Impacts of Corn Straw Compost on Rice Growth and Soil Microflora under Saline-Alkali Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Determination of Soil Physiochemical Properties and Enzymatic Activities
2.3. Preparation and 16S rRNA Sequencing of Soil Bacteria
2.4. Bioinformatics Analyses
3. Results
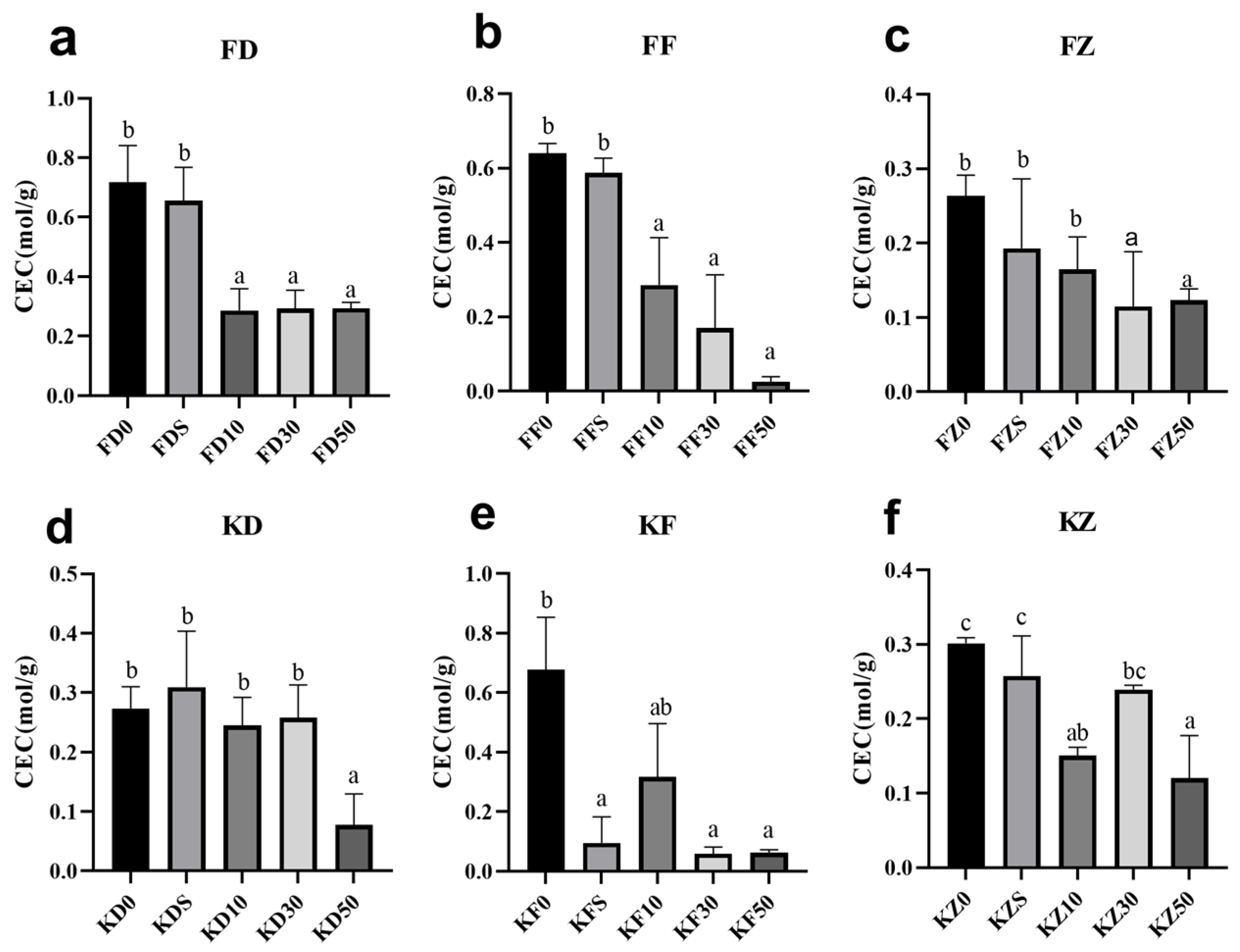
3.1. Effects of Corn Straw Compost on Soil Nutrients and Structure
3.2. Effects of Corn Straw Compost on Soil pH Value and Electrical Conductivity
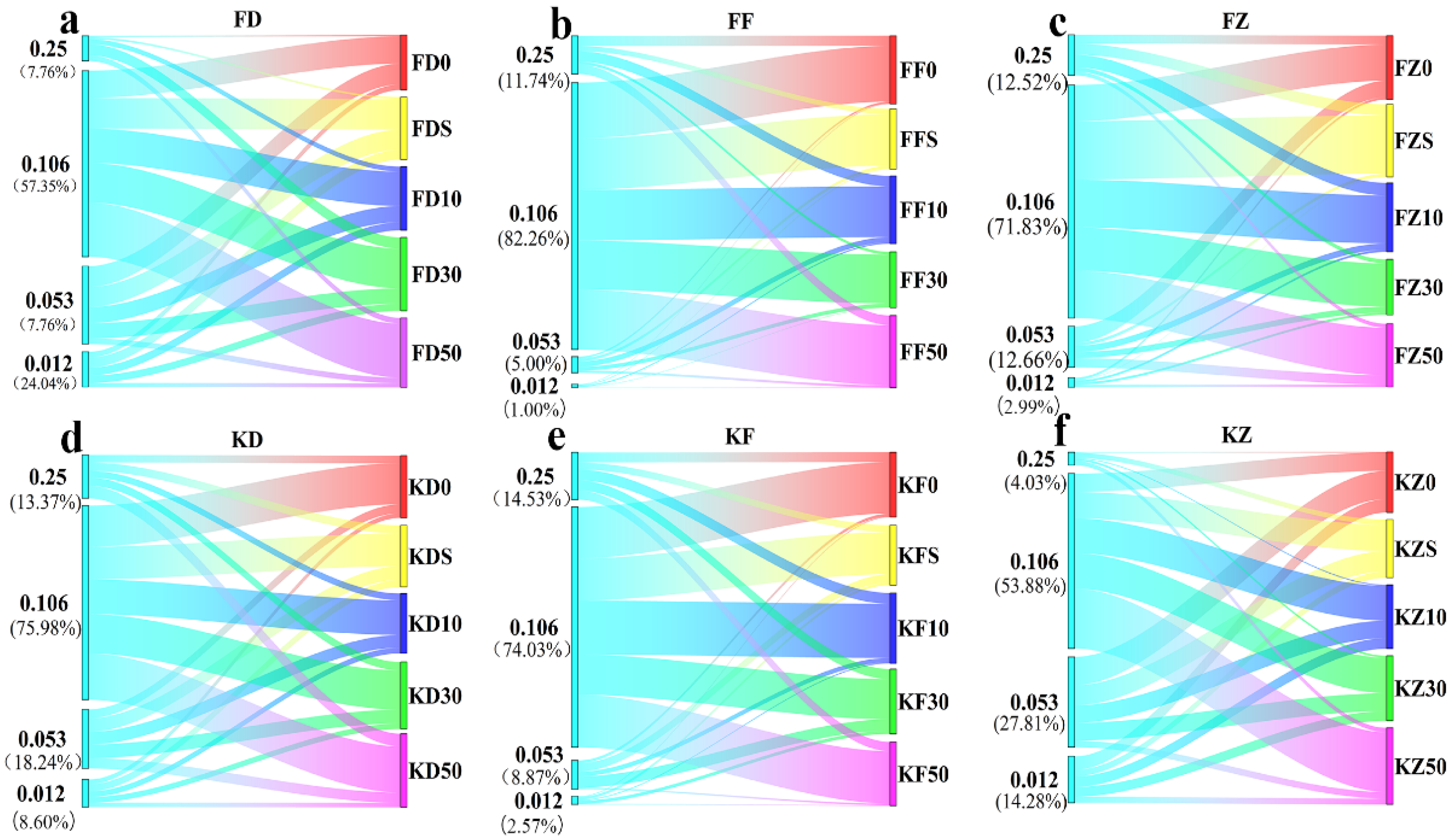
3.3. Effects of Corn Straw Compost on Soil Bacterial Diversity
3.4. Effects of Corn Straw Compost on Bacterial Community Composition
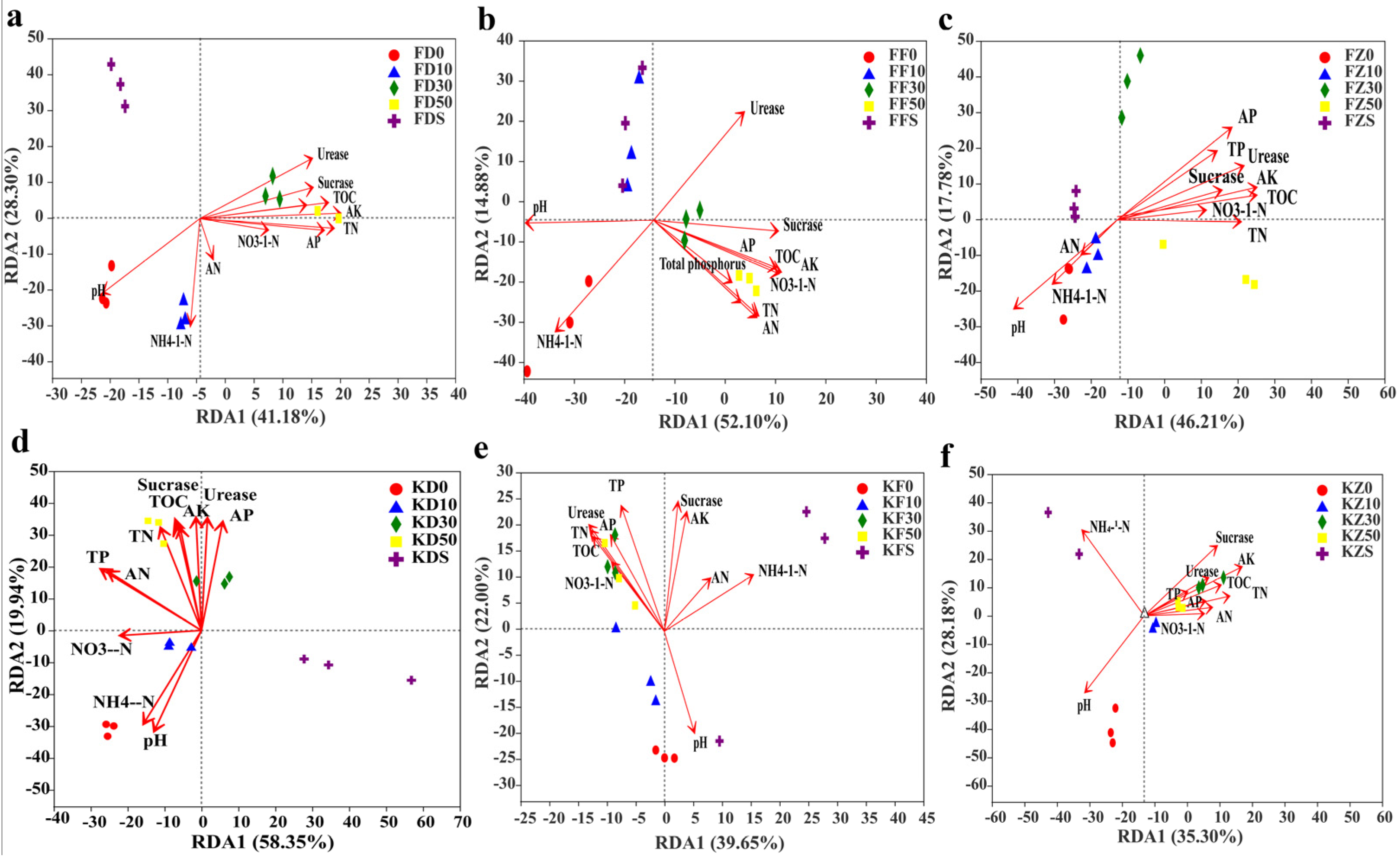
3.5. Correlation between Soil Properties and Bacterial Genera Levels
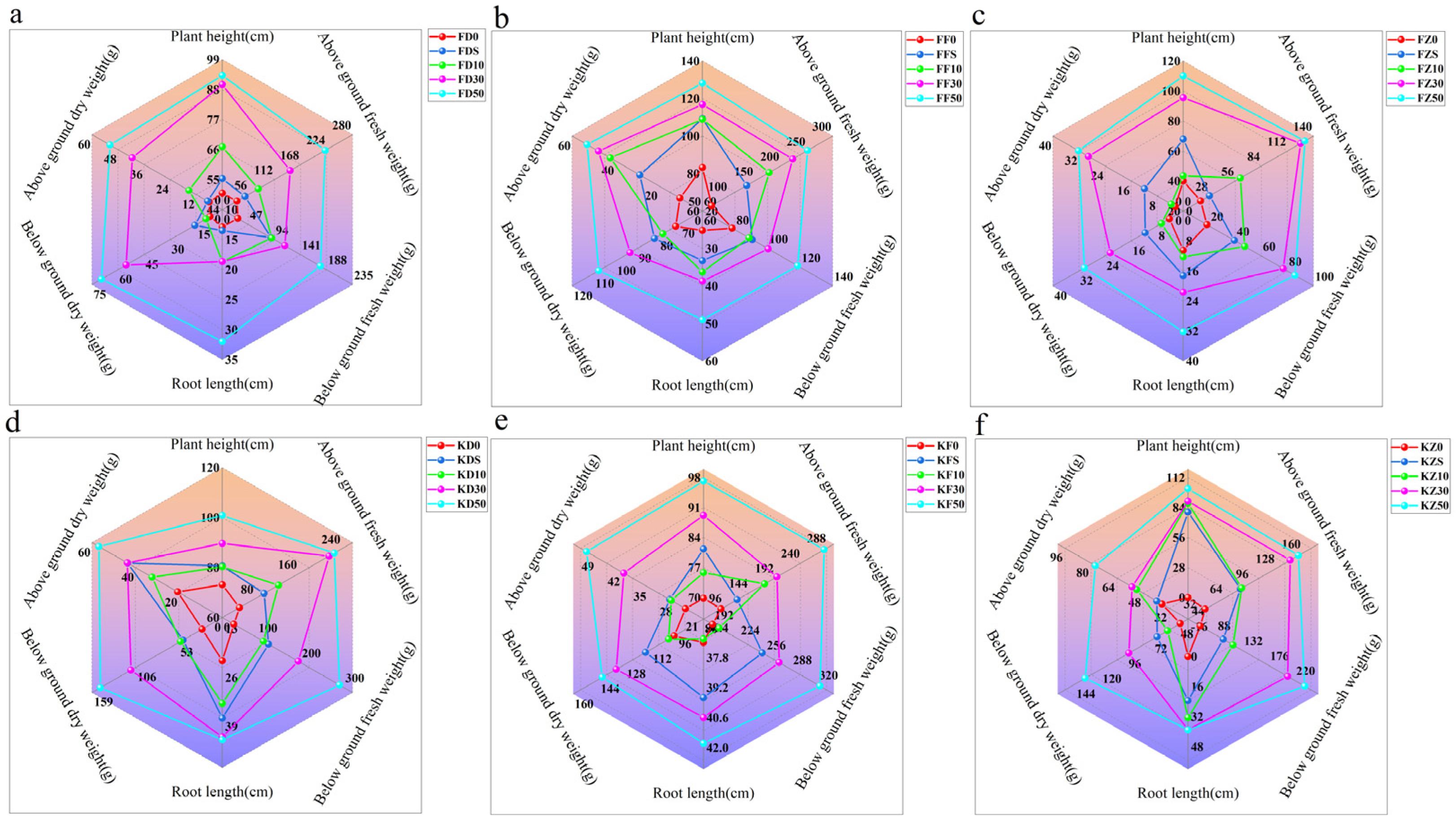
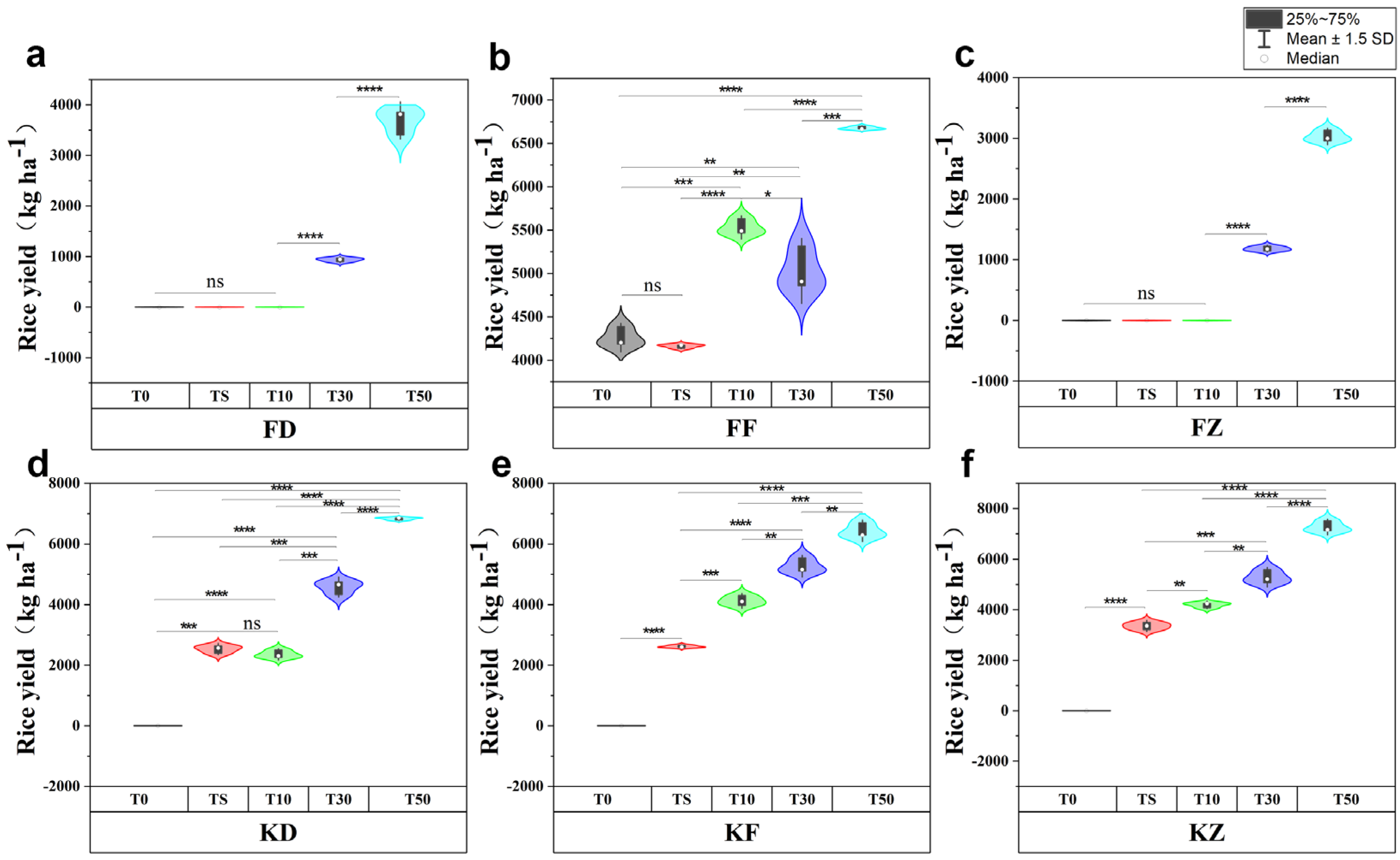
3.6. Effects of Corn Straw Compost on Rice Biomass and Yields
4. Discussion
4.1. Corn Straw Compost Improved Soil Microenvironment by Increasing Soil Nutrients and Adjusting pH
4.2. Corn Straw Compost Affected Diversity and Composition of Bacterial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Cai, C.P.; Wu, S.; Zhang, F.; Hou, S.; Guo, W.Z. Evaluation and Exploration of Favorable QTL Alleles for Salt Stress Related Traits in Cotton Cultivars (G-hirsutum L.). PLoS ONE 2016, 11, 0151076. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yao, Q.; Cai, Z.P.; Xie, X.L.; Zhu, H.H. Isolation and Identification of Myxobacteria from Saline-Alkaline Soils in Xinjiang, China. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Stille, L.; Smeets, E.; Wicke, B.; Singh, R.; Singh, G. The economic performance of four (agro-) forestry systems on alkaline soils in the state of Haryana in India. Energy Sustain. Dev. 2011, 15, 388–397. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Liu, Q.; Deng, S.; Jin, X.; Yin, Y.; Guo, J.; Li, N.; Liu, Y.; Han, S.; et al. A Na(2)CO(3)-Responsive Chitinase Gene From Leymus chinensis Improve Pathogen Resistance and Saline-Alkali Stress Tolerance in Transgenic Tobacco and Maize. Front. Plant Sci. 2020, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; An, F.H.; Ma, H.Y.; Wang, Z.C.; Zhou, X.; Liu, Z.J. Variations on Soil Salinity and Sodicity and Its Driving Factors Analysis under Microtopography in Different Hydrological Conditions. Water 2016, 8, 227. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Zu, Y.G.; Guan, Y.; Liu, Z.G.; Zhang, Z.H.; Xu, H.N.; Yu, X.Y. Addition of HPMA affects seed germination, plant growth and properties of heavy saline-alkali soil in northeastern China: Comparison with other agents and determination of the mechanism. Plant Soil 2011, 339, 177–191. [Google Scholar] [CrossRef]
- Shi, S.H.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.S.; Yang, F.; Wang, Z.C.; Tian, C.J. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Yin, J.; Liu, Y.; Yu, F.; Cai, J.; Liu, T. Screening and identification of a lignin degrading bacterium and its application in composting. Chin. Soil Fertil. 2019, 281, 179–185. [Google Scholar]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, G.; Heitman, J.L. The effects of compost incorporation on soil physical properties in urban soils—A concise review. J. Environ. Manag. 2020, 261, 10. [Google Scholar] [CrossRef] [PubMed]
- Gaind, S.; Pandey, A.K.; Lata. Microbial biomass, P-nutrition, and enzymatic activities of wheat soil in response to phosphorus enriched organic and inorganic manures. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2006, 41, 177–187. [Google Scholar] [CrossRef]
- Das, A.; Baiswar, P.; Patel, D.P.; Munda, G.C.; Ghosh, P.K.; Ngachan, S.V.; Panwar, A.S.; Chandra, S. Compost Quality Prepared from Locally Available Plant Biomass and their Effect on Rice Productivity under Organic Production System. J. Sustain. Agric. 2010, 34, 466–482. [Google Scholar] [CrossRef]
