 5 | n.d. | n.d. | 6.7 ± 1.1 |
| 14 | 626 ± 56 | 0.36 ± 0.02 | <0.005 | 4.8 ± 0.7 |
| 15 | 753.3 ± 34 | n.d. | <0.005 | 8.2 ± 0.8 |
| UGP | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acid | ||||||||||||||||
| P-hydroxybenzoic acid | 1 ± 1 | 43 ± 1 | 42 ± 2 | 61 ± 2 | 65 ± 4 | 70 ± 3 | 63 ± 4 | 90 ± 5 | 68 ± 3 | 56 ± 3 | 66 ± 2 | 62 ± 2 | 72 ± 6 | 61 ± 4 | 60 ± 4 | 55 ± 3 |
| Gallic acid hexoside | 2 ± 1 | 138 ± 3 | 138 ± 5 | 134 ± 3 | 52 ± 2 | 116 ± 4 | 102 ± 5 | 128 ± 7 | 123 ± 3 | 111 ± 5 | 108 ± 3 | 110 ± 3 | 111 ± 2 | 114 ± 1 | 73 ± 2 | 103 ± 2 |
| Protocatechuic acid hexoside | n.d. | 1 ± 1 | 22 ± 1 | 5 ± 1 | 2 ± 0.3 | 4 ± 1 | 7 ± 1 | 18 ± 1 | 5 ± 1 | 7 ± 1 | 10 ± 1 | 10 ± 1 | 20 ± 1 | 3 ± 1 | 20 ± 01 | 4 ± 0.4 |
| Vanillic acid | 2 ± 1 | 26 ± 1 | 25 ± 1 | 64 ± 1 | 56 ± 1 | 58 ± 1 | 52 ± 1 | 54 ± 1 | 53 ± 1 | 46 ± 1 | 46 ± 1 | 42 ± 1 | 66 ± 3 | 37 ± 1 | 52 ± 2 | 55 ± 0.3 |
| Coutaric acid | n.d. | 1 ± 1 | <1 | 2 ± 1 | 3 ± 1 | 4 ± 0.1 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 4 ± 1 | 4 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 1 | 4 ± 1 | 3 ± 0.2 |
| Caffeic acid | n.d. | 6 ± 1 | 6 ± 1 | n.d. | 5 ± 1 | 4 ± 0.3 | n.d. | n.d. | n.d. | 4 ± 1 | n.d. | 4 ± 1 | 5 ± 1 | 3 ± 1 | 3 ± 1 | n.d. |
| Caftaric acid | n.d. | n.d. | n.d. | 4 ± 1 | 9 ± 1 | n.d. | 4 ± 1 | 5 ± 1 | 5 ± 1 | 4 ± 1 | 5 ± 1 | n.d. | 7 ± 1 | n.d. | 8 ± 1 | 5 ± 0.2 |
| P-coumaric acid | n.d. | 2 ± 1 | 2 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ferulic acid | <1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Chlorogenic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ellagic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Phenolic Derivatives | ||||||||||||||||
| 3,4-dihydroxyphenylglycol | 49 ± 2 | 144 ± 3 | 138 ± 2 | 159 ± 4 | 43 ± 3 | 151 ± 4 | 213 ± 5 | 264 ± 5 | 244 ± 4 | 168 ± 7 | 177 ± 8 | 132 ± 8 | 74 ± 5 | 160 ± 12 | 60 ± 5 | 168 ± 12 |
| 3-hydroxytyrosol | <1 | 35 ± 1 | 36 ± 1 | 80 ± 2 | 1330 ± 22 | 198 ± 2 | 425 ± 5 | 504 ± 7 | 465 ± 12 | 150 ± 3 | 204 ± 8 | 241 ± 2 | 109 ± 2 | 200 ± 10 | 830 ± 16 | 187 ± 11 |
| p-tyrosol | n.d. | 35 ± 1 | 13 ± 1 | 49 ± 1 | 107 ± 2 | 286 ± 3 | 51 ± 1 | 5 ± 0.1 | 53 ± 1 | 91 ± 2 | 78 ± 2 | 92 ± 2 | 1 ± 0.01 | 29 ± 2 | 102 ± 1 | 90 ± 0.1 |
| Vanillin | 1 ± 1 | 6 ± 1 | 7 ± 1 | 8 ± 1 | 26 ± 1 | 15 ± 1 | 10 ± 1 | 10 ± 1 | 11 ± 1 | 10 ± 1 | 10 ± 1 | 13 ± 1 | 21 ± 1 | 8 ± 1 | 24 ± 1 | 10 ± 0.1 |
| 4-methyl catechol | n.d. | n.d. | n.d. | 11 ± 1 | 80 ± 2 | n.d. | n.d. | 84 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Syringol | n.d. | n.d. | n.d. | n.d. | 21 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 24 ± 1 | n.d. |
| 3,4-dimethyl benzyl alcohol | 1 ± 1 | n.d. | n.d. | n.d. | 21 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 24 ± 1 | n.d. |
| Flavonols | ||||||||||||||||
| Catechin | n.d. | n.d. | n.d. | 19 ± 1 | n.d. | n.d. | n.d. | 4 ± 0.1 | 10 ± 1 | n.d. | n.d. | n.d. | 8 ± 1 | n.d. | 2 ± 1 | n.d. |
| Epicatechin | 1 ± 1 | 5 ± 1 | 4 ± 1 | 10 ± 1 | 4 ± 1 | 14 ± 1 | 8 ± 1 | 5 ± 1 | 9 ± 1 | 9 ± 1 | 10 ± 1 | 9 ± 1 | 16 ± 1 | 8 ± 1 | 12 ± 1 | 7 ± 0.2 |
| Quercetin-3-rutinoside | 2 ± 1 | 5 ± 1 | n.d. | 5 ± 0.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-glucoside | 2 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-glucuronide | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-3-glucoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3 ± 1 | n.d. | n.d. | n.d. | 2 ± 1 | n.d. | 2 ± 1 | n.d. |
| Kaempferol-3-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; Díaz-Navarrete, P.; Mora, R.; Ciudad, G.; Ortega, J.C.; Pinto-Ibieta, F. Acid Hydrothermal Amendment of Grape Wine Pomace: Enhancement of Phenol and Carbohydrate Co-Solubilization. Agronomy 2023, 13, 1501. https://doi.org/10.3390/agronomy13061501
Serrano A, Díaz-Navarrete P, Mora R, Ciudad G, Ortega JC, Pinto-Ibieta F. Acid Hydrothermal Amendment of Grape Wine Pomace: Enhancement of Phenol and Carbohydrate Co-Solubilization. Agronomy. 2023; 13(6):1501. https://doi.org/10.3390/agronomy13061501
Chicago/Turabian StyleSerrano, Antonio, Paola Díaz-Navarrete, Roberto Mora, Gustavo Ciudad, Juan Carlos Ortega, and Fernanda Pinto-Ibieta. 2023. "Acid Hydrothermal Amendment of Grape Wine Pomace: Enhancement of Phenol and Carbohydrate Co-Solubilization" Agronomy 13, no. 6: 1501. https://doi.org/10.3390/agronomy13061501





