Comprehensive Evaluation and Physiological Response of Quinoa Genotypes to Low Nitrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Comprehensive Screening of Quinoa Genotypes using Low Nitrogen
2.1.1. Genotypes and Treatments
2.1.2. Observations
2.2. Application of Three Nitrogen Levels to Selected Quinoa Genotypes
2.3. Measurement of Morphological Indices
2.4. Measurement of Root Vitality
2.5. Measurement of Physiological Traits
2.6. Activities of Nitrogen Metabolizing Enzymes
2.7. Antioxidant Enzyme Activities and Measurement of Lipid Peroxidation
2.8. Nitrogen Use Efficiency
2.9. Relative Value of Indices and Comprehensive Value of Genotypes
2.10. Statistical Analysis
3. Results
3.1. Comprehensive Evaluation of Low-N Tolerance of Quinoa Genotypes
3.2. Morphological Indices of Quinoa Genotypes
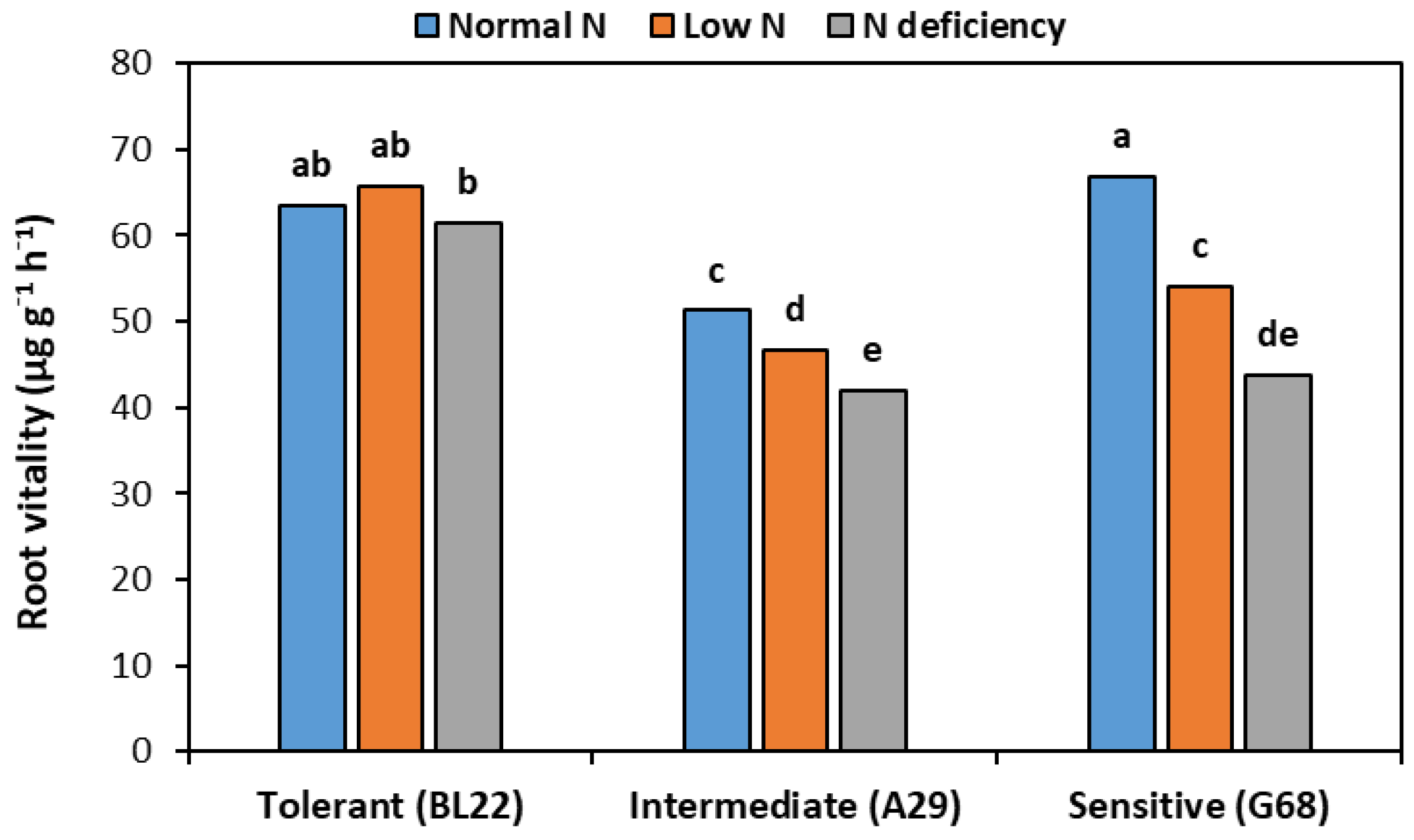
3.3. Root Vitality
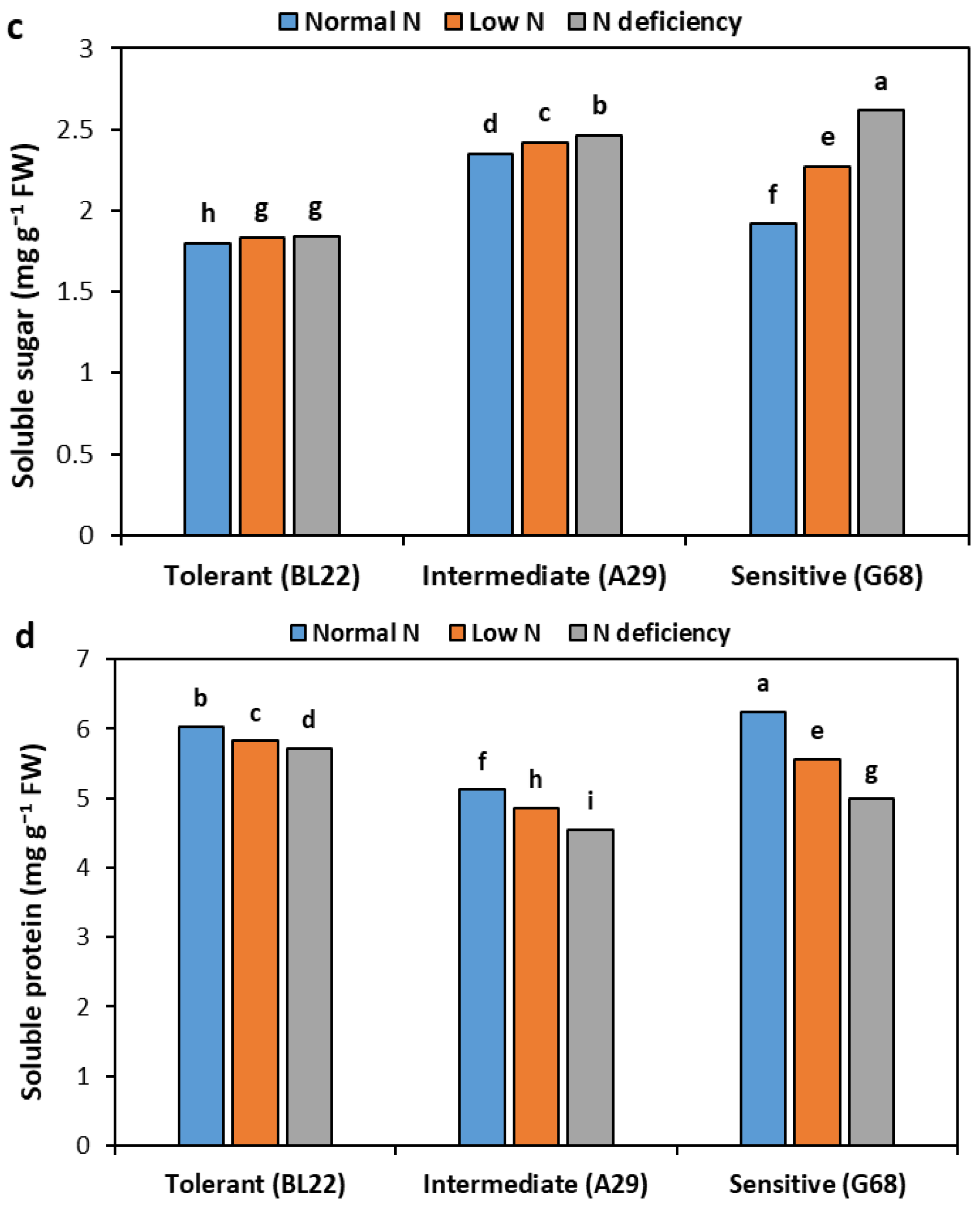
3.4. Metabolites
3.5. Antioxidant Enzyme Activities and Lipid Peroxidation
3.6. Nitrogen-Metabolizing Enzymes
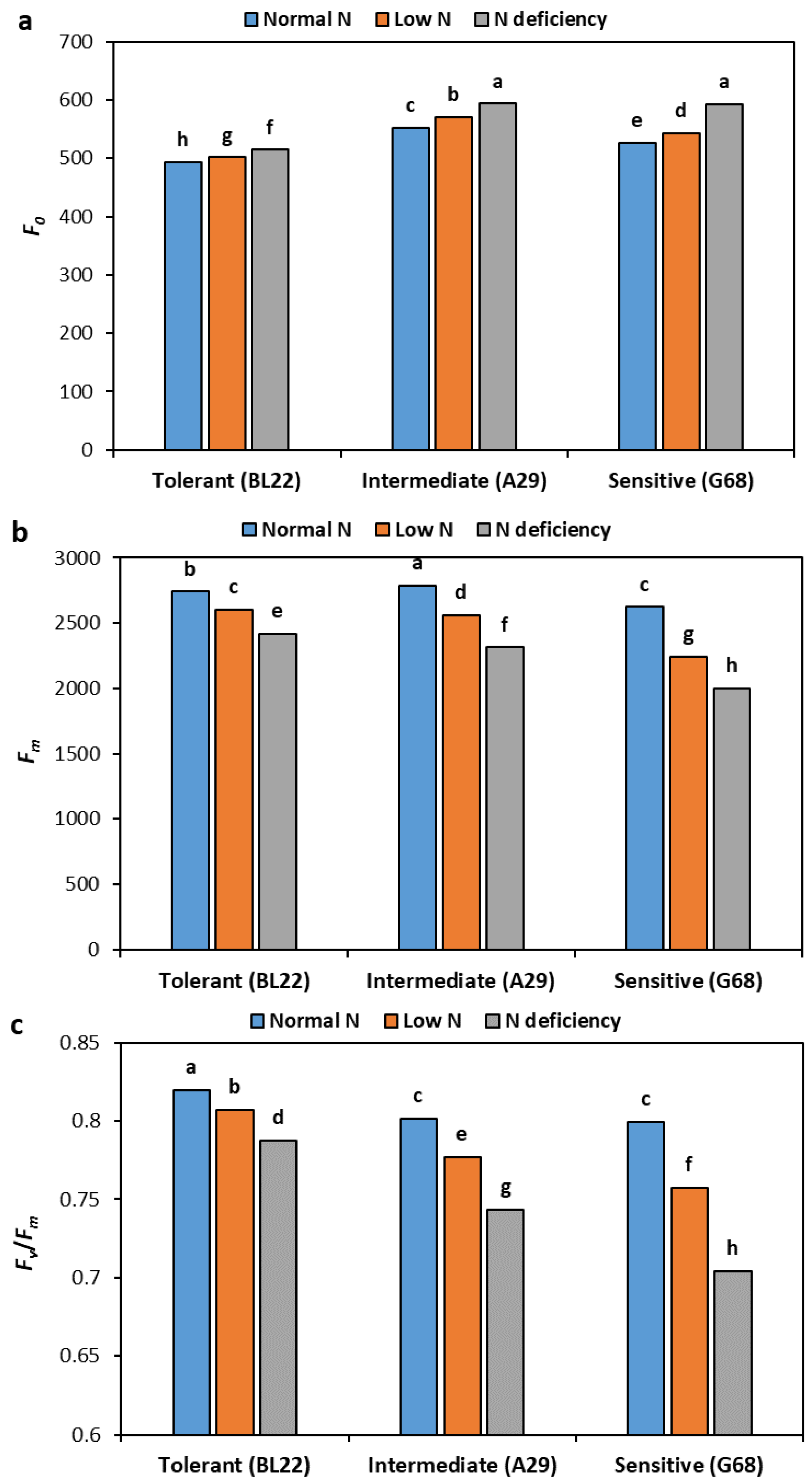
3.7. Chlorophyll Fluorescence
3.8. Nitrogen Accumulation, Nitrogen Content, and Use Efficiency
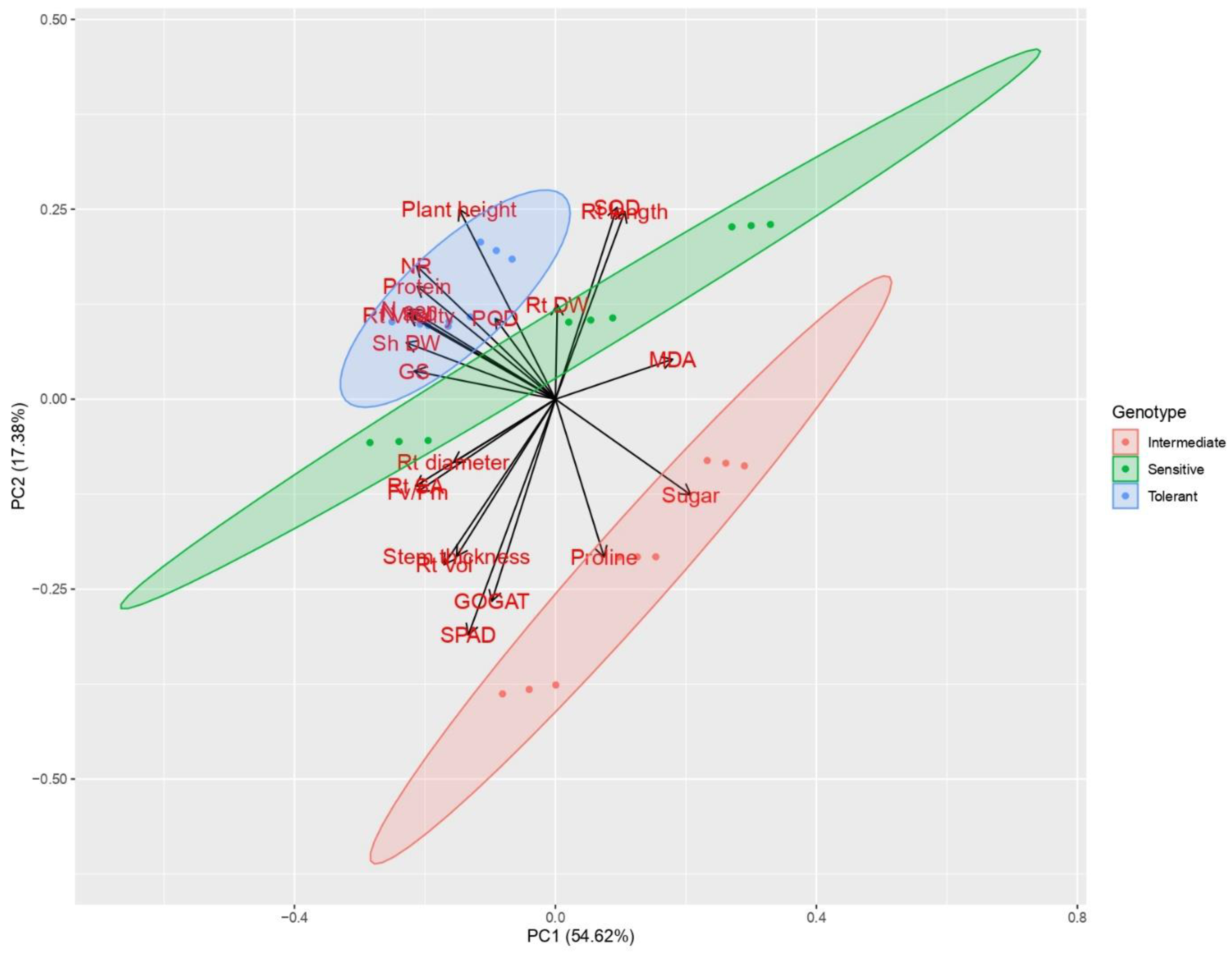
3.9. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Gong, X.; Wang, H.; Dang, K.; Deng, X.; Feng, B. Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling. Plant Physiol. Biochem. 2020, 151, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Zhang, L.; Gao, J.; Zhang, W.; Yi, J.; Zhen, X.; Bi, C.; He, D.; Liu, S.; Zhao, X. Adaptation mechanism of roots to low and high nitrogen revealed by proteomic analysis. Rice 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner's Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 191–248. [Google Scholar]