- Xie, K.Z.; Xu, P.Z.; Yang, S.H.; Lu, Y.S.; Jiang, R.P.; Gu, W.J.; Li, W.Y.; Sun, L.L. Effects of Supplementary Composts on Microbial Communities and Rice Productivity in Cold Water Paddy Fields. J. Microbiol. Biotechnol. 2015, 25, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Marecik, R.; Blaszczyk, L.; Bieganska-Marecik, R.; Piotrowska-Cyplik, A. Screening and Identification of Trichoderma Strains Isolated from Natural Habitats with Potential to Cellulose and Xylan Degrading Enzymes Production. Pol. J. Microbiol. 2018, 67, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Takakai, F.; Hatakeyama, K.; Nishida, M.; Nagata, O.; Sato, T.; Kaneta, Y. Effect of the long-term application of organic matter on soil carbon accumulation and GHG emissions from a rice paddy field in a cool-temperate region, Japan-II. Effect of different compost applications. Soil Sci. Plant Nutr. 2020, 66, 96–105. [Google Scholar] [CrossRef]
- Sarangi, S.; Swain, H.; Adak, T.; Bhattacharyya, P.; Mukherjee, A.K.; Kumar, G.; Mehetre, S.T. Trichoderma-mediated rice straw compost promotes plant growth and imparts stress tolerance. Environ. Sci. Pollut. Res. 2021, 28, 44014–44027. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Lin, X.G.; Wang, Y.M.; Hu, J.L. Mitigating methane emissions from irrigated paddy fields by application of aerobically composted livestock manures in eastern China. Soil Use Manag. 2011, 27, 103–109. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Gillespie, J.L.; Morina, J.C.; Franklin, R.B. Salinity affects microbial activity and soil organic matter content in tidal wetlands. Glob. Chang. Biol. 2014, 20, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Pang, H.C.; Zhao, Y.G.; Wang, J.; Lu, C.; Li, Y.Y. Buried straw layer plus plastic mulching improves soil organic carbon fractions in an arid saline soil from Northwest China. Soil Tillage Res. 2017, 165, 286–293. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Wong, V.N.L.; Dalal, R.C.; Greene, R.S.B. Salinity and sodicity effects on respiration and microbial biomass of soil. Biol. Fertil. Soils 2008, 44, 943–953. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Yu, S.; Hawke, B.G.; Harch, B.D. Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol. Fertil. Soils 2001, 33, 204–217. [Google Scholar] [CrossRef]
- Xu, M.; Xian, Y.; Wu, J.; Gu, Y.F.; Yang, G.; Zhang, X.H.; Peng, H.; Yu, X.Y.; Xiao, Y.L.; Li, L. Effect of biogas slurry addition on soil properties, yields, and bacterial composition in the rice-rape rotation ecosystem over 3 years. J. Soils Sediments 2019, 19, 2534–2542. [Google Scholar] [CrossRef]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellappan, K.P.; Balasubramanian, T. Restoration of saline land by halophytes for Indian soils. Soil Biol. Biochem. 2007, 39, 2661–2664. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Effects of Phosphate-Solubilizing Bacteria, Rock Phosphate and Chemical Fertilizers on Maize-Wheat Cropping Cycle and Economics. Pedosphere 2015, 25, 428–437. [Google Scholar] [CrossRef]
- Shen, C.C.; Xiong, J.B.; Zhang, H.Y.; Feng, Y.Z.; Lin, X.G.; Li, X.Y.; Liang, W.J.; Chu, H.Y. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Ding, S.J.; Zhang, X.F.; Yang, W.L.; Xin, X.L.; Zhu, A.N.; Huang, S.M. Soil Nutrients and Aggregate Composition of Four Soils with Contrasting Textures in a Long-Term Experiment. Eurasian Soil Sci. 2021, 54, 1746–1755. [Google Scholar] [CrossRef]
- Yang, L.; Tan, L.L.; Zhang, F.H.; Gale, W.J.; Cheng, Z.B.; Sang, W. Duration of continuous cropping with straw return affects the composition and structure of soil bacterial communities in cotton fields. Can. J. Microbiol. 2018, 64, 167–181. [Google Scholar] [CrossRef]
