Bnt05G007257, a Novel NAC Transcription Factor, Predicts Developmental and Synthesis Capabilities of Fiber Cells in Ramie (Boehmeria nivea L.)
Abstract
1. Introduction
2. Results
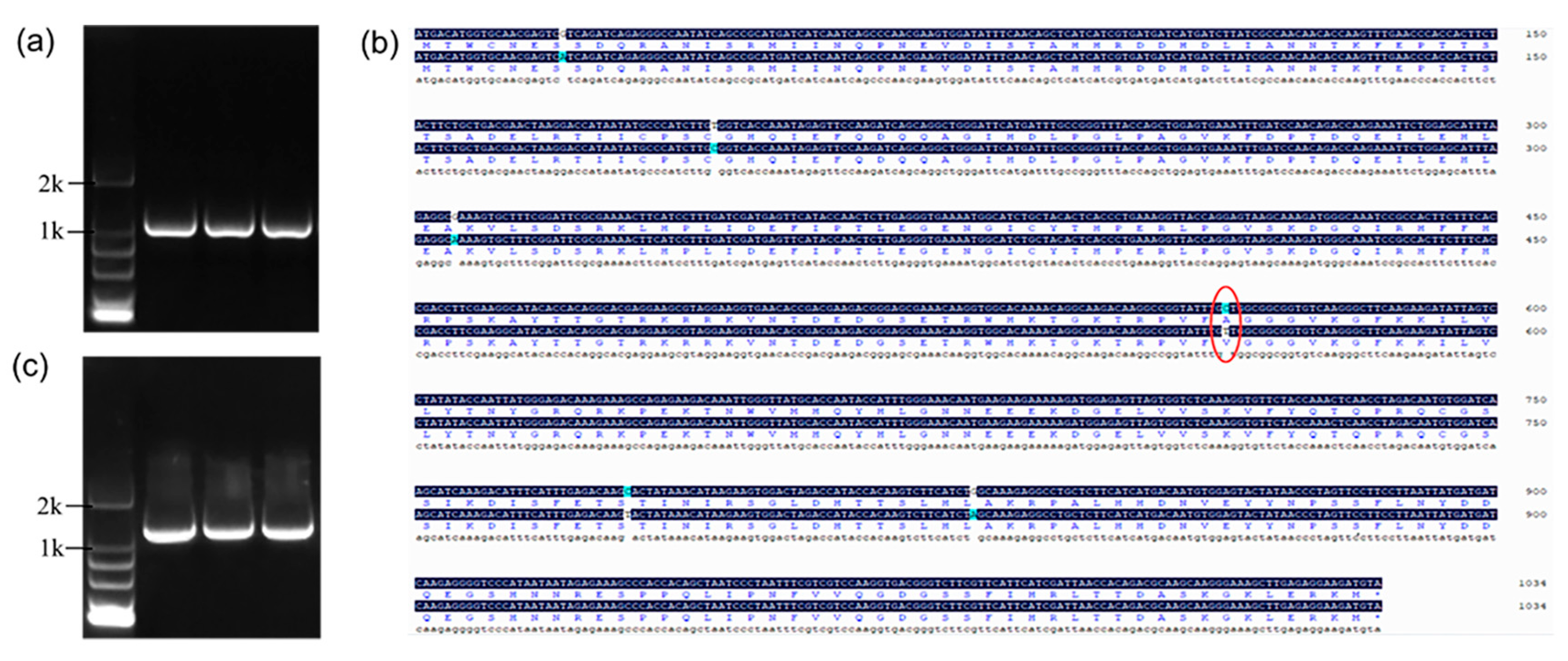
2.1. Isolation and Characterization of Bnt05G007257
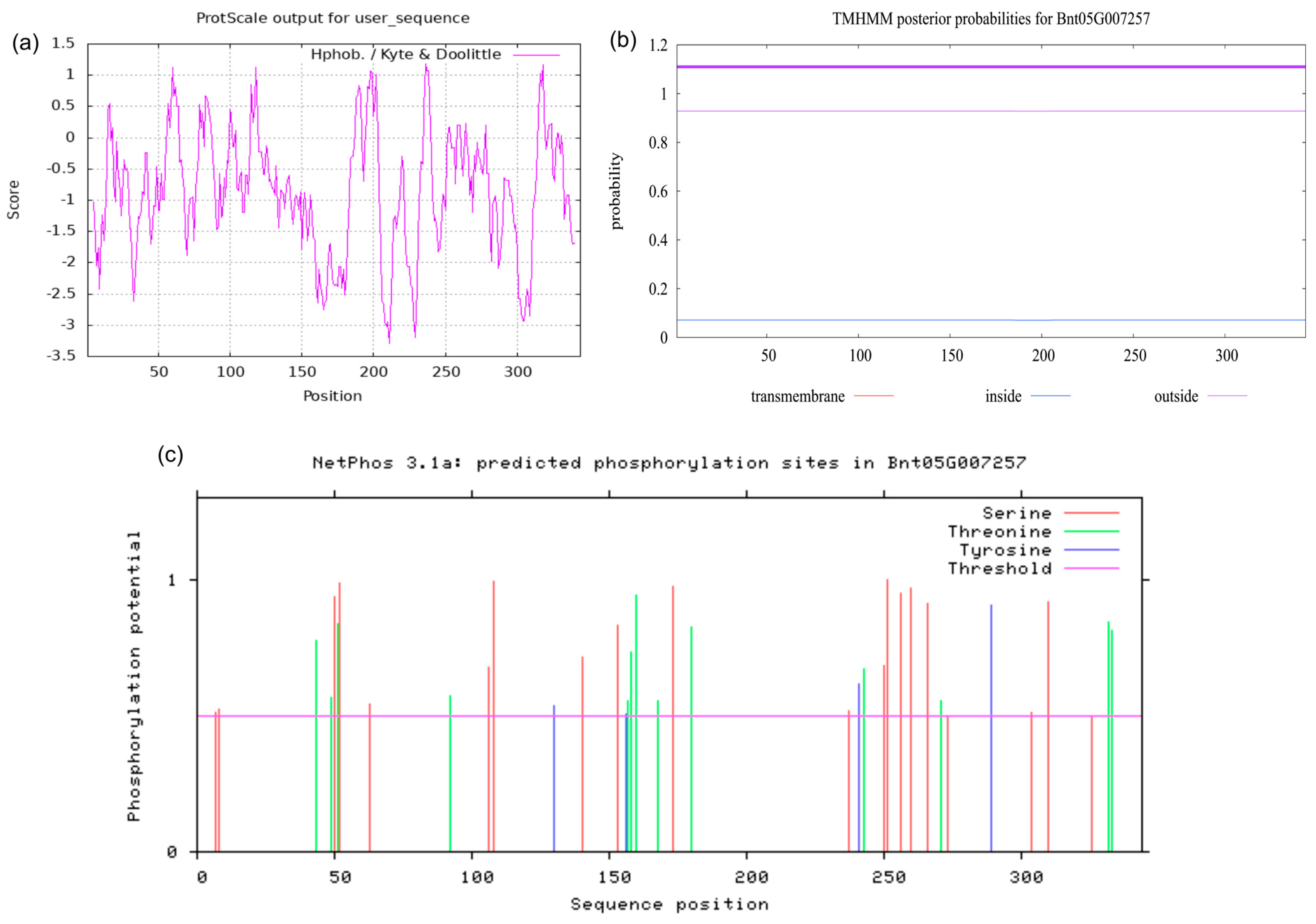
2.2. Protein Physicochemical Property Analysis
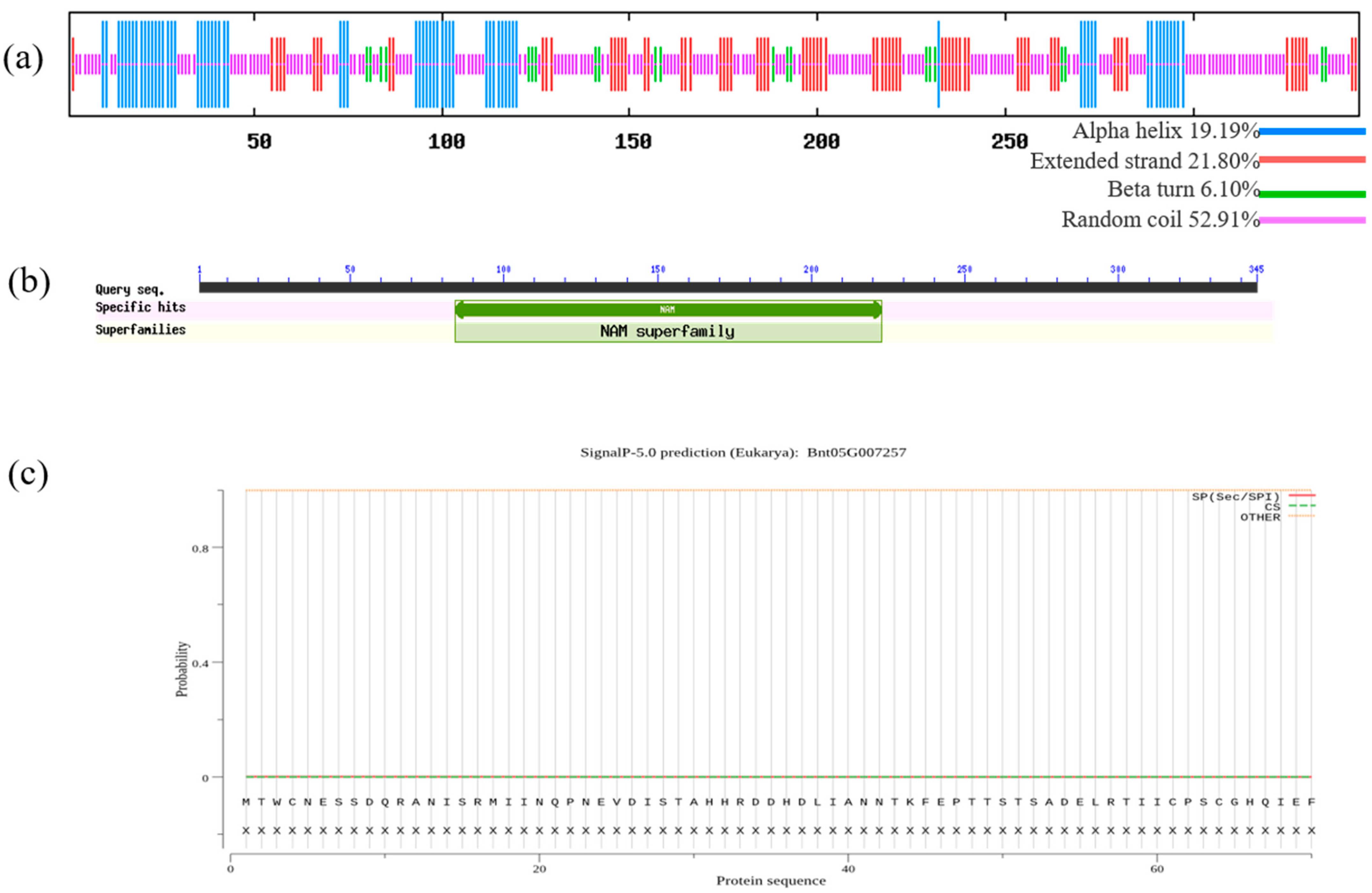
2.3. Protein Structure and Function Prediction
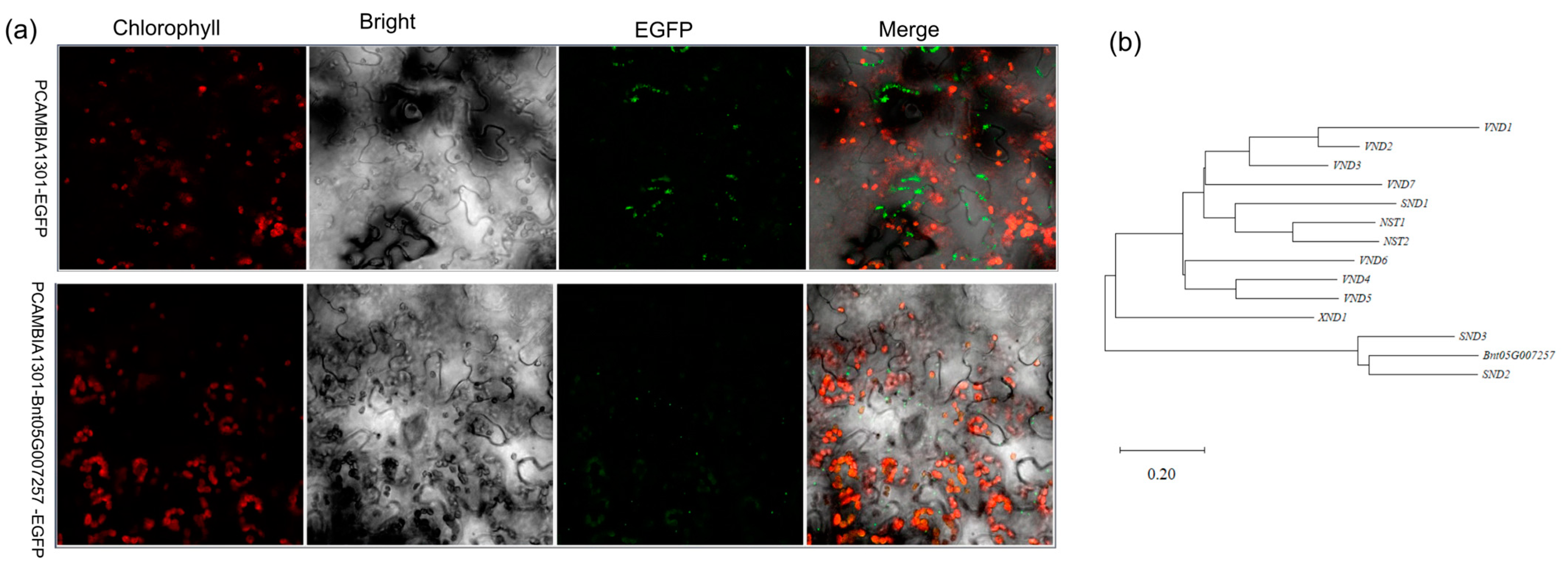
2.4. Evolutionary Analysis of Protein Homology
2.5. Subcellular Localization of Bnt05G007257
2.6. Function Validation of Bnt05G007257
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material Collection
5.2. RNA Extraction and cDNA Synthesis
5.3. Biological Information Analysis and Phylogenetic Tree Analysis
5.4. Gene Cloning and Overexpression Vector Construction
5.5. Subcellular Localization of Bnt05G007257
5.6. Molecular Validation of Transgenic Plants
5.7. Plant Guidelines Statement
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ni, J.-L.; Zhu, A.-G.; Wang, X.-F.; Xu, Y.; Sun, Z.-M.; Chen, J.-H.; Luan, M.-B. Genetic diversity and population structure of ramie (Boehmeria nivea L.). Ind. Crop. Prod. 2018, 115, 340–347. [Google Scholar] [CrossRef]
