Deciphering the Interactions in the Root–Soil Nexus Caused by Urease and Nitrification Inhibitors: A Review
Abstract
:1. Introduction
2. Nitrogen as an Important Plant Nutrient
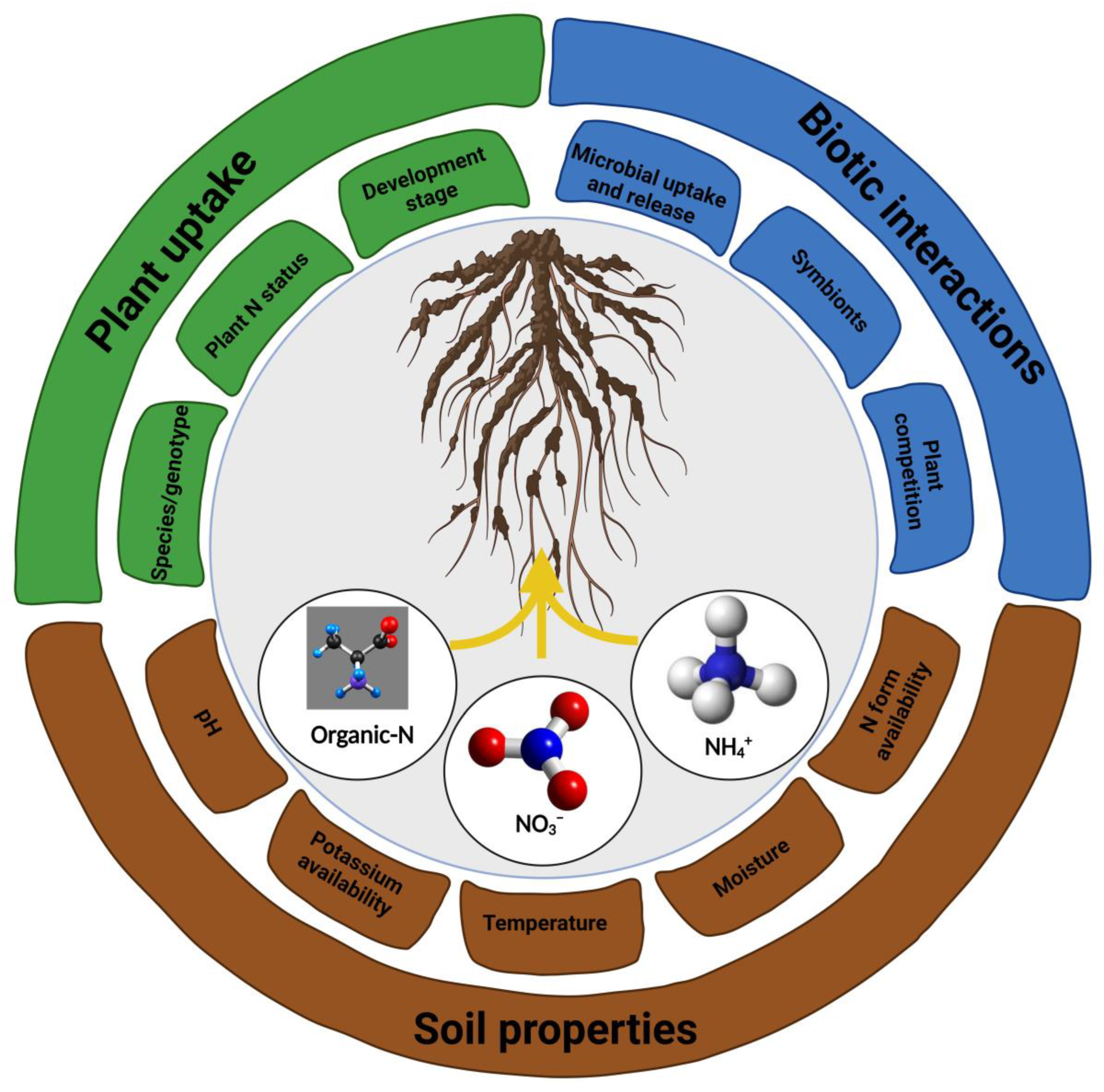
2.1. Acquisition and Assimilation of N by Plants
2.2. Plants Influence Microbial N Transformations in Soils
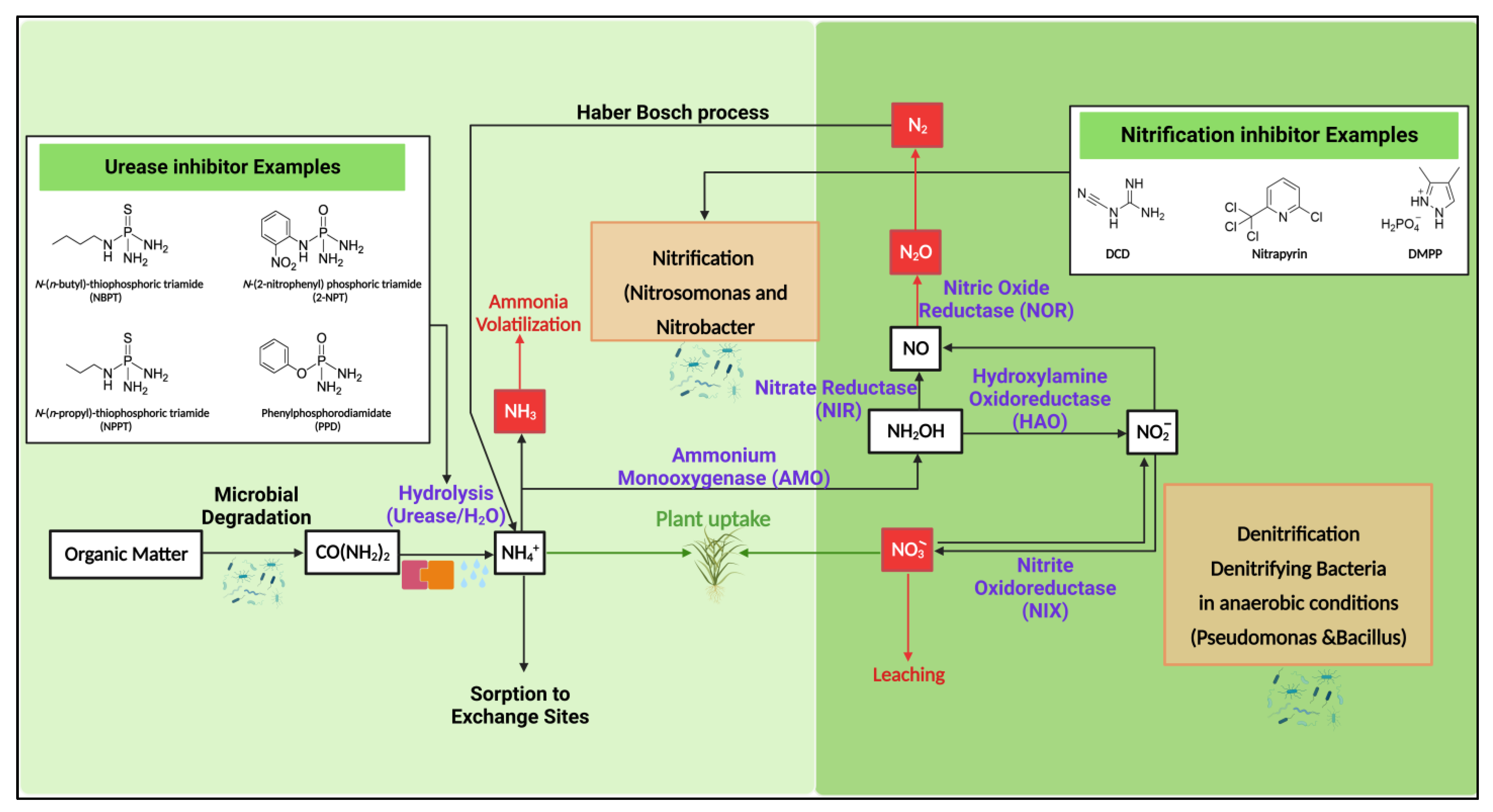
3. Influence of Soil Nitrogen Conversions on Nr Loss Control
4. Use of Inhibitors
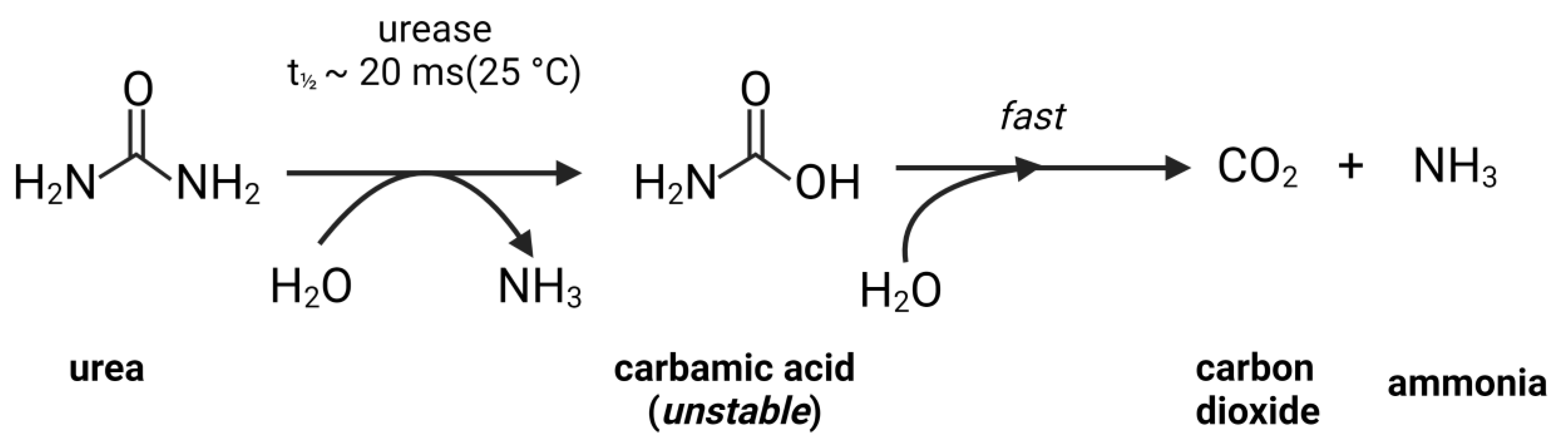
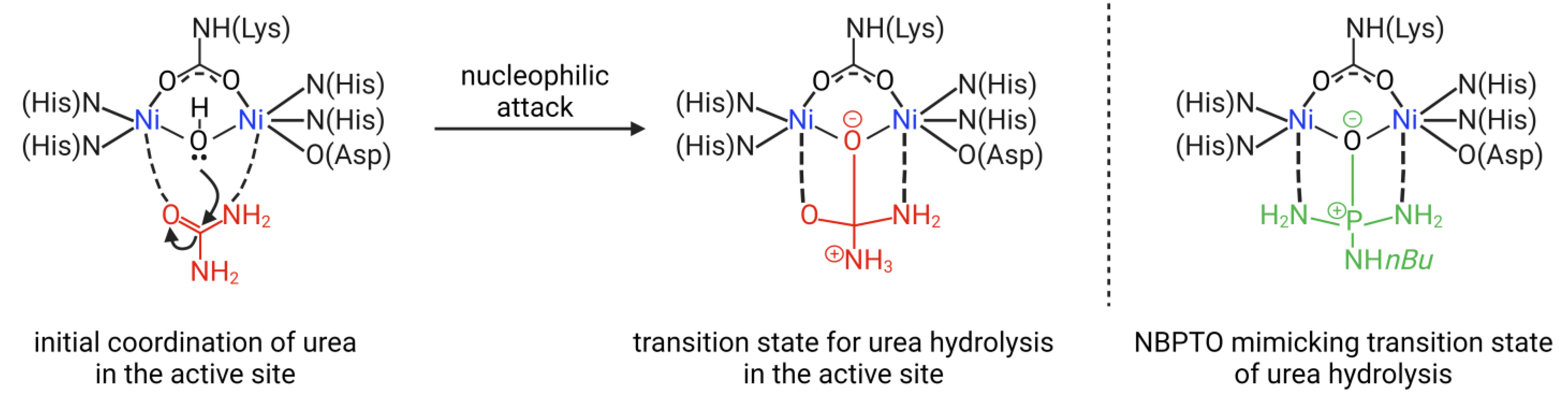
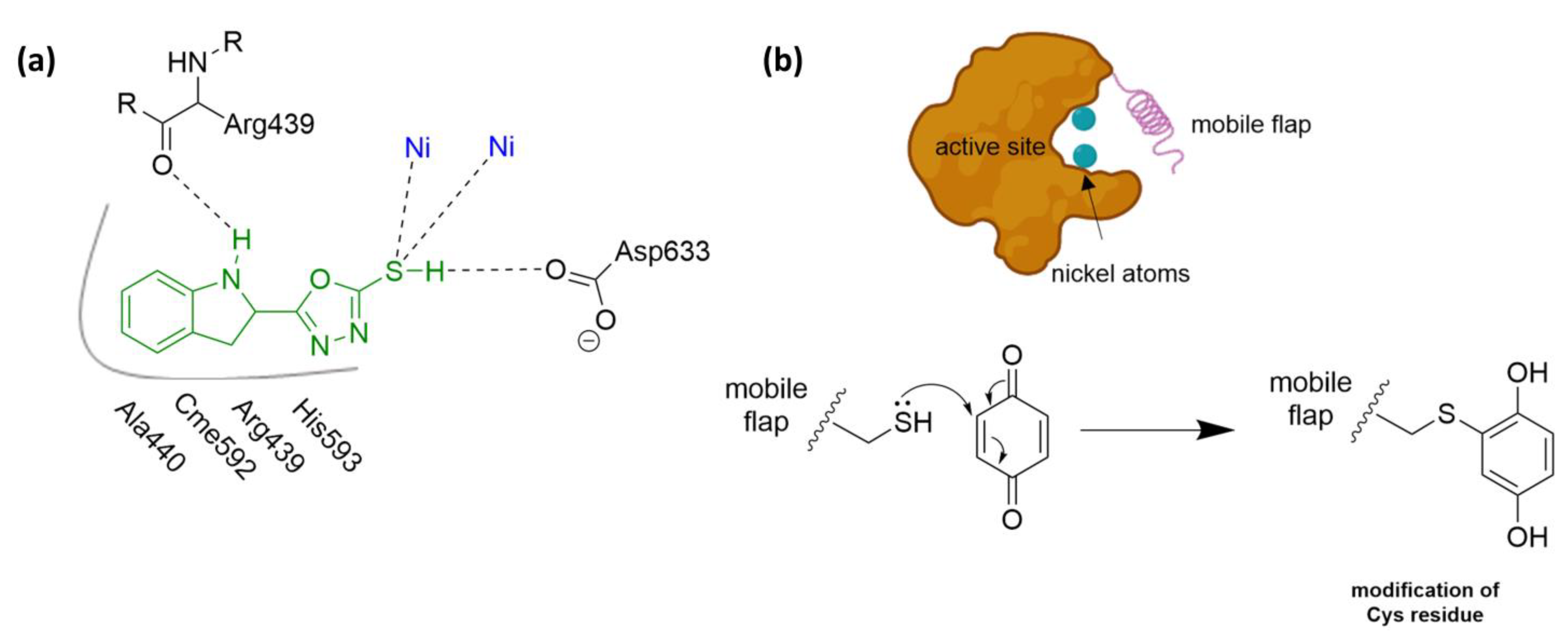
4.1. Mechanism of Urease Inhibition (UI)
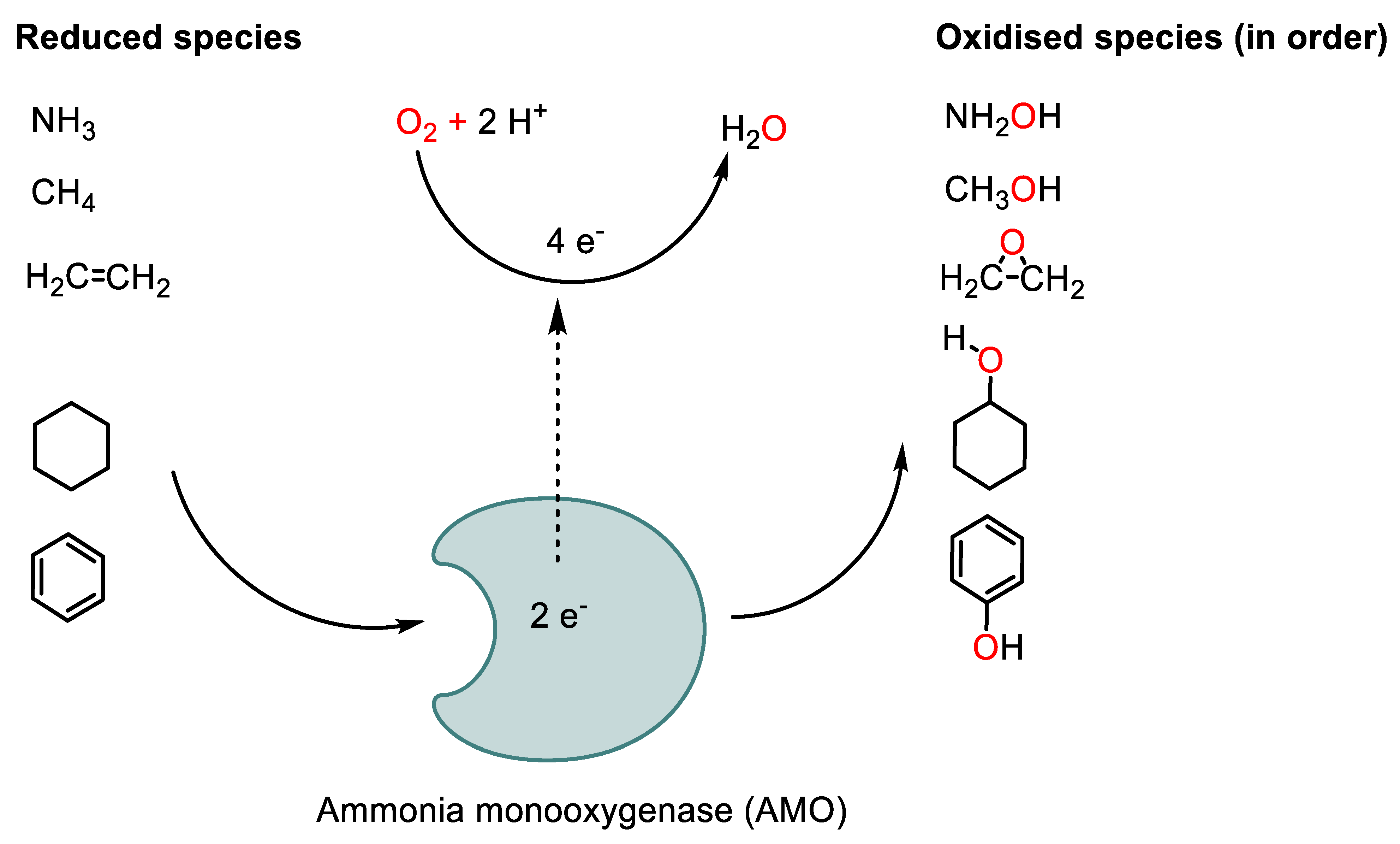
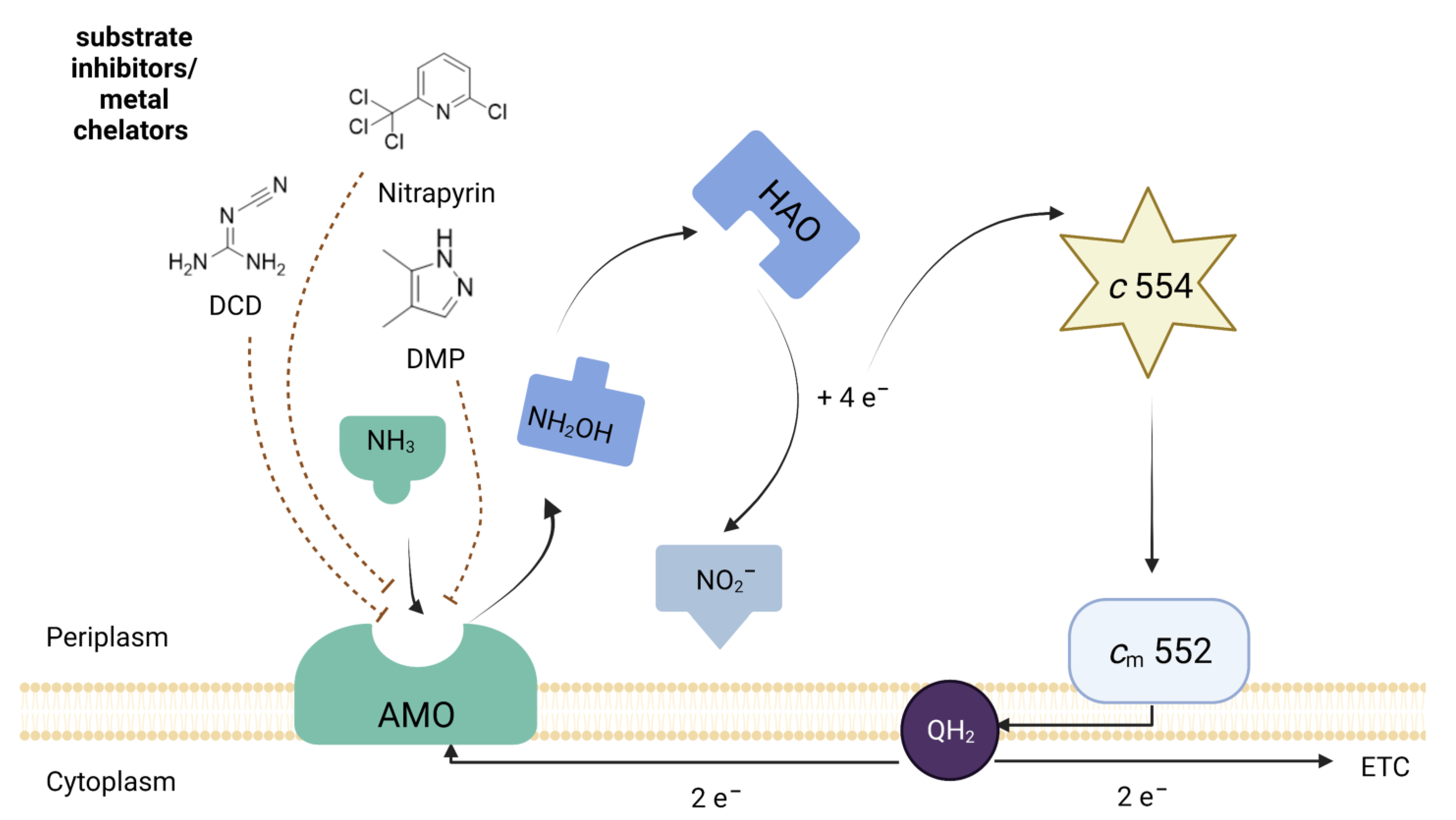
4.2. Mechanism of Nitrification Inhibition (NI)
4.3. Factors Affecting the Effectiveness of UIs and NIs
4.3.1. Temperature
4.3.2. Soil pH
4.3.3. Clay Content and Organic Matter
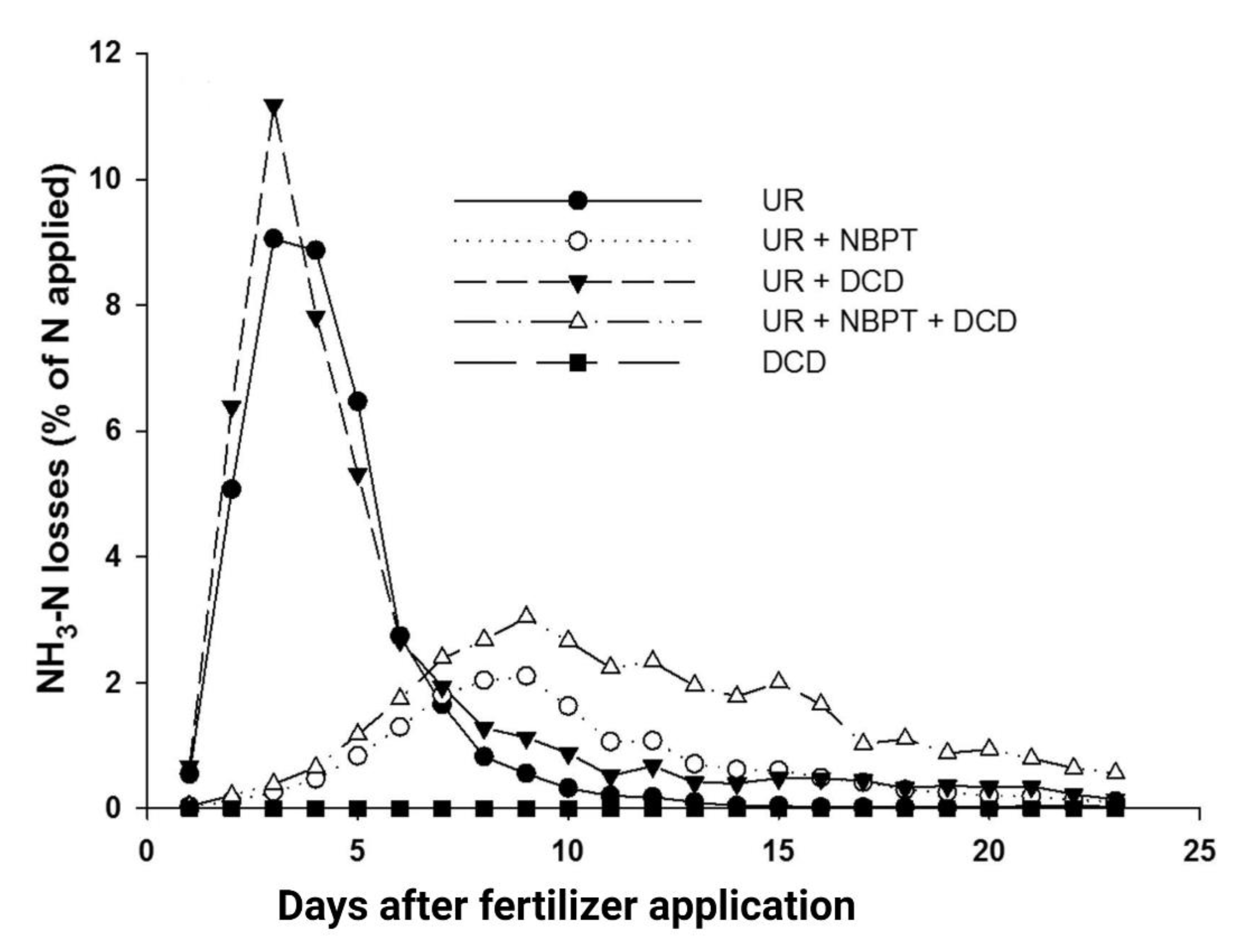
4.4. Use of Combined UIs and NIs
5. Use of Nitrification and Urease Inhibitors in Different Plant Genotypes—What Is Known?
6. Future Outlook/Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhard, A. The nitrogen cycle: Processes. Play. Hum. 2010, 3, 10–25. [Google Scholar]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Roxana Petrescu, A.M.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. AMBIO 2002, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants. 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Rai, A.K. Agricultural nitrogen management for sustainable development and global food security. J. Sci. Res. 2019, 63, 79–87. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Rolinski, S.; Weindl, I.; Schmitz, C.; Muller, C.; Bonsch, M.; Humpenoder, F.; et al. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Comm. 2014, 5, 3858. [Google Scholar] [CrossRef]
- Senauer, B. Feeding the World: A Challenge for the Twenty-First Century; Oxford University Press: New York, NY, USA; Oxford, UK, 2001; Volume 83, pp. 790–792. [Google Scholar]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating worldwide use of urea—A global change contributing to coastal eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Erisman, J.W.; Galloway, J.; Seitzinger, S.; Bleeker, A.; Butterbach-Bahl, K. Reactive nitrogen in the environment and its effect on climate change. Curr. Opin Environ. Sustain. 2011, 3, 281–290. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzeti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Prod. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M. Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment Science. Total Environ. 2010, 408, 3569–3575. [Google Scholar] [CrossRef]
- Gong, L.; Lewicki, R.; Griffin, R.J.; Tittel, F.K.; Lonsdale, C.R.; Stevens, R.G.; Pierce, J.R.; Malloy, Q.G.J.; Travis, S.A.; Bobmanuel, L.M.; et al. Role of atmospheric ammonia in particulate matter formation in Houston during summertime. Atmos. Environ. 2013, 77, 893–900. [Google Scholar] [CrossRef]
- Wang, S.; Nan, J.; Shi, C.; Fu, Q.; Gao, S.; Wang, D.; Cui, H.; Saiz-Lopez, A.; Zhou, B. Atmospheric ammonia and its impacts on regional air quality over the megacity of Shanghai, China. Sci. Rep. 2015, 5, 15842. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chao, Y.Y.; Huang, W.D.; Kao, C.H. Effect of nitrogen deficiency on antioxidant status and Cd toxicity in rice seedlings. Plant Growth Regul. 2011, 64, 263–273. [Google Scholar] [CrossRef]