- Hong, Y.H.; Zhao, D.; Zhang, F.Z.; Shen, G.N.; Yuan, Y.; Gao, Y.M.; Yan, L.; Wei, D.; Wang, W.D. Soil water-stable aggregates and microbial community under long-term tillage in black soil of Northern China. Ecotoxicology 2021, 30, 1754–1768. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Lakhdar, A.; Hafsi, C.; Debez, A.; Montemurro, F.; Jedidi, N.; Abdelly, C. Assessing solid waste compost application as a practical approach for salt-affected soil reclamation. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 284–288, Erratum in Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 389. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M.; Simunek, J. Leaching and reclamation of a biochar and compost amended saline-sodic soil with moderate SAR reclaimed water. Agric. Water Manag. 2015, 158, 255–265. [Google Scholar] [CrossRef]
- Walker, D.J.; Bernal, M.P. The effects of olive mill waste compost and poultry manure on the availability and plant uptake of nutrients in a highly saline soil. Bioresour. Technol. 2008, 99, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sax, M.S.; Bassuk, N.; van Es, H.; Rakow, D. Long-term remediation of compacted urban soils by physical fracturing and incorporation of compost. Urban For. Urban Green. 2017, 24, 149–156. [Google Scholar] [CrossRef]
- Cogger, C.; Hummel, R.; Hart, J.; Bary, A. Soil and Redosier Dogwood Response to Incorporated and Surface-applied Compost. Hortscience 2008, 43, 2143–2150. [Google Scholar] [CrossRef]
- Aggelides, S.M.; Londra, P.A. Effects of compost produced from town wastes and sewage sludge on the physical properties of a loamy and a clay soil. Bioresour. Technol. 2000, 71, 253–259. [Google Scholar] [CrossRef]
- Cogger, C.G. Potential compost benefits for restoration of soils disturbed by urban development. Compos. Sci. Util. 2005, 13, 243–251. [Google Scholar] [CrossRef]
- Garcia, R.A.; Li, Y.C.; Rosolem, C.A. Soil Organic Matter and Physical Attributes Affected by Crop Rotation Under No-till. Soil Sci. Soc. Am. J. 2013, 77, 1724–1731. [Google Scholar] [CrossRef]
