Dairy Manure-Derived Biochar in Soil Enhances Nutrient Metabolism and Soil Fertility, Altering the Soil Prokaryote Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Data Collection and Chemical Analysis
2.3. Sequence Library Preparation and Sequencing
2.4. Bioinformatics Analysis
2.4.1. Creating Feature Tables and Assigning Taxonomy Using QIIME2
2.4.2. Diversity Analysis
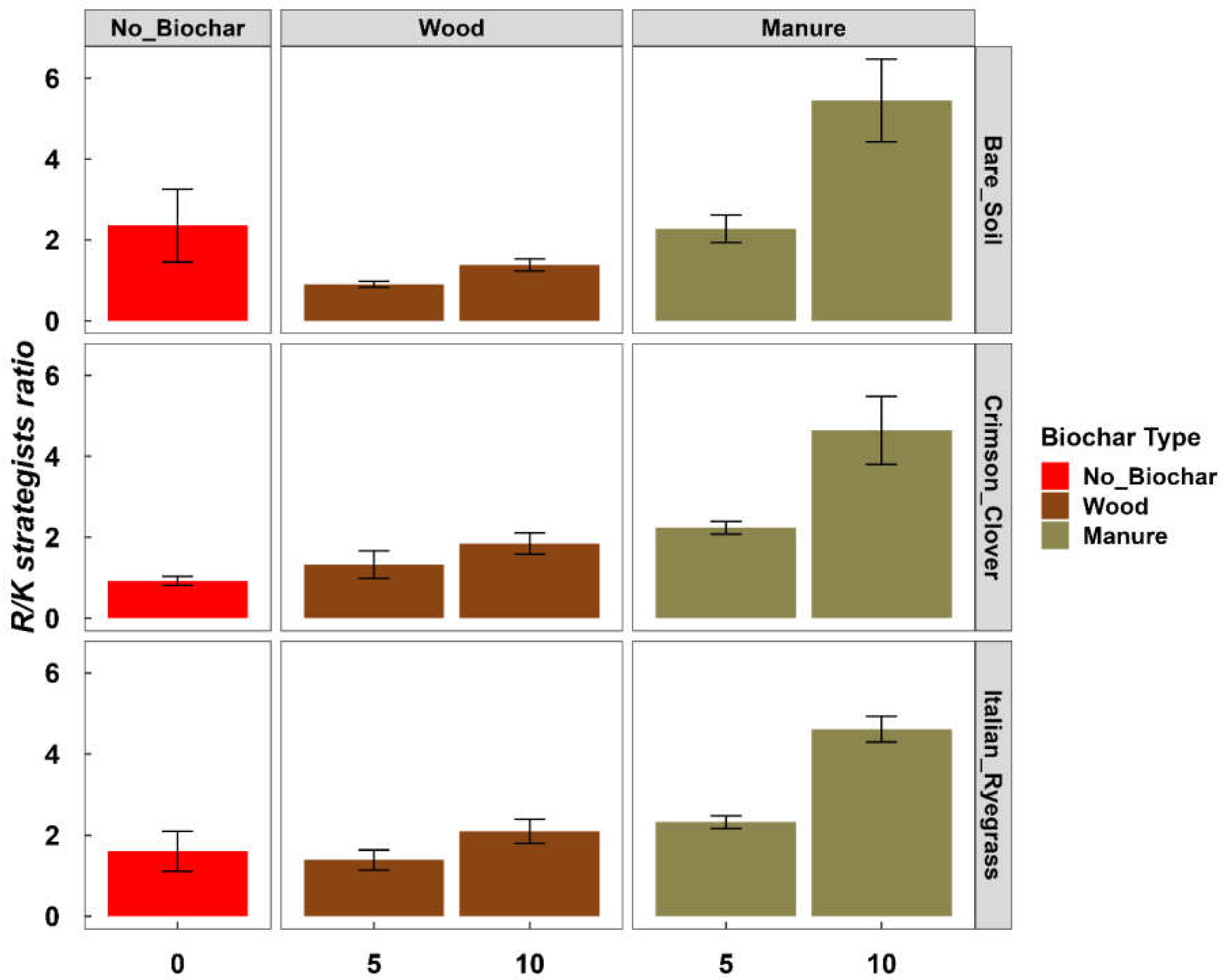
2.4.3. Estimating the Ratio between r- and K-Strategists
2.4.4. Functional Profiling Using PICRUSt2
2.4.5. Statistical Analysis
3. Results
3.1. Outcome of Sequencing
3.2. Alpha Diversity
| Terms | DF | Richness | Diversity | ||
|---|---|---|---|---|---|
| Observed | Chao1 | Shannon | InvSimpson | ||
| Crop type | 2 | 0.183 | 0.164 | 0.379 | 0.227 |
| Biochar Type | 2 | 0.000 | 0.000 | 0.001 | 0.041 |
| Loading percent | 1 | 0.028 | 0.033 | 0.016 | 0.000 |
| Crop type × Biochar type | 4 | 0.226 | 0.182 | 0.756 | 0.710 |
| Crop type × Loading percent | 2 | 0.001 | 0.001 | 0.05 | 0.011 |
| Biochar type × Loading percent | 1 | 0.785 | 0.806 | 0.615 | 0.100 |
| Crop type × Biochar type × Loading percent | 1 | 0.609 | 0.565 | 0.912 | 0.654 |
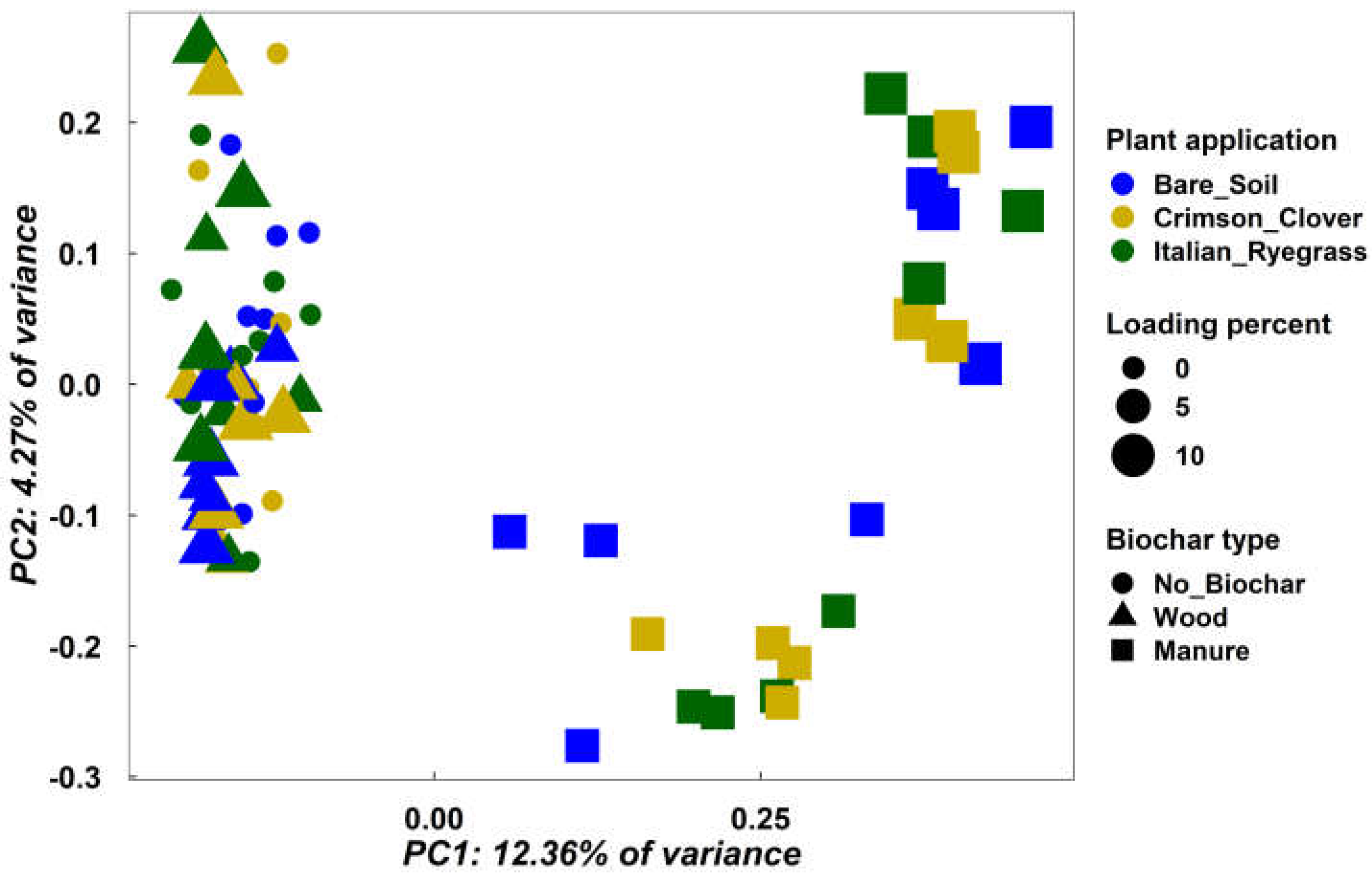
3.3. Microbial Community Structure
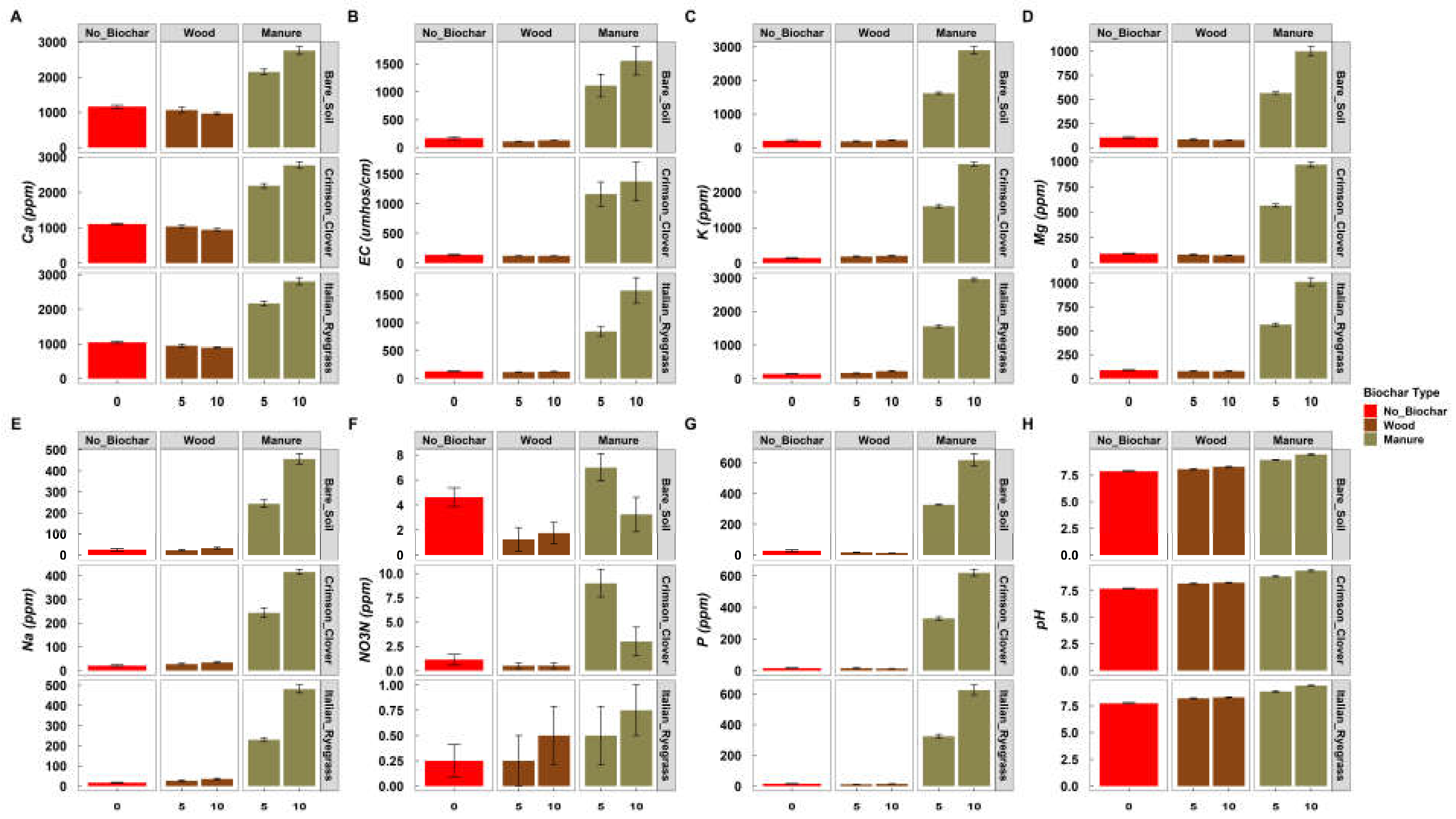
3.4. Soil Physicochemical Properties
3.5. Correlating Prokaryote Community Structure with Physicochemical Properties
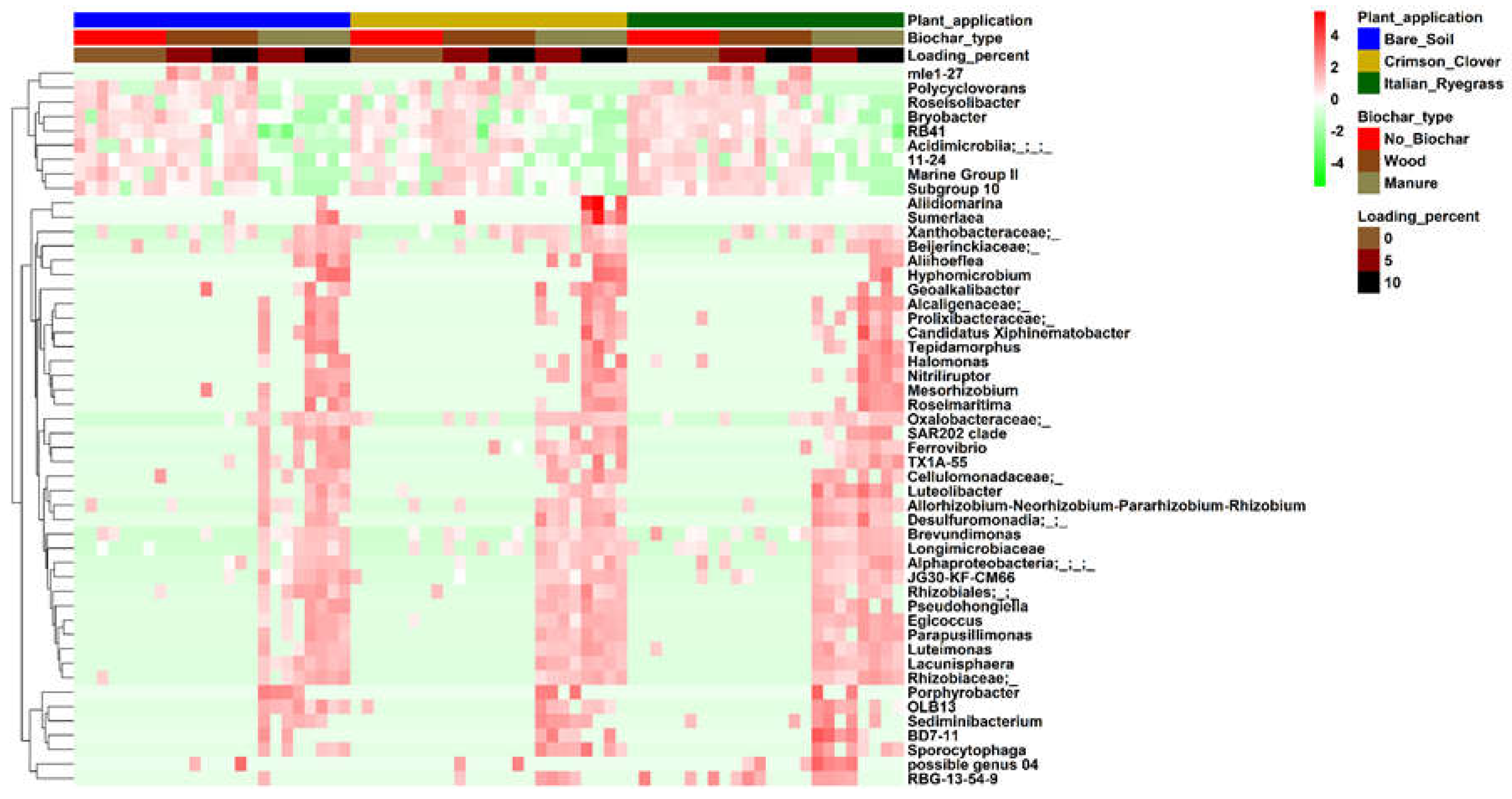
3.6. Microbial Community Composition
3.7. Function Prediction Based on 16S Sequences
4. Discussion
4.1. Effect of BC on the Soil Physiochemical Properties
4.2. Effect of BC on Prokaryote Community Diversity and Structure
4.3. Effect of BC on Microbial Community Composition
4.4. Effect of BC on the Prokaryote Functional Potential