- Decouard, B.; Bailly, M.; Rigault, M.; Marmagne, A.; Arkoun, M.; Soulay, F.; Caïus, J.; Paysant-Le Roux, C.; Louahlia, S.; Jacquard, C.; et al. Genotypic variation of nitrogen use efficiency and amino acid metabolism in barley. Front. Plant Sci. 2022, 12, 807798. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.H.; Gruber, B.D.; von Wirén, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Shi, F.; Li, W.; Zhong, M.; Li, C.; Chen, S. Comprehensive screening of low nitrogen tolerant maize based on multiple traits at the seedling stage. PeerJ 2022, 10, e14218. [Google Scholar] [CrossRef]
- Tyagi, B.S.; Foulkes, J.; Singh, G.; Sareen, S.; Kumar, P.; Broadley, M.; Gupta, V.; Krishnappa, G.; Ojha, A.; Khokhar, J.; et al. Identification of wheat cultivars for low nitrogen tolerance using multivariable screening approaches. Agronomy 2020, 10, 417. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Das, B.; Cairns, J.E.; Vinayan, M.T.; Tarekegne, A.T.; Magorokosho, C.; Zaidi, P.H.; Seetharam, K. Phenotyping for Abiotic Stress Tolerance in Maize Low Nitrogen Stress: A Field Manual; CIMMYT: Harare, Zimbabwe, 2018. [Google Scholar]
- Du, Q.G.; Juan, Y.A.N.G.; Sadiq, S.S.M.; Yang, R.X.; Yu, J.J.; Li, W.X. Comparative transcriptome analysis of different nitrogen responses in low-nitrogen sensitive and tolerant maize genotypes. J. Integ. Agric. 2021, 20, 2043–2055. [Google Scholar] [CrossRef]
- Sharma, N.; Sinha, V.B.; Gupta, N.; Rajpal, S.; Kuchi, S.; Sitaramam, V.; Parsad, R.; Raghuram, N. Phenotyping for nitrogen use efficiency: Rice genotypes differ in N-responsive germination, oxygen consumption, seed urease activities, root growth, crop duration, and yield at low N. Front. Plant Sci. 2018, 9, 1452. [Google Scholar] [CrossRef]
- Ajmera, I.; Henry, A.; Radanielson, A.M.; Klein, S.P.; Ianevski, A.; Bennett, M.J.; Band, L.R.; Lynch, J.P. Integrated root phenotypes for improved rice performance under low nitrogen availability. Plant Cell Environ. 2022, 45, 805–822. [Google Scholar] [CrossRef]