- Horner-Devine, M.C.; Carney, K.M.; Bohannan, B.J.M. An ecological perspective on bacterial biodiversity. Proc. R. Soc. B Biol. Sci. 2004, 271, 113–122. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kasai, T.; Nakagawa, G.; Yamamuro, A.; Abe, T.; Watanabe, K. Comparative Metagenomics of Anode-Associated Microbiomes Developed in Rice Paddy-Field Microbial Fuel Cells. PLoS ONE 2013, 8, e0077443. [Google Scholar] [CrossRef]
- Sun, R.B.; Zhang, X.X.; Guo, X.S.; Wang, D.Z.; Chu, H.Y. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Barzegar, A.R.; Nelson, P.N.; Oades, J.M.; Rengasamy, P. Organic matter, sodicity, and clay type: Influence on soil aggregation. Soil Sci. Soc. Am. J. 1997, 61, 1131–1137. [Google Scholar] [CrossRef]
- Wong, V.N.L.; Dalal, R.C.; Greene, R.S.B. Carbon dynamics of sodic and saline soils following gypsum and organic material additions: A laboratory incubation. Appl. Soil Ecol. 2009, 41, 29–40. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shao, T.Y.; Zhou, Y.J.; Cao, Y.C.; Hu, H.Y.; Sun, Q.K.; Long, X.H.; Yue, Y.; Gao, X.M.; Rengel, Z. Effects of planting Melia azedarach L. on soil properties and microbial community in saline-alkali soil. Land Degrad. Dev. 2021, 32, 2951–2961. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C.; Leinweber, P. Density, metabolic activity, and identity of cultivable rhizosphere bacteria on Salix viminalis in disturbed arable and landfill soils. J. Plant Nutr. Soil Sci. 2010, 173, 747–756. [Google Scholar] [CrossRef]
- Zahran, H.H. Diversity, adaptation and activity of the bacterial flora in saline environments. Biol. Fertil. Soils 1997, 25, 211–223. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag. 2016, 48, 115–126. [Google Scholar] [CrossRef]
- Li, M.X.; He, X.S.; Tang, J.; Li, X.; Zhao, R.; Tao, Y.Q.; Wang, C.; Qiu, Z.P. Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 2021, 264, 128549. [Google Scholar] [CrossRef]