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niraula, S.; Choi, Y.K.; Payne, K.; Muir, J.P.; Kan, E.; Chang, W.S. Dairy Effluent-Saturated Biochar Alters Microbial Communities and Enhances Bermudagrass Growth and Soil Fertility. Agronomy 2021, 11, 1794. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Gnansounou, E.; Chaturvedi, P. Biochar and Environmental Sustainability: Emerging Trends and Techno-Economic Perspectives. Bioresour. Technol. 2021, 332, 125102. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Adsorption and Regeneration on Iron-Activated Biochar for Removal of Microcystin-LR. Chemosphere 2021, 273, 129649. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Bio-Energy in the Black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; De Freitas, R.A.; Mangrich, A.S. Effect of Surface and Porosity of Biochar on Water Holding Capacity Aiming Indirectly at Preservation of the Amazon Biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of Biochar Amendment on Soil Carbon Balance and Soil Microbial Activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in Agriculture—A Systematic Review of 26 Global Meta-Analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing Potential of Biochar for Increasing Water-Holding Capacity of Sandy Soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative Effects of Biochar and Compost Applications on Water Holding Capacity and Crop Yield of Rice under Evaporation Stress: A Two-Years Field Study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.W.; Mitra, S. Sustainable Improvement of Soil Health Utilizing Biochar and Arbuscular Mycorrhizal Fungi: A Review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C.W. A Critical Review on Performance Indicators for Evaluating Soil Biota and Soil Health of Biochar-Amended Soils. J. Hazard Mater. 