- Shah, J.M.; Asgher, Z.; Zeng, J.; Quan, X.; Ali, E.; Shamsi, I.H.; Zhang, G. Growth and physiological characterization of low nitrogen responses in Tibetan wild barley (Hordeum spontaneum) and cultivated barley (Hordeum vulgare). J. Plant Nutr. 2017, 40, 861–868. [Google Scholar] [CrossRef]
- Zhang, H.; FU, X.; Wang, X.; Gui, H.; Dong, Q.; Pang, N.; Wang, Z.; Zhang, X.; Song, M. Identification and screening of nitrogen-efficient cotton genotypes under low and normal nitrogen environments at the seedling stage. J. Cotton Res. 2018, 1, 6. [Google Scholar] [CrossRef]
- Saudy, H.S.; El–Samad, G.A.A.; El–Temsah, M.E.; El–Gabry, Y.A.E.G. Effect of Iron, Zinc, and Manganese Nano-Form Mixture on the Micronutrient Recovery Efficiency and Seed Yield Response Index of Sesame Genotypes. J. Soil Sci. Plant Nutr. 2022, 22, 732–742. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Sanhueza, C.; Pinto, K.; Cifuentes, L.; Reguera, M.; Briones, V.; Zurita-Silva, A.; Álvarez, R.; Morales, A.; Silva, H. Nitrogen physiology of contrasting genotypes of Chenopodium quinoa Willd. (Amaranthaceae). Sci. Rep. 2018, 8, 17524. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Gracia-Romero, A.; Vergara-Diaz, O.; Zaman-Allah, M.A.; Tarekegne, A.; Cairns, J.E.; Prasanna, B.M.; Araus, J.L.; Kefauver, S.C. Evaluating maize genotype performance under low nitrogen conditions using RGB UAV phenotyping techniques. Sensors 2019, 19, 1815. [Google Scholar] [CrossRef] [PubMed]
- Debaeke, P.; Route, P.; Justes, E. Relationship between the normalize SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for marginal environments: Toward future food and nutritional security in MENA and Central Asia regions. Front. Plant Sci. 2016, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Juan-Ling, W.; Anwar, S.; Chuang-Yun, W.; Li, Z.; Li-Guang, Z.; Hong-Xia, G.; Li-Xia, Q.; Hua, L.; Mei-Xia, W. Phenology, lodging and yield traits of Chenopodium quinoa under the effect of planting density and row spacings. Fresenius Environ. Bull. 2021, 30, 11757–11767. [Google Scholar]