- Luo, B.F.; Du, S.T.; Lu, K.X.; Liu, W.J.; Lin, X.Y.; Jin, C.W. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J. Exp Bot. 2012, 63, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Terrestrial nutrient cycling. In Principles of Terrestrial Ecosystem Ecology, 1st ed.; Springer: New York, NY, USA, 2002; pp. 97–122. [Google Scholar]
- Courty, P.E.; Smith, P.; Koegel, S.; Redecker, D.; Wipf, D. Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. Crit. Rev. Plant Sci. 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil. Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- von Wirén, N.; Gazzarrini, S.; Frommer, W.B. Regulation of mineral nitrogen uptake in plants. Plant Soil 1997, 196, 191–199. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Cui, J.; Yu, C.; Qiao, N.; Xu, X.; Tian, Y.; Ouyang, H. Plant preference for NH4+ versus NO3− at different growth stages in an alpine agroecosystem. Field Crops Res. 2017, 201, 192–199. [Google Scholar] [CrossRef]
- Miller, A.E.; Bowman, W.D.; Suding, K.N. Plant uptake of inorganic and organic nitrogen: Neighbor identity matters. Ecology 2007, 88, 1832–1840. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-M.; Moulin, L.; Bontemps, C.; Vandamme, P.; Béna, G.; Boivin-Masson, C. Legume symbiotic nitrogen fixation by β-Proteobacteria is widespread in nature. J. Bacteriol. 2003, 185, 7266–7272. [Google Scholar] [CrossRef]
- Moulin, L.; Munive, A.; Dreyfus, B.; Boivin-Masson, C. Nodulation of legumes by members of the β-subclass of Proteobacteria. Nature 2001, 411, 948–950. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Phys. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Wang, M.Y.; Siddiqi, M.Y.; Ruth, T.J.; Glass, A.D.M. Ammonium uptake by rice roots. (I. Fluxes and subcellular distribution of 13NH4+). Plant Physiol. 1993, 103, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Unkles, S.E.; Wang, R.; Wang, Y.; Glass, A.D.; Crawford, N.M.; Kinghorn, J.R. Nitrate reductase activity is required for nitrate uptake into fungal but not plant cells. J. Biol. Chem. 2004, 279, 28182–28186. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Noguero, M.; Lacombe, B. Transporters involved in root nitrate uptake and sensing by Arabidopsis. Front. Plant. Sci. 2016, 7, 1391. [Google Scholar] [CrossRef] [PubMed]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; Lacombe, B. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Cerezo, M.; Tillard, P.; Filleur, S.; Munos, S.; Daniel-Vedele, F.; Gojon, A. Major alterations of the regulationof root NO3 uptake are associated with the mutation of NRT21 and NRT22 genes in Arabidopsis. Plant Phys. 2001, 127, 262–271. [Google Scholar] [CrossRef]
- Filleur, S.; Dorbe, M.F.; Cerezo, M.; Orsel, M.; Granier, F.; Gojon, A.; Daniel-Vedele, F. An Arabidopsis T-DNA mutant affected in NRT2 genes is impaired in nitrate uptake. FEBS Lett. 2001, 489, 220–224. [Google Scholar] [CrossRef]
- Little, D.Y.; Rao, H.Y.; Oliva, S.; Daniel-Vedele, F.; Krapp, A.; Malamy, J.E. The putative high-affinity nitrate transporter NRT21 represses lateral root initiation in response to nutritional cues. Prod. Natl. Acad. Sci. USA 2005, 102, 13693–13698. [Google Scholar] [CrossRef]