- Liu, L.-J.; Lao, C.-Y.; Zhang, N.; Chen, H.-Q.; Deng, G.; Zhu, C.; Peng, D.-X. The effect of new continuous harvest technology of ramie (Boehmeria nivea L. Gaud.) on fiber yield and quality. Ind. Crop. Prod. 2013, 44, 677–683. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Chen, L.; Abbasi, A.M.; Guo, X.; Liu, R.H. Comparative Study of Phenolic Profiles, Antioxidant and Antiproliferative Activities in Different Vegetative Parts of Ramie (Boehmeria nivea L.). Molecules 2019, 24, 1551. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Nho, J.W.; Hwang, I.G.; Kim, W.J.; Lee, Y.J.; Jeong, H.S. Chemical Composition and Antioxidant Activity of Ramie Leaf (Boehmeria nivea L.). Food Sci. Biotechnol. 2009, 18, 1096–1099. [Google Scholar]
- Liu, T.; Tang, S.; Zhu, S.; Tang, Q.; Zheng, X. Transcriptome comparison reveals the patterns of selection in domesticated and wild ramie (Boehmeria nivea L. Gaud). Plant Mol. Biol. 2014, 86, 85–92. [Google Scholar] [CrossRef]
- Mu, L.; Cai, M.; Wang, Z.; Liu, J.; Liu, T.; Wanapat, M.; Huang, B. Assessment of ramie leaf (Boehmeria nivea L. gaud) as an animal feed supplement in P.R. China. Trop. Anim. Health Prod. 2019, 52, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Mokshina, N.; Chernova, T.; Galinousky, D.; Gorshkov, O.; Gorshkova, T. Key Stages of Fiber Development as Determinants of Bast Fiber Yield and Quality. Fibers 2018, 6, 20. [Google Scholar] [CrossRef]
- Liu, D.-D.; Wang, J.-Y.; Tang, R.-J.; Chen, J.-D.; Liu, Z.; Chen, L.; Yao, M.-Z.; Ma, C.-L. Transcriptomic and Metabolomic Analyses Provide Insights into an Aberrant Tissue of Tea Plant (Camellia sinensis). Front. Plant Sci. 2021, 12, 730651. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, S.; Wang, Y.; Bai, X.; Liu, C.; Chen, J.; Zhang, T.; Wei, Y.; Li, F.; Bao, Z.; et al. Resequencing of 301 ramie accessions identifies genetic loci and breeding selection for fiber yield traits. Plant Biotechnol. J. 2022, 20, 323–334. [Google Scholar] [CrossRef]
- Chen, J.; Pei, Z.; Dai, L.; Wang, B.; Liu, L.; An, X.; Peng, D. Transcriptome profiling using pyrosequencing shows genes associated with bast fiber development in ramie (Boehmeria nivea L.). BMC Genom. 2014, 15, 919. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, S.; Fu, L.; Tang, Q.; Yu, Y.; Chen, P.; Luan, M.; Wang, C.; Tang, S. Development and Characterization of 1827 Expressed Sequence Tag-Derived Simple Sequence Repeat Markers for Ramie (Boehmeria nivea L. Gaud). PLoS ONE 2013, 8, e60346. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, F.; Xing, H.; Mao, K.; Chen, G.; Guo, Q.; Chen, J. Correlation Analysis of Lignin Accumulation and Expression of Key Genes Involved in Lignin Biosynthesis of Ramie (Boehmeria nivea). Genes 2019, 10, 389. [Google Scholar] [CrossRef]
- Nie, G.; Yang, X.; Yang, Z.; Zhong, M.; Zhu, Y.; Zhou, J.; Appiah, C.; Liao, Z.; Feng, G.; Zhang, X. Genome-wide investigation of the NAC transcript factor family in perennial ryegrass (Lolium perenne L.) and expression analysis under various abiotic stressor. Genomics 2020, 112, 4224–4231. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P.; et al. Comprehensive Analysis of NAC Family Genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Ying, L.; Jin, J.; Li, Q.; Cai, W.M. Determining the Transcriptional Regulation Pattern of PgTIP1 in Transgenic Arabidopsis Thaliana by Constructing Gene Coexpression Networks. Adv. Biosci. Biotechnol. 2010, 1, 384–390. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Wei, W.; Song, Q.-X.; Chen, H.-W.; Zhang, Y.-Q.; Wang, F.; Zou, H.-F.; Lei, G.; Tian, A.-G.; Zhang, W.-K.; et al. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011, 68, 302–313. [Google Scholar] [CrossRef]
- Hendelman, A.; Stav, R.; Zemach, H.; Arazi, T. The tomato NAC transcription factor SlNAM2 is involved in flower-boundary morphogenesis. J. Exp. Bot. 2013, 64, 5497–5507. [Google Scholar] [CrossRef]
- Ma, N.; Feng, H.; Meng, X.; Li, D.; Yang, D.; Wu, C.; Meng, Q. Overexpression of tomato SlNAC1transcription factor alters fruit pigmentation and softening. BMC Plant Biol. 2014, 14, 351. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Tanaka, T.; Ogiwara, I.; Kanekatsu, M.; van Doorn, W.G.; Yamada, T. Expression of an AtNAP gene homolog in senescing morning glory (Ipomoea nil) petals of two cultivars with a different flower life span. J. Plant Physiol. 2014, 171, 633–638. [Google Scholar] [CrossRef]
- Chai, M.; Bellizzi, M.; Wan, C.; Cui, Z.; Li, Y.; Wang, G.-L. The NAC transcription factor OsSWN1 regulates secondary cell wall development in Oryza sativa. J. Plant Biol. 2015, 58, 44–51. [Google Scholar] [CrossRef]