- Alvar-Beltrán, J.; Verdi, L.; Dalla Marta, A.; Dao, A.; Vivoli, R.; Sanou, J.; Orlandini, S. The effect of heat stress on quinoa (cv. Titicaca) under controlled climatic conditions. J. Agric. Sci. 2020, 158, 255–261. [Google Scholar] [CrossRef]
- Geren, H. Effects of different nitrogen levels on the grain yield and some yield components of quinoa (Chenopodium quinoa Willd.) under Mediterranean climatic conditions. Turk. J. Field Crops 2015, 20, 59–64. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, L.; Anwar, S.; Zhang, L.G.; Shafiq, F.; Guo, H.X.; Qin, L.X.; Wang, M.X.; Wang, C.Y. Phosphorus fertigation conferred lodging tolerance and improved grain quality in Chenopodium quinoa via enhanced root proliferation and stalk strength. J. Soil Sci. Plant Nutr. 2022, 22, 5099–5110. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Davis, K.E.; Patterson, C.; Oo, S.; Liu, W.; Liu, J.; Wang, G.; Fontana, J.E.; Thornburg, T.E.; et al. Response of root growth and development to nitrogen and potassium deficiency as well as microRNA-mediated mechanism in peanut (Arachis hypogaea L.). Front. Plant Sci. 2021, 12, 695234. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.K.; Lim, H.M.; Oh, K.H.; Yang, H.J.; Jeong, J.S.; Kim, S.K. Interpretation of protein quantitation using the Bradford assay: Comparison with two calculation models. Anal. Biochem. 2013, 434, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K. Analytical Techniques in Biochemistry and Molecular Biology; (No. PA572. 0724 R16.); Springer: Berlin/Heidelberg, Germany, 2011; p. 180. [Google Scholar]