- Orsel, M.; Chopin, F.; Leleu, O.; Smith, S.J.; Krapp, A.; Daniel-Vedele, F.; Miller, A.J. Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis Physiology and protein-protein interaction. Plant Phys. 2006, 142, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. Acentral role for the nitrate transporter NRT21 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Phys. 2006, 140, 909–921. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, A.D.M. Dissection of the AtNRT21: AtNRT22 inducible high-affinity nitrate transporter gene cluster. Plant Phys. 2007, 143, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Brehaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H. The Arabidopsis nitrate transporter NRT24 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 2012, 24, 245–258. [Google Scholar] [CrossRef]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT25 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014, 80, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.W.; Quilliam, R.S.; DeLuca, T.H.; Farrar, J.; Farrell, M.; Roberts, P.; Newsham, K.K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS ONE 2011, 6, e19220. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Rentsch, D.; Schmidt, S. Plants can use protein as a nitrogen source without assistance from other organisms. Prod. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Lipson, D.; Näsholm, T. The unexpected versatility of plants: Organic nitrogen use and availability in terrestrial ecosystems. Oecologia 2001, 128, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Raab, T.K.; Lipson, D.A.; Monson, R.K. Soil amino acid utilization among species of the cyperaceae: Plant and soil processes. Ecology 1999, 80, 2408–2419. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1998, 26, 1–15. [Google Scholar] [CrossRef]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.; Espen, L.; Prinsi, B. Nitrogen uptake in plants: The plasma membrane root transport systems from a physiological proteomic perpective. Plants 2021, 10, 681. [Google Scholar] [CrossRef]
- Yang, G.; Wei, Q.; Huang, H.; Xia, J. Amino acid transporters in plant cells: A brief review. Plants 2020, 9, 967. [Google Scholar] [CrossRef]
- Greenfield, L.M.; Hill, P.W.; Paterson, E.; Baggs, E.M.; Jones, D.L. Do plants use root-derived proteases to promote the uptake of soil organic nitrogen? Plant Soil 2020, 456, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Miflin, B.J. Nitrogen assimilation and its relevance to crop improvement. Annu. Plant Rev. Online 2018, 42, 1–40. [Google Scholar]
- Phillips, D.A.; Fox, T.C.; Six, J. Root exudation (net efflux of amino acids) may increase rhizodeposition under elevated CO2. Glob. Change Biol. 2006, 12, 561–567. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- Warren, C.R. Organic N molecules in the soil solution: What is known, what is unknown and the path forwards. Soil Biol. Biochem. 2014, 375, 1–19. [Google Scholar] [CrossRef]
- Warren, C.R. Variation in small organic N compounds and amino acid enantiomers along an altitudinal gradient. Soil Biol. Biochem. 2017, 115, 197–212. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef]
- Roberts, P.; Jones, D. Microbial and plant uptake of free amino sugars in grassland soils. Soil Biol. Biochem. 2012, 49, 139–149. [Google Scholar] [CrossRef]