2021, 414, 125378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar Reduces Soil Heterotrophic Respiration in a Subtropical Plantation through Increasing Soil Organic Carbon Recalcitrancy and Decreasing Carbon-Degrading Microbial Activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting Effects of Biochar versus Manure on Soil Microbial Communities and Enzyme Activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar Increases Soil Microbial Biomass with Changes in Extra- and Intracellular Enzyme Activities: A Global Meta-Analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Soil Microbial Responses to Biochars Varying in Particle Size, Surface and Pore Properties. Pedosphere 2015, 25, 770–780. [Google Scholar] [CrossRef]
- Ye, J.; Joseph, S.D.; Ji, M.; Nielsen, S.; Mitchell, D.R.; Donne, S.; Horvat, J.; Wang, J.; Munroe, P.; Thomas, T. Chemolithotrophic Processes in the Bacterial Communities on the Surface of Mineral-Enriched Biochars. ISME J. 2017, 11, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Awad, Y.M.; Ok, Y.S.; Abrigata, J.; Beiyuan, J.; Beckers, F.; Tsang, D.C.W.; Rinklebe, J. Pine Sawdust Biomass and Biochars at Different Pyrolysis Temperatures Change Soil Redox Processes. Sci. Total Environ. 2018, 625, 147–154. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S.; Méndez, A.; Gascó, G. Interactive Effects of Biochar and the Earthworm Pontoscolex Corethrurus on Plant Productivity and Soil Enzyme Activities. J. Soils Sediments 2014, 14, 483–494. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of Biochar on Mineralisation of C and N from Soil and Willow Litter and Its Relationship with Microbial Community Biomass and Structure. Biol. Fertil. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