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.A.; Ohtani, M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front. Plant Sci. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; He, Q.; Bao, Z.; Zeng, Z.; An, D.; Zhang, T.; Yan, L.; Wang, H.; Zhu, S.; et al. Genomic analyses provide comprehensive insights into the domestication of bast fiber crop ramie (Boehmeria nivea). Plant J. 2021, 107, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kudapa, H.; Garg, V.; Varshney, R.K. Comprehensive analysis and identification of drought-responsive candidate NAC genes in three semi-arid tropics (SAT) legume crops. BMC Genom. 2021, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A New Tomato NAC (NAM/ATAF1/2/CUC2) Transcription Factor, SlNAC4, Functions as a Positive Regulator of Fruit Ripening and Carotenoid Accumulation. Plant Cell Physiol. 2014, 55, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Greve, K.; La Cour, T.; Jensen, M.K.; Poulsen, F.M.; Skriver, K. Interactions between plant RING-H2 and plant-specific NAC (NAM/ATAF1/2/CUC2) proteins: RING-H2 molecular specificity and cellular localization. Biochem. J. 2003, 371 Pt 1, 97–108. [Google Scholar] [CrossRef]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Genome-Wide Survey and Expression Analysis of the Plant-Specific NAC Transcription Factor Family in Soybean during Development and Dehydration Stress. DNA Res. 2011, 18, 263–276. [Google Scholar] [CrossRef]