- Glanville, H.; Hill, P.; Schnepf, A.; Oburger, E.; Jones, D. Combined use of empirical data and mathematical modelling to better estimate the microbial turnover of isotopically labelled carbon substrates in soil. Soil Biol.Biochem. 2016, 94, 154–168. [Google Scholar] [CrossRef]
- Farzadfar, S.; Knight, J.D.; Congreves, K.A. Soil organic nitrogen: An overlooked but potentially significant contribution to crop nutrition. Plant Soil 2021, 462, 7–23. [Google Scholar] [CrossRef]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- de Vries, F.T.; Bardgett, R.D. Plant community controls on short-term ecosystem nitrogen retention. New Phytol. 2016, 210, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Abalos, D.; van Groenigen, J.W.; De Deyn, G.B. What plant functional traits can reduce nitrous oxide emissions from intensively managed grasslands? Glob. Chang. Biol. 2018, 24, e248–e258. [Google Scholar] [CrossRef]
- Cantarel, A.A.M.; Pommier, T.; Desclos-Theveniau, M.; Diquelou, S.; Dumont, M.; Grassein, F.; Kastl, E.-M.; Grigulis, K.; Laine, P.; Lavorel, S.; et al. Using plant traits to explain plant–microbe relationships involved in nitrogen acquisition. Ecology 2015, 96, 788–799. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Harty, M.A.; Forrestal, P.J.; Watson, C.J.; McGeough, K.L.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.J.; Richards, K.G.; Lanigan, G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016, 563, 576–586. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Snyder, C.S.; Blaylock, A.D.; Del Grosso, S.J. Enhanced-efficiency nitrogen fertilizers: Potential role in nitrous oxide emission mitigation. J. Agron. 2014, 106, 715–722. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.; Liu, X.; Roescke, M.; Pasda, G.; Zerulla, W.; Wissemeier, A.H.; Chen, X.; Goulding, K.; Zhang, F. A new urease-inhibiting formulation decreases ammonia volatilization and improves maize nitrogen utilization in North China Plain. Sci. Rep. 2017, 7, 43853. [Google Scholar] [CrossRef] [PubMed]
- Heffer, P.; Prud’homme, M. Fertilizer outlook 2013–2017. In Proceedings of the 81st International Fertilizer Industry Association Conference, Chicago, IL, USA, 13 March 2013; Paper No.: A/13/78. p. 8. [Google Scholar]
- Mazzei, L.; Cianci, M.; Contaldo, U.; Ciurli, S. Insights into urease inhibition by N-(n-Butyl) phosphoric triamide through an integrated structural and kinetic approach. J. Agric. Food Chem. 2019, 67, 2127–2138. [Google Scholar] [CrossRef]
- Svane, S.; Sigurdarson, J.J.; Finkenwirth, F.; Eitinger, T.; Karring, H. Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Chen, A.; Lei, B.; Hu, W.; Lu, Y.; Mao, Y.; Duan, Z.; Shi, Z. Characteristics of ammonia volatilization on rice grown under different nitrogen application rates and its quantitative predictions in Erhai Lake Watershed, China. Nutr. Cycl. Agroecosys. 2015, 101, 139–152. [Google Scholar] [CrossRef]
- Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’Callaghan, T.F. Urease and nitrification inhibitors—As mitigation tools for greenhouse gas emissions in sustainable dairy systems: A review. Sustainability 2020, 12, 6018. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. J. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lücker, S.; Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lucker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Seitzinger, S.; Harrison, J.A.; Bohlke, J.K.; Baouwman, A.F.; Lowrance, R.; Peterson, B.; Tobias, C.; Van Drecht, G. Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC Climate Chang. 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Netz, B.; Davidson, O.; Bosch, P.; Dave, R.; Meyer, L. Climate Change 2007: Mitigation. In Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Summary for Policymakers; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2007. [Google Scholar]