- Gillikin, J.W.; Graham, J.S. Purification and developmental analysis of the major anionic peroxidase from the seed coat of Glycine max. Plant Physiol. 1991, 96, 214–220. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Bremner, J.M.; Edwards, A.P. Determination and isotope-ratio analysis of different forms of nitrogen in soils: I. Apparatus and procedure for distillation and determination of ammonium. Soil Sci. Soc. Am. J. 1965, 29, 504–507. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass, A.D.M. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]
- Dong, G.; Chen, C.; Wu, G. A Method of Determining Membership Function in Fuzzy Comprehensive Evaluation. In Emerging Trends in Intelligent and Interactive Systems and Applications, Proceedings of the 5th International Conference on Intelligent, Interactive Systems and Applications (IISA2020), Shanghai, China, 25–27 September 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 39–46. [Google Scholar]
- Jiang, Q.; Chen, Z.; Liu, C.; He, T.; Guo, G.; Gao, R.; Xu, H.; Li, Y.; Lu, R.; Huang, J. Screening and identification indices of low-nitrogen tolerance for barley landraces at seedling stage. Acta Agric. Boreali Sin. 2019, 34, 148–155. [Google Scholar]
- Berti, M.; Wilckens, R.; Hevia, F.; Serri, H.; Vidal, I.; Mendez, C. Nitrogen fertilization in quinoa (Chenopodium quinoa Willd). Fertilizacion nitrogenada en quinoa (Chenopodium quinoa Willd). Cien. Investig. Agrar. 2000, 27, 81–90. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Vadez, V. Physiological traits for crop yield improvement in low N and P environments. Plant Soil 2002, 245, 1–15. [Google Scholar] [CrossRef]
- Jia, Z.; von Wirén, N. Signaling pathways underlying nitrogen-dependent changes in root system architecture: From model to crop species. J. Exp. Bot. 2020, 71, 4393–4404. [Google Scholar] [CrossRef] [PubMed]
- Saudy, H.S.; Mohamed El–Metwally, I. Effect of Irrigation, Nitrogen Sources, and Metribuzin on Performance of Maize and Its Weeds. Commun Soil. Sci. Plant Anal. 2023, 54, 22–35. [Google Scholar] [CrossRef]