- Gao, M.; Liang, F.; Yu, A.; Li, B.; Yang, L. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 2010, 78, 614–619. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Kuramae, E.E.; de Hollander, M.; Pijl, A.S.; van Veen, J.A.; Tsai, S.M. Acidobacterial community responses to agricultural management of soybean in Amazon forest soils. Fems Microbiol. Ecol. 2013, 83, 607–621. [Google Scholar] [CrossRef]




| Abbreviation | Mixture Components |
|---|---|
| T0 | 0% corn straw compost + 100% saline soil |
| T10 | 10% corn straw compost + 90% saline soil |
| T30 | 30% corn straw compost + 70% saline soil |
| T50 | 50% corn straw compost + 50% saline soil, |
| TS | 7.7% gypsum + 92.3% saline soil (control) |
| Abbreviation | The Rice Varieties and Grown Soil |
|---|---|
| FD | Wuyoudao No. 4 in soil from Dumeng |
| KD | Tongxi926 in soil from Dumeng |
| FF | Wuyoudao No. 4 in soil from Fularki |
| KF | Tongxi926 in soil from Fularki |
| FZ | Wuyoudao No. 4 in soil from Zhaoyuan |
| KZ | Tongxi926 in soil from Zhaoyuan |
| Genus of Bacteria | Treatments | Genus of Bacteria | Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FD0 | FD10 | FD30 | FD50 | FDS | KD0 | KD10 | KD30 | KD50 | KDS | ||
| thermobacillus | 0.00% | 0.00% | 1.80% | 2.62% | 0.00% | thermobacillus | 0.00% | 0.00% | 1.84% | 2.30% | 0.00% |
| thermobispora | 0.00% | 0.00% | 3.24% | 5.35% | 0.00% | thermobispora | 0.00% | 0.00% | 3.35% | 5.07% | 0.00% |
| thermopolyspora | 0.00% | 1.24% | 3.98% | 8.79% | 0.00% | thermopolyspora | 0.00% | 0.00% | 3.63% | 5.63% | 0.00% |
| micromonosporacease | 0.00% | 0.00% | 1.54% | 3.19% | 0.00% | micromonosporacease | 0.00% | 0.00% | 1.37% | 1.84% | 0.00% |
| desulfobacterota | 0.00% | 0.00% | 0.00% | 1.72% | 0.00% | desulfobacterota | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| FF0 | FF10 | FF30 | FF50 | FFS | KF0 | KF10 | KF30 | KF50 | KFS | ||