- Singh, M.; Yadav, D.S.; Kumar, V. Leaching and transformation of urea in dry and wet soils as affected by irrigation water. Plant Soil 1984, 81, 411–420. [Google Scholar] [CrossRef]
- Dawar, K.; Zaman, M.; Rowarth, J.S.; Blennerhassett, J.; Turnbull, M.H. Urea hydrolysis and lateral and vertical movement in the soil: Effects of urease inhibitor and irrigation. Biol. Fertil. Soils 2011, 47, 139–146. [Google Scholar] [CrossRef]
- Domínguez, M.J.; Sanmartín, C.; Font, M.; Palop, J.A.; San Francisco, S.; Urrutia, O.; Houdusse, F.; García-Mina, J.M. Design, synthesis, and biological evaluation of phosphoramide derivatives as urease inhibitors. J. Agric. Food Chem. 2008, 56, 3721–3731. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, L.H.; Dai, F. Influence of a new phosphoramide urease inhibitor on urea-N transformation in different texture soil. J. Appl. Ecol. 2016, 27, 4003–4012. [Google Scholar]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)–A new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Subbarao, G.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.; Berry, W. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria Humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Prod. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Zakir, H.; Subbarao, G.V.; Pearse, S.J.; Gopalakrishnan, S.; Ito, O.; Ishikawa, T.; Kawano, N.; Nakahara, K.; Yoshihashi, T.; Ono, H.; et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (sorghum bicolor). New Phytol. 2008, 5, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jones, C.M.; Meijer, J.; Lundquist, P.-O.; Fransson, P.; Carlsson, G.; Hallin, S. Intercropping affects genetic potential for inorganic nitrogen cycling by root-associated microorganisms in Medicago sativa and Dactylis glomerata. Appl. Soil Ecol. 2017, 119, 260–266. [Google Scholar] [CrossRef]
- Nardi, P.; Laanbroek, H.J.; Nicol, G.W.; Renella, G.; Cardinale, M.; Pietramellara, G.; Weckwerth, W.; Trinchera, A.; Ghatak, A.; Nannipieri, P. Biological nitrification inhibition in the rhizosphere: Determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol. Rev. 2020, 44, 874–908. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Arango, J.; Masahiro, K.; Hooper, A.M.; Yoshihashi, T.; Ando, Y.; Nakahara, K.; Deshpande, S.; Ortiz-Monasterio, I.; Ishitani, M.; et al. Genetic mitigation strategies to tackle agricultural GHG emissions: The case for biological nitrification inhibition technology. Plant Sci. 2017, 262, 165–168. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.M.; Hash, C.T.; Geouge, T.S.; Srinivasa Roa, P.; Nardi, P.; et al. Biological nitrification inhibition: A novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012, 114, 249–302. [Google Scholar]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Rao, I.M.; Ishitani, M.; Hash, C.T.; Kishii, M.; Bonnett, D.G.; Berry, W.L.; Lata, J.C. A paradigm shift towards low-nitrifying production systems: The role of biological nitrification inhibition (BNI). Ann. Bot. 2013, 112, 297–316. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Xie, T.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: A review. Ecotoxicol. Environ. Saf. 2021, 220, 112338. [Google Scholar] [CrossRef]