- Lutze, J.L.; Gifford, R.M. Acquisition and allocation of carbon and nitrogen by Danthonia richardsonii in response to restricted nitrogen supply and CO2 enrichment. Plant Cell Environ. 1998, 21, 1133–1141. [Google Scholar] [CrossRef]
- Ye, X.; Hong, J.; Shi, L.; Xu, F. Adaptability mechanism of nitrogen-efficient germplasm of natural variation to low nitrogen stress in Brassica napus. J. Plant Nutr. 2010, 33, 2028–2040. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef]




| Index | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Soluble protein content | 0.711 | −0.288 | 0.243 | −0.382 |
| Soluble sugar content | −0.837 | −0.133 | −0.296 | 0.393 |
| MDA content | −0.869 | 0.419 | 0.114 | 0.16 |
| NR activity | 0.930 | −0.095 | −0.157 | −0.176 |
| Proline content | 0.717 | 0.412 | −0.347 | 0.01 |
| POD activity | 0.803 | 0.476 | −0.325 | −0.03 |
| SOD activity | 0.403 | 0.826 | −0.26 | 0.19 |
| Root vitality | 0.757 | −0.167 | −0.548 | −0.207 |
| GS activity | 0.618 | 0.326 | 0.395 | −0.285 |
| GOGT activity | 0.085 | −0.853 | 0.296 | 0.315 |
| Plant height | 0.507 | −0.126 | 0.598 | 0.531 |
| Stem thickness | 0.494 | 0.468 | 0.579 | 0.239 |
| SPAD | 0.523 | −0.076 | 0.748 | 0.188 |
| F0 | −0.839 | −0.185 | −0.151 | −0.425 |
| Fm | 0.304 | 0.343 | 0.489 | −0.698 |
| Fv/Fm | 0.815 | 0.406 | 0.362 | 0.029 |
| Maximum root length | −0.85 | 0.079 | 0.021 | 0.359 |
| Average root diameter | −0.274 | −0.927 | 0.169 | −0.073 |
| Root surface area | 0.366 | 0.37 | −0.425 | 0.567 |
| Root volume | 0.578 | −0.388 | −0.277 | 0.145 |
| Root dry weight | −0.903 | 0.219 | −0.078 | −0.253 |
| Stem and leaf dry weight | 0.244 | −0.196 | −0.3 | −0.415 |
| Root-to-shoot ratio | −0.856 | 0.289 | 0.111 | 0.042 |
| Total nitrogen content | 0.886 | −0.285 | −0.149 | 0.176 |
| Nitrogen accumulation | 0.910 | −0.312 | −0.204 | 0.085 |
| Nitrogen use efficiency | −0.878 | 0.291 | 0.196 | −0.188 |
| Cumulative contribution rate | 47.463 | 65.91 | 75.976 | 83.959 |