- Shan, W.; Kuang, J.-F.; Chen, L.; Xie, H.; Peng, H.-H.; Xiao, Y.-Y.; Li, X.-P.; Chen, W.-X.; He, Q.-G.; Chen, J.-Y.; et al. Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J. Exp. Bot. 2012, 63, 5171–5187. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.S.P.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004, 39, 863–876. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Ye, Z.-H. Evolutionary conservation of the transcriptional network regulating secondary cell wall biosynthesis. Trends Plant Sci. 2010, 15, 625–632. [Google Scholar] [CrossRef]
- Zhao, Q.; Gallego-Giraldo, L.; Wang, H.; Zeng, Y.; Ding, S.-Y.; Chen, F.; Dixon, R.A. An NAC transcription factor orchestrates multiple features of cell wall development in Medicago truncatula. Plant J. 2010, 63, 100–114. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; McCarthy, R.L.; Reeves, C.K.; Jones, E.G.; Ye, Z.-H. Transcriptional Activation of Secondary Wall Biosynthesis by Rice and Maize NAC and MYB Transcription Factors. Plant Cell Physiol. 2011, 52, 1856–1871. [Google Scholar] [CrossRef]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, S.; Zhang, B.; Shang-Guan, K.; Shi, Y.; Zhang, D.; Liu, X.; Wu, K.; Xu, Z.; Fu, X.; et al. A Gibberellin-Mediated DELLA-NAC Signaling Cascade Regulates Cellulose Synthesis in Rice. Plant Cell 2015, 27, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Demura, T.; Ye, Z.H. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 2006, 18, 3158–3170. [Google Scholar] [CrossRef]
- Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. The NAC Transcription Factors NST1 and NST2 of Arabidopsis Regulate Secondary Wall Thickenings and Are Required for Anther Dehiscence. Plant Cell 2005, 17, 2993–3006. [Google Scholar] [CrossRef]
- Ko, J.-H.; Yang, S.H.; Park, A.H.; Lerouxel, O.; Han, K.-H. ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana. Plant J. 2007, 50, 1035–1048. [Google Scholar] [CrossRef]
- Sundell, D.; Street, N.R.; Kumar, M.; Mellerowicz, E.J.; Kucukoglu, M.; Johnsson, C.; Kumar, V.; Mannapperuma, C.; Delhomme, N.; Nilsson, O.; et al. Aspwood: High-spatial-resolution transcriptome profiles reveal uncharacterized modularity of wood formation inpopulus tremula. Plant Cell 2017, 29, 1585–1604. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.-H. A Battery of Transcription Factors Involved in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef]
- Zhong, R.; McCarthy, R.L.; Lee, C.; Ye, Z.-H. Dissection of the Transcriptional Program Regulating Secondary Wall Biosynthesis during Wood Formation in Poplar. Plant Physiol. 2011, 157, 1452–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Lee, C.; Haghighat, M.; Ye, Z.H. Xylem vessel-specific SND5 and its homologs regulate second-ary wall biosynthesis through activating secondary wall NAC binding elements. New Phytol. 2021, 231, 1496–1509. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