- Prasad, R.; Power, J.F. Nitrification inhibitors for agriculture, health, and the environment. Adv. Agron. 1995, 54, 233–281. [Google Scholar]
- Dong, G.; Xiao, D.; Zhang, C.-H. The effect of nitrification inhibitors on nitrogen cycle: A comprehensive review. In Proceedings of the IOP Conference Series: Earth and Environmental Science 2020 International Symposium on water, Ecology and Environment, Beijing, China, 6–8 December 2021; Volume 690, p. 012012. [Google Scholar]
- Mazzei, L.; Musiani, F.; Ciurli, S. The structure-based reaction mechanism of urease, a nickel dependent enzyme: Tale of a long debate. J. Biol. Inorg. Chem. 2020, 25, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, A.A.; Akinremi, O.O. Kinetics and thermodynamics of urea hydrolysis in the presence of urease and nitrification inhibitors. Can. J. Soil Sci. 2021, 101, 192–202. [Google Scholar] [CrossRef]
- Suter, H.C.; Pengthamkeerati, P.; Walker, C.; Chen, D. Influence of temperature and soil type on inhibition of urea hydrolysis by N-(n-butyl) thiophosphoric triamide in wheat and pasture soils in south-eastern Australia. Soil Res. 2011, 49, 315–319. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total Environ. 2021, 771, 145483. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Talma, M. Recent advances in design of new urease inhibitors: A review. J. Adv. Res. 2018, 13, 101–112. [Google Scholar] [CrossRef]
- Modolo, L.V.; da-Silva, C.J.; Brandão, D.S.; Chaves, I.S. A minireview on what we have learned about urease inhibitors of agricultural interest since mid-2000s. J. Adv. Res. 2018, 13, 29–37. [Google Scholar] [CrossRef]
- Brito, T.O.; Souza, A.X.; Mota, Y.C.C.; Morais, V.S.S.; De Souza, L.T.; De Fátima, Â.; Macedo, F.; Modolo, L.V. Design, syntheses and evaluation of benzoylthioureas as urease inhibitors of agricultural interest. RSC Adv. 2015, 5, 44507–44515. [Google Scholar] [CrossRef]
- Imran, M.; Waqar, S.; Ogata, K.; Ahmed, M.; Noreen, Z.; Javed, S.; Bibi, N.; Bokhari, H.; Amjad, A.; Muddassar, M. Identification of novel bacterial urease inhibitors through molecular shape and structure based virtual screening approaches. RSC Adv. 2020, 10, 16061–16070. [Google Scholar] [CrossRef]
- Hanif, M.; Shoaib, K.; Saleem, M.; Hasan Rama, N.; Zaib, S.; Iqbal, J. Synthesis, urease inhibition, antioxidant, antibacterial, and molecular docking studies of 1,3,4-oxadiazole derivatives. ISRN Pharmacol. 2012, 2012, 928901. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Musiani, F.; Ciurli, S. Inactivation of urease by 1, 4-benzoquinone: Chemistry at the protein surface. Dalton Trans. 2016, 45, 5455–5459. [Google Scholar] [CrossRef] [PubMed]
- Babu, T.M.C.; Rajesh, S.S.; Bhaskar, B.V.; Devi, S.; Rammohan, A.; Sivaraman, T.; Rajendra, W. Molecular docking, molecular dynamics simulation, biological evaluation and 2D QSAR analysis of flavonoids from Syzygium alternifolium as potent anti-Helicobacter pylori agents. RSC Adv. 2017, 7, 18277–18292. [Google Scholar] [CrossRef]
- Christianson, C.B.; Howard, R.G. Use of soil thin-layer chromatography to assess the mobility of the phosphoric triamide urease inhibitors and urea in soil. Soil Biol. Biochem. 1994, 26, 1161–1164. [Google Scholar] [CrossRef]