- Ren, T.; Feng, H.; Xu, C.; Xu, Q.; Fu, B.; Azwar, E.; Wei, Y.; Lam, S.S.; Liu, G. Exogenous Application and Interaction of Biochar with Environmental Factors for Improving Functional Diversity of Rhizosphere’s Microbial Community and Health. Chemosphere 2022, 294, 133710. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C Mineralization and Microbial Activity in Four Biochar Field Experiments Several Years after Incorporation. Soil Biol. Biochem. 2014, 78, 195–203. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar Addition Rate Influences Soil Microbial Abundance and Activity in Temperate Soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, Y.G.; Tan, X.F.; Zeng, G.M.; Zhou, Y.H.; Liu, S.B.; Yin, Z.H.; Jiang, L.H.; Li, M.F.; Wen, J. The Effect of Several Activated Biochars on Cd Immobilization and Microbial Community Composition during In-Situ Remediation of Heavy Metal Contaminated Sediment. Chemosphere 2018, 208, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize Biochar Addition Rate Influences Soil Enzyme Activity and Microbial Community Composition in a Fluvo-Aquic Soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Calderon, R.B.; Jeong, C.; Ku, H.H.; Coghill, L.M.; Ju, Y.J.; Kim, N.; Ham, J.H. Changes in the Microbial Community in Soybean Plots Treated with Biochar and Poultry Litter. Agronomy 2021, 11, 1428. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. Enhanced Removal of Antibiotic Resistance Genes and Human Bacterial Pathogens during Anaerobic Digestion of Dairy Manure via Addition of Manure Biochar. Chemosphere 2022, 304, 135178. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zeng, J.; Zhang, D.; Wang, F.; Li, Y.; Yi, W. Effect of Pyrolysis Temperature on Characteristics, Chemical Speciation and Environmental Risk of Cr, Mn, Cu, and Zn in Biochars Derived from Pig Manure. Sci. Total Environ. 2020, 704, 135283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of Pyrolysis Temperature on Chemical Speciation, Leaching Ability, and Environmental Risk of Heavy Metals in Biochar Derived from Cow Manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.B.; Muir, J.P.; Brady, J.A.; Kan, E.; Mitchell, A.B.; Obayomi, O. Impacts of Biochar on Trifolium Incarnatum and Lolium Multiflorum: Soil Nutrient Retention and Loss in Sandy Loam Amended with Dairy Manure. Agronomy 2023, 13, 26. [Google Scholar] [CrossRef]
- Mehlich, A. New Extractant for Soil Test Evaluation of Phosphorus, Potassium, Magnesium, Calcium, Sodium, Manganese and Zinc. Commun. Soil Sci. Plant Anal. 1978, 9, 477–492. [Google Scholar] [CrossRef]
- Kachurina, O.; Zhang, H.; Raun, W.; Krenzer, E. Simultaneous Determination of Soil Aluminum, Ammonium- and Nitrate-nitrogen Using 1 M Potassium Chloride Extraction. Commun. Soil Sci. Plant Anal. 2000, 31, 893–903. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 643–698. ISBN 9780891189770. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Navas-Molina, J.A.; Peralta-Sanchez, J.M.; Gonzalez, A.; McMurdie, P.J.; Vazquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing Our Understanding of the Human Microbiome Using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E. Qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://rdrr.io/github/jbisanz/qiime2R/ (accessed on 1 April 2023).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package 2018. Available online: http://cran.nexr.com/web/packages/vegan/index.html (accessed on 1 April 2023).