| Quinoa Genotypes | FAC1 | FAC2 | FAC3 | FAC4 | D-Value | Sorting |
|---|---|---|---|---|---|---|
| HL58 | 0.043 | −0.166 | −2.285 | −1.087 | 0.413 | 7 |
| G68 | −1.492 | −1.838 | 0.258 | 0.564 | 0.204 | 9 |
| A29 | 0.027 | 0.316 | −0.039 | 0.944 | 0.615 | 5 |
| G36 | 0.092 | 1.361 | 0.548 | −0.717 | 0.655 | 4 |
| BL23 | 0.5 | −0.638 | −0.065 | −0.434 | 0.592 | 6 |
| A86 | −1.655 | 0.995 | 0.747 | −0.638 | 0.324 | 8 |
| HL93 | 0.914 | −0.446 | 0.751 | −0.914 | 0.699 | 2 |
| BL77 | 0.191 | 0.901 | −0.757 | 1.869 | 0.682 | 3 |
| BL22 | 1.38 | −0.483 | 0.842 | 0.413 | 0.835 | 1 |
| Weights | 0.558 | 0.194 | 0.14 | 0.108 |
| N Levels | Low N Tolerance (Varieties) | Plant Height (cm) | Stem Diameter (mm) | Aboveground (Stem + Leaves) DW (g) | Root DW (g) | Maximum Root Length (cm) | Root Diameter (mm) | Root Surface Area | Root Volume |
|---|---|---|---|---|---|---|---|---|---|
| Normal N | - | 24.83 a | 5.61 a | 1.44 a | 0.197 c | 11.02 c | 0.250 a | 46.93 a | 0.548 a |
| Low N | - | 20.65 b | 5.43 b | 1.37 b | 0.201 b | 11.27 b | 0.237 b | 44.74 b | 0.523 b |
| N deficiency | - | 19.74 c | 5.26 c | 1.28 c | 0.211 a | 11.56 a | 0.228 c | 42.54 c | 0.506 c |
| - | Tolerant (BL22) | 22.85 b | 5.60 a | 1.48 a | 0.217 a | 11.25 b | 0.263 a | 45.72 a | 0.525 ab |
| - | Intermediate (A29) | 15.93 c | 5.55 b | 1.27 c | 0.203 b | 11.15 c | 0.241 b | 44.09 b | 0.528 a |
| - | Sensitive (G68) | 24.09 a | 5.14 c | 1.34 b | 0.188 c | 11.44 a | 0.212 c | 44.40 b | 0.524 b |
| Interaction | |||||||||
| Normal N | Tolerant (BL22) | 23.49 b | 5.68 b | 1.50 a | 0.218 a | 11.24 d | 0.270 a | 46.33 b | 0.537 c |
| Intermediate (A29) | 17.12 f | 5.75 a | 1.35 d | 0.197 d | 10.82 g | 0.256 c | 46.76 b | 0.549 b | |
| Sensitive (G68) | 26.84 a | 5.40 d | 1.47 b | 0.175 f | 10.99 f | 0.225 e | 47.69 a | 0.559 a | |
| Low N | Tolerant (BL22) | 22.79 c | 5.61 b | 1.49 ab | 0.212 b | 11.12 de | 0.260 b | 46.33 b | 0.522 de |