- McCarty, G.W. Modes of action of nitrification inhibitors. Biol. Fertil. Soils 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Lees, H. Effect of Copper-Enzyme Poisons on Soil Nitrification. Nature 1946, 158, 97. [Google Scholar] [CrossRef]
- Yildirim, S.C.; Walker, R.M.; Roessner, U.; Wille, U. Assessing the efficacy, acute toxicity, and binding modes of the agricultural nitrification inhibitors 3, 4-Dimethyl-1 H-pyrazole (DMP) and Dicyandiamide (DCD) with Nitrosomonas europaea. ACS J. Agric. Sci. 2023, 3, 222–231. [Google Scholar] [CrossRef]
- Fisher, O.S.; Kenney, G.E.; Ross, M.O.; Ro, S.Y.; Lemma, B.E.; Batelu, S.; Thomas, P.M.; Sosnowski, V.C.; DeHart, C.J.; Kelleher, N.L.; et al. Characterization of a long overlooked copper protein from methane- and ammonia-oxidizing bacteria. Nat. Commun. 2018, 9, 4276. [Google Scholar] [CrossRef]
- Lieberman, R.L.; Rosenzweig, A.C. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 2005, 434, 177–182. [Google Scholar] [CrossRef]
- Suzuki, I.; Dular, U.; Kwok, S.C. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J. Bacteriol. 1974, 120, 556–558. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Duncan, E.G.; Whisson, K.; Treble, K.; Ward, P.R.; Roper, M.M. A colourimetric microplate assay for simple, high throughput assessment of synthetic and biological nitrification inhibitors. Plant Soil 2017, 413, 275–287. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Schatteman, A.; Crombie, A.T.; Murrell, J.C.; Lehtovirta-Morley, L.E. Inhibition of ammonia monooxygenase from ammonia-oxidizing archaea by linear and aromatic alkynes. Appl. Environ. Microbiol. 2020, 86, e02388-19. [Google Scholar] [CrossRef]
- Kaur-Bhambra, J.; Wardak, D.L.R.; Prosser, J.I.; Gubry-Rangin, C. Revisiting plant biological nitrification inhibition efficiency using multiple archaeal and bacterial ammonia-oxidising cultures. Biol Fertil. Soils 2021, 58, 241–249. [Google Scholar] [CrossRef]
- Bédard, C.; Knowles, R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 1989, 53, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Juliette, L.Y.; Hyman, M.R.; Arp, D.J. Inhibition of ammonia oxidation in Nitrosomonas europaea by sulfur compounds: Thioethers are oxidized to sulfoxides by ammonia monooxygenase. Appl. Environ. Microbiol. 1993, 59, 3718–3727. [Google Scholar] [CrossRef]
- Hubley, J.H.; Mitton, J.R.; Wilkinson, J.F. The oxidation of carbon monoxide by methane-oxidizing bacteria. Arch. Microbiol. 1974, 95, 365–368. [Google Scholar] [CrossRef]
- Hyman, M.R.; Wood, P.M. Methane oxidation by Nitrosomonas europaea. J. Biochem. 1983, 212, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.R.; Wood, P.M. Ethylene oxidation by Nitrosomonas europaea. Arch. Microbiol. 1984, 137, 155–158. [Google Scholar] [CrossRef]
- Stirling, D.I.; Dalton, H. The fortuitous oxidation and cometabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus(Bath). FEMS Microbiol Lett. 1979, 5, 315–318. [Google Scholar] [CrossRef]
- Voysey, P.A.; Wood, P.M. Methanol and formaldehyde oxidation by an autotrophic nitrifying bacterium. Microbiol. 1987, 133, 283–290. [Google Scholar] [CrossRef]
- Ward, B.B. Kinetic studies on ammonia and methane oxidation by Nitrosococcus oceanus. Arch. Microbiol. 1987, 147, 126–133. [Google Scholar] [CrossRef]