- Chen, H.; Jing, Q.; Liu, X.; Zhou, X.; Fang, C.; Li, B.; Zhou, S.; Nie, M. Microbial Respiratory Thermal Adaptation Is Regulated by R-/K-Strategy Dominance. Ecol. Lett. 2022, 25, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R. A Language and Environment for Statistical Computing; Austria R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- McArdle, B.H.; Anderson, M.J. Fitting Multivariate Models to Community Data: A Comment on Distance-Based Redundancy Analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pheatmap: Pretty Heatmaps 2019. Available online: https://rdrr.io/cran/pheatmap/ (accessed on 1 April 2023).
- Zhang, X.; Zhang, Q.; Zhan, L.; Xu, X.; Bi, R.; Xiong, Z. Biochar Addition Stabilized Soil Carbon Sequestration by Reducing Temperature Sensitivity of Mineralization and Altering the Microbial Community in a Greenhouse Vegetable Field. J. Environ. Manag. 2022, 313, 114972. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Jang, H.M.; Kan, E.; Wallace, A.R.; Sun, W. Adsorption of Phosphate in Water on a Novel Calcium Hydroxide-Coated Dairy Manure-Derived Biochar. Environ. Eng. Res. 2019, 24, 434–442. [Google Scholar] [CrossRef]
- Zeng, S.; Harris, R.; Kan, E. Effect of Alfalfa-Derived Biochar on Anaerobic Digestion of Dairy Manure. Agronomy 2022, 12, 911. [Google Scholar] [CrossRef]
- Algren, M.; Costello, C.; Landis, A.E. Phosphorus (P) in Animal Diets as a Driver of Embodied P in Animal Products and Net Anthropogenic P Inputs. J. Ind. Ecol. 2022, 26, 1123–1135. [Google Scholar] [CrossRef]
- Flachowsky, G.; Gruen, M.; Meyer, U. Feed-Efficient Ruminant Production: Opportunities and Challenges. J. Anim. Feed Sci. 2013, 22, 177–187. [Google Scholar] [CrossRef]
- Brewer, C.E.; Brown, R.C. Biochar. Compr. Renew. Energy 2012, 5, 357–384. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of Biochar on Chemical Properties of Acidic Soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-Chemical Properties and Microbial Responses in Biochar-Amended Soils: Mechanisms and Future Directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from Water Hyacinth (Eichornia Crassipes) and Its Impact on Soil Biological Activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Stewart, C.E.; Zheng, J.; Botte, J.; Cotrufo, M.F. Co-Generated Fast Pyrolysis Biochar Mitigates Green-House Gas Emissions and Increases Carbon Sequestration in Temperate Soils. GCB Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Wong, F.; Chen, Z.; Chen, X.; Wang, C.; Ng, W.; Wong, M.H.; Luo, Y.; Po, T.; Kong, H. Soil-Water Retention Behavior of Compacted Biochar-Amended Clay: A Novel Landfill Final Cover Material. J. Soils Sediments 2017, 17, 590–598. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.N.; Shao, H.B.; Chang, S.X. Biochar Had Effects on Phosphorus Sorption and Desorption in Three Soils with Differing Acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, S.; Tian, L.; Li, S.; Li, X.; Shen, Y.; Tian, C. Long-Term Biochar Application Influences Soil Microbial Community and Its Potential Roles in Semiarid Farmland. Appl. Soil Ecol. 2017, 117–118, 10–15. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X.; Emam, A.E.R. Engineered Biochar from Microwave-Assisted Catalytic Pyrolysis of Switchgrass for Increasing Water-Holding Capacity and Fertility of Sandy Soil. Sci. Total Environ. 2016, 566–567, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Waller, L.P. What Drives Differences in Arbuscular Mycorrhizal Fungal Communities among Plant Species? Fungal Ecol. 2016, 24, 135–138. [Google Scholar] [CrossRef]
- McNicol, J.W.; Singh, B.K.; Prosser, J.I.; Nunan, N.; Papert, A.; Daniell, T.J. Links between Plant and Rhizoplane Bacterial Communities in Grassland Soils, Characterized Using Molecular Techniques. Appl. Environ. Microbiol. 2005, 71, 6784–6792. [Google Scholar] [CrossRef]