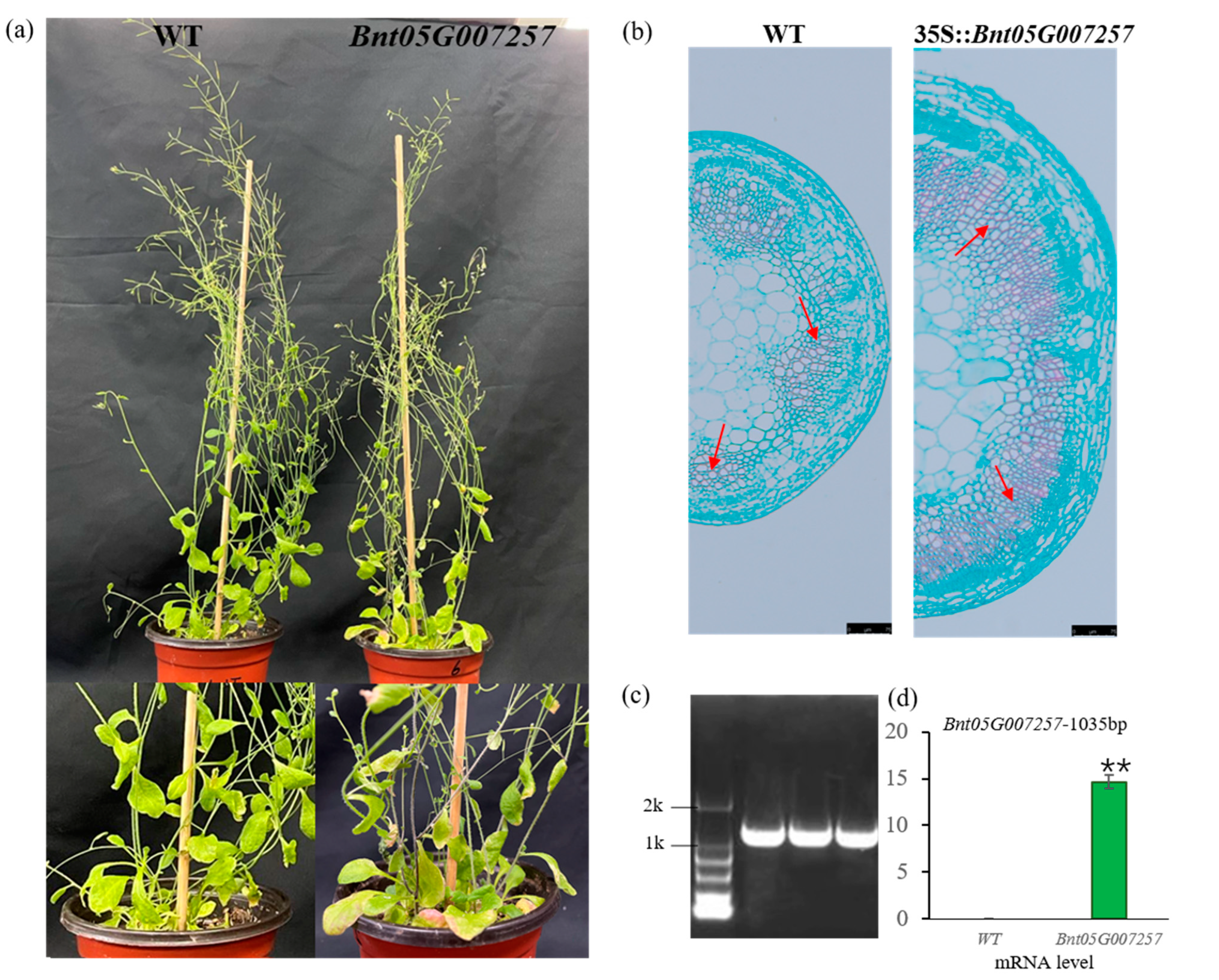
| Samples | Radial Width of Fiber Cells (μm) | Fiber Cell Wall Thickness (μm) |
|---|---|---|
| WT | 9.93 ± 3.35 * | 1.27 ± 0.35 ** |
| 35S:Bnt05G007257 | 12.09 ± 1.96 * | 1.67 ± 0.40 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Fu, Y.; Wang, X.; Chen, G.; Wang, Y.; Liu, T.; Li, G.; Zhu, S. Bnt05G007257, a Novel NAC Transcription Factor, Predicts Developmental and Synthesis Capabilities of Fiber Cells in Ramie (Boehmeria nivea L.). Agronomy 2023, 13, 1575. https://doi.org/10.3390/agronomy13061575
Bai X, Fu Y, Wang X, Chen G, Wang Y, Liu T, Li G, Zhu S. Bnt05G007257, a Novel NAC Transcription Factor, Predicts Developmental and Synthesis Capabilities of Fiber Cells in Ramie (Boehmeria nivea L.). Agronomy. 2023; 13(6):1575. https://doi.org/10.3390/agronomy13061575
Chicago/Turabian StyleBai, Xuehua, Yafen Fu, Xin Wang, Guangyao Chen, Yanzhou Wang, Tongying Liu, Guang Li, and Siyuan Zhu. 2023. "Bnt05G007257, a Novel NAC Transcription Factor, Predicts Developmental and Synthesis Capabilities of Fiber Cells in Ramie (Boehmeria nivea L.)" Agronomy 13, no. 6: 1575. https://doi.org/10.3390/agronomy13061575
APA StyleBai, X., Fu, Y., Wang, X., Chen, G., Wang, Y., Liu, T., Li, G., & Zhu, S. (2023). Bnt05G007257, a Novel NAC Transcription Factor, Predicts Developmental and Synthesis Capabilities of Fiber Cells in Ramie (Boehmeria nivea L.). Agronomy, 13(6), 1575. https://doi.org/10.3390/agronomy13061575