| thermobacillus | 0.00% | 0.00% | 0.00% | 1.78% | 0.00% | thermobacillus | 0.00% | 0.00% | 0.00% | 1.61% | 0.00% |
| thermobispora | 0.00% | 0.00% | 2.09% | 3.67% | 0.00% | thermobispora | 0.00% | 0.00% | 1.97% | 3.02% | 0.00% |
| thermopolyspora | 0.00% | 1.22% | 3.15% | 5.59% | 0.00% | thermopolyspora | 0.00% | 0.00% | 2.67% | 4.70% | 0.00% |
| micromonosporacease | 0.00% | 0.00% | 1.14% | 1.63% | 0.00% | micromonosporacease | 0.00% | 0.00% | 1.58% | 1.42% | 0.00% |
| desulfobacterota | 0.00% | 0.00% | 0.00% | 1.17% | 0.00% | desulfobacterota | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| FZ0 | FZ10 | FZ30 | FZ50 | FZS | KZ0 | KZ10 | KZ30 | KZ50 | KZS | ||
| thermobacillus | 0.00% | 0.00% | 1.62% | 2.36% | 0.00% | thermobacillus | 0.00% | 0.00% | 1.44% | 2.66% | 0.00% |
| thermobispora | 0.00% | 0.00% | 1.94% | 3.63% | 0.00% | thermobispora | 0.00% | 1.18% | 2.53% | 5.42% | 0.00% |
| thermopolyspora | 0.00% | 0.00% | 2.08% | 3.72% | 0.00% | thermopolyspora | 0.00% | 1.00% | 2.76% | 7.11% | 0.00% |
| micromonosporacease | 0.00% | 0.00% | 0.90% | 1.84% | 0.00% | micromonosporacease | 0.00% | 0.00% | 1.45% | 3.34% | 0.00% |
| desulfobacterota | 0.00% | 0.00% | 0.77% | 1.13% | 0.00% | desulfobacterota | 0.00% | 0.00% | 0.00% | 1.64% | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, L.; Wang, Z.; Sun, J.; Zhang, H. Impacts of Corn Straw Compost on Rice Growth and Soil Microflora under Saline-Alkali Stress. Agronomy 2023, 13, 1525. https://doi.org/10.3390/agronomy13061525
Li S, Li L, Wang Z, Sun J, Zhang H. Impacts of Corn Straw Compost on Rice Growth and Soil Microflora under Saline-Alkali Stress. Agronomy. 2023; 13(6):1525. https://doi.org/10.3390/agronomy13061525
Chicago/Turabian StyleLi, Shenglin, Lixin Li, Zhigang Wang, Jing Sun, and Hailong Zhang. 2023. "Impacts of Corn Straw Compost on Rice Growth and Soil Microflora under Saline-Alkali Stress" Agronomy 13, no. 6: 1525. https://doi.org/10.3390/agronomy13061525