- Obayomi, O.; Edelstein, M.; Safi, J.; Mihiret, M.; Ghazaryan, L.; Vonshak, A.; Bernstein, N.; Gillor, O. The Combined Effects of Treated Wastewater Irrigation and Plastic Mulch Cover on Soil and Crop Microbial Communities. Biol. Fertil. Soils 2020, 56, 729–742. [Google Scholar] [CrossRef]
- Singh, B.K.; Munro, S.; Potts, J.M.; Millard, P. Influence of Grass Species and Soil Type on Rhizosphere Microbial Community Structure in Grassland Soils. Appl. Soil Ecol. 2007, 36, 147–155. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Crowther, T.W.; Maynard, D.S.; Leff, J.W.; Oldfield, E.E.; McCulley, R.L.; Fierer, N.; Bradford, M.A. Predicting the Responsiveness of Soil Biodiversity to Deforestation: A Cross-Biome Study. Glob. Chang. Biol. 2014, 20, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Zheng, J.; Zhang, X.; Liu, X.; Bian, R.; Li, L.; Cheng, K.; Zheng, J.; Pan, G. Biochar Amendment Changes Temperature Sensitivity of Soil Respiration and Composition of Microbial Communities 3 Years after Incorporation in an Organic Carbon-Poor Dry Cropland Soil. Biol. Fertil. Soils 2018, 54, 175–188. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Fan, M.; Wu, Y.; Shangguan, Z. Interactions between Biochar and Nitrogen Impact Soil Carbon Mineralization and the Microbial Community. Soil Tillage Res. 2020, 196, 104437. [Google Scholar] [CrossRef]
- Obayomi, O.; Seyoum, M.M.; Ghazaryan, L.; Tebbe, C.C.; Murase, J.; Bernstein, N.; Gillor, O. Soil Texture and Properties Rather than Irrigation Water Type Shape the Diversity and Composition of Soil Microbial Communities. Appl. Soil Ecol. 2021, 161, 103834. [Google Scholar] [CrossRef]
- Wasmund, K.; Schreiber, L.; Lloyd, K.G.; Petersen, D.G.; Schramm, A.; Stepanauskas, R.; Jørgensen, B.B.; Adrian, L. Genome Sequencing of a Single Cell of the Widely Distributed Marine Subsurface Dehalococcoidia, Phylum Chloroflexi. ISME J. 2014, 8, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.F.; Chen, L.-X.; Jessen, G.L.; Rudderham, S.B.; McBeth, J.M.; Lindsay, M.B.J.; Slater, G.F.; Banfield, J.F.; Warren, L.A. Putative Mixotrophic Nitrifying-Denitrifying Gammaproteobacteria Implicated in Nitrogen Cycling Within the Ammonia/Oxygen Transition Zone of an Oil Sands Pit Lake. Front. Microbiol. 2019, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Kirby, R. Actinomycetes and Lignin Degradation. Adv. Appl. Microbiol. 2005, 58, 125–168. [Google Scholar] [CrossRef]
- O’Neill, B.; Grossman, J.; Tsai, M.T.; Gomes, J.E.; Lehmann, J.; Peterson, J.; Neves, E.; Thies, J.E. Bacterial Community Composition in Brazilian Anthrosols and Adjacent Soils Characterized Using Culturing and Molecular Identification. Microb. Ecol. 2009, 58, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar Soil Amendment Increased Bacterial but Decreased Fungal Gene Abundance with Shifts in Community Structure in a Slightly Acid Rice Paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Chapman, S.J.; Khan, S.; Yao, H. Microbial Utilization of Rice Straw and Its Derived Biochar in a Paddy Soil. Sci. Total Environ. 2016, 559, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-Term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems across the Globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy Cycle: An Oxidative Process in Plants for Nutrient Extraction from Symbiotic Microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global Biogeography and Quantitative Seasonal Dynamics of Gemmatimonadetes in Soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of Biochar and Biochar-Based Fertilizer on Yield, Quality of Tea and Microbial Community in an Acid Tea Orchard Soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of Biochar on Carbon Fractions and Enzyme Activity of Red Soil. Catena 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s Stability and Effect on the Content, Composition and Turnover of Soil Organic Carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Jing, Y.; Zhang, R. Biochar Amendment Effects on the Activities of Soil Carbon, Nitrogen, and Phosphorus Hydrolytic Enzymes: A Meta-Analysis. Environ. Sci. Pollut. Res. 2019, 26, 22990–23001. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Zhang, B.; Li, Z.; Du, H.; Tie, B.; Lei, M. Bacterial Diversity Rather than Available Cd Is the Main Driver of Exoenzyme Activity and Stoichiometry after Soil Amendments in Mildly Contaminated Soil. J. Soils Sediments 2022, 22, 443–456. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of Carbon and Microbial Activity in Salt-Induced Soil by Application of Peanut Shell Biochar during Short-Term Incubation Study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Hussain, Q.; Khan, K.S.; Akmal, M.; Ijaz, S.S.; Hayat, R.; Khalid, A.; Azeem, M.; Rashid, M. Response of Soil Microbial Biomass and Enzymatic Activity to Biochar Amendment in the Organic Carbon Deficient Arid Soil: A 2-Year Field Study. Arab. J. Geosci. 2019, 12, 95. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of Poultry Litter Biochar on Soil Enzymatic Activity, Ecotoxicity and Plant Growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Cely, P.; Plaza, C.; Méndez, A. Influence of Pig Manure and Its Biochar on Soil CO2 Emissions and Soil Enzymes. Ecol. Eng. 2016, 95, 19–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Zhang, D.; Li, R.; Cheng, T.; Zhang, Y.; Liu, X.; Wong, G.; Tang, Y.; Wang, H.; et al. Comparative Transcriptome Analysis Reveals Relationship of Three Major Domesticated Varieties of Auricularia Auricula-Judae. Sci. Rep. 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, D.; Xu, Q.; Fuhrmann, J.J.; Li, L.; Pan, G.; Li, Y.; Qin, H.; Liang, C.; Sun, X. Organic Carbon Quality, Composition of Main Microbial Groups, Enzyme Activities, and Temperature Sensitivity of Soil Respiration of an Acid Paddy Soil Treated with Biochar. Biol. Fertil. Soils 2019, 55, 185–197. [Google Scholar] [CrossRef]




| Terms | DF | R2 | Pseudo F-Value | p-Value |
|---|---|---|---|---|
| Crop type | 2 | 0.027 | 1.082 | 0.183 |
| Biochar Type | 2 | 0.132 | 5.358 | 0.001 |
| Loading percent | 1 | 0.028 | 2.244 | 0.001 |
| Crop type × Biochar type | 4 | 0.048 | 0.970 | 0.571 |
| Crop type × Loading percent | 2 | 0.021 | 0.846 | 0.949 |
| Biochar type × Loading percent | 1 | 0.026 | 2.101 | 0.001 |
| Crop type × Biochar type × Loading percent | 2 | 0.019 | 0.786 | 0.997 |
| Residuals | 57 | 0.700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obayomi, O.; Taggart, C.B.; Zeng, S.; Sefcik, K.; Willis, B.; Muir, J.P.; Kan, E.; Brady, J.A. Dairy Manure-Derived Biochar in Soil Enhances Nutrient Metabolism and Soil Fertility, Altering the Soil Prokaryote Community. Agronomy 2023, 13, 1512. https://doi.org/10.3390/agronomy13061512
Obayomi O, Taggart CB, Zeng S, Sefcik K, Willis B, Muir JP, Kan E, Brady JA. Dairy Manure-Derived Biochar in Soil Enhances Nutrient Metabolism and Soil Fertility, Altering the Soil Prokaryote Community. Agronomy. 2023; 13(6):1512. https://doi.org/10.3390/agronomy13061512
Chicago/Turabian StyleObayomi, Olabiyi, Cosette B. Taggart, Shengquan Zeng, Kristin Sefcik, Bianca Willis, James P. Muir, Eunsung Kan, and Jeff A. Brady. 2023. "Dairy Manure-Derived Biochar in Soil Enhances Nutrient Metabolism and Soil Fertility, Altering the Soil Prokaryote Community" Agronomy 13, no. 6: 1512. https://doi.org/10.3390/agronomy13061512
APA StyleObayomi, O., Taggart, C. B., Zeng, S., Sefcik, K., Willis, B., Muir, J. P., Kan, E., & Brady, J. A. (2023). Dairy Manure-Derived Biochar in Soil Enhances Nutrient Metabolism and Soil Fertility, Altering the Soil Prokaryote Community. Agronomy, 13(6), 1512. https://doi.org/10.3390/agronomy13061512