| Intermediate (A29) | 15.51 g | 5.52 c | 1.29 e | 0.202 c | 11.10 ef | 0.239 d | 43.97 c | 0.523 d | |
| Sensitive (G68) | 23.66 b | 5.16 e | 1.34 d | 0.188 e | 11.59 b | 0.212 f | 43.91 c | 0.523 d | |
| N deficiency | Tolerant (BL22) | 22.27 d | 5.52 c | 1.45 c | 0.221 a | 11.39 c | 0.258 bc | 44.49 c | 0.515 ef |
| Intermediate (A29) | 15.17 g | 5.39 d | 1.18 g | 0.211 b | 11.54 b | 0.228 e | 41.54 d | 0.513 f | |
| Sensitive (G68) | 21.78 e | 4.87 f | 1.21 f | 0.201 c | 11.73 a | 0.199 g | 41.59 d | 0.489 g | |
| ANOVA | Varieties | 4318.2 *** | 358.1 *** | 828.2 *** | 815.0 *** | 32.9 *** | 1481.7 *** | 59.9 *** | 3.72 * |
| (F-value) | N levels | 437.0 *** | 176.0 *** | 489.1 *** | 204.2 *** | 115.0 *** | 279.2 *** | 387.3 *** | 262.0 *** |
| Var × N levels | 79.7 *** | 17.0 *** | 67.0 *** | 52.3 *** | 23.6 *** | 16.14 *** | 40.3 *** | 28.6 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Sun, X.; Zhang, Q.; Anwar, S.; Lu, J.; Guo, H.; Qin, L.; Zhang, L.; Wang, C. Comprehensive Evaluation and Physiological Response of Quinoa Genotypes to Low Nitrogen. Agronomy 2023, 13, 1597. https://doi.org/10.3390/agronomy13061597
Deng Y, Sun X, Zhang Q, Anwar S, Lu J, Guo H, Qin L, Zhang L, Wang C. Comprehensive Evaluation and Physiological Response of Quinoa Genotypes to Low Nitrogen. Agronomy. 2023; 13(6):1597. https://doi.org/10.3390/agronomy13061597
Chicago/Turabian StyleDeng, Yan, Xiaojing Sun, Qi Zhang, Sumera Anwar, Jingying Lu, Hongxia Guo, Lixia Qin, Liguang Zhang, and Chuangyun Wang. 2023. "Comprehensive Evaluation and Physiological Response of Quinoa Genotypes to Low Nitrogen" Agronomy 13, no. 6: 1597. https://doi.org/10.3390/agronomy13061597
APA StyleDeng, Y., Sun, X., Zhang, Q., Anwar, S., Lu, J., Guo, H., Qin, L., Zhang, L., & Wang, C. (2023). Comprehensive Evaluation and Physiological Response of Quinoa Genotypes to Low Nitrogen. Agronomy, 13(6), 1597. https://doi.org/10.3390/agronomy13061597