- Hyman, M.R.; Sansome-Smith, A.W.; Shears, J.H.; Wood, P.M. A kinetic study of benzene oxidation to phenol by whole cells of Nitrosomonas europaea and evidence for the further oxidation of phenol to hydroquinone. Arch. Microbiol. 1985, 143, 302–306. [Google Scholar] [CrossRef]
- Rasche, M.E.; Hicks, R.E.; Hyman, M.R.; Arp, D.J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J. Bacteriol. 1990, 172, 5368–5373. [Google Scholar] [CrossRef]
- Fernández, M.L.; Estrin, D.A.; Bari, S.E. Theoretical insight into the hydroxylamine oxidoreductase mechanism. J. Inorg. Biochem. 2008, 102, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.; Bergmann, D.; Arciero, D.; Hooper, A.B. Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochem. Biophys. Acta. 2000, 1459, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J.; Ham, J.; Sun, T.; Rosenzweig, A.C. Structural conservation of the B subunit in the ammonia monooxygenase/particulate methane monooxygenase superfamily. Proteins 2014, 82, 2263–2267. [Google Scholar] [CrossRef]
- Mario, C.M.; Carmen, G.M.; Adrián, B.L.; Luis, L.; Beñat, A. Mechanism of action of nitrification inhibitors based on dimethylpyrazole: A matter of chelation. Sci. Total Environ. 2021, 752, 141885. [Google Scholar]
- Ensign, S.A.; Hyman, M.R.; Arp, D.J. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J. Bacteriol. 1993, 175, 1971–1980. [Google Scholar] [CrossRef]
- Huérfano, X.; Fuertes-Mendizábal, T.; Fernández-Diez, K.; Estavillo, J.M.; González-Murua, C.; Menéndez, S. The new nitrification inhibitor 3, 4-dimethylpyrazole succinic (DMPSA) as an alternative to DMPP for reducing N2O emissions from wheat crops under humid Mediterranean conditions. Eur. J. Agron. 2016, 80, 78–87. [Google Scholar] [CrossRef]
- Recio, J.; Alvarez, J.M.; Rodriguez-Quijano, M.; Vallejo, A. Nitrification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat. Environ. Pollut. 2019, 245, 199–207. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Xu, F. Synthesis of a novel polymer nitrification inhibitor with acrylic acid and 3,4-Dimethylpyrazole. J. Agric. Food Chem. 2021, 69, 307–3311. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Asgedom, H.; Tenuta, M.; Flaten, D.N.; Gao, X.; Kebreab, E. Nitrous oxide emissions from a clay soil receiving granular urea formulations and dairy manure. Agron. J. 2014, 106, 732–774. [Google Scholar] [CrossRef]
- Dougherty, W.; Collins, D.; Zwieten, L.V.; Rowlings, D. Nitrification (DMPP) and urease (NBPT) inhibitors had no effect on pasture yield, nitrous oxide emissions, or nitrate leaching under irrigation in a hot-dry climate. Soil Res. 2016, 54, 675–683. [Google Scholar] [CrossRef]
- Guardia, G.; Marsdden, K.A.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci. Total. Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Suter, H.; Liu, R.; Yuan, S.; Chen, D. Effects of nitrification inhibitors on gross N nitrification rate, ammonia oxidizers, and N2O production under different temperatures in two pasture soils. Environ. Sci. Pollut. Res. 2018, 25, 28344–28354. [Google Scholar] [CrossRef]
- Sha, Z.; Li, Q.; Lv, T.; Misselbrook, T.; Liu, X. Response of ammonia volatilization to biochar addition: A meta-analysis. Sci. Total Environ. 2019, 655, 1387–1396. [Google Scholar] [CrossRef]
- Sha, Z.; Ma, X.; Wang, J.; Lv, T.; Li, Q.; Misselbrook, T.; Liu, X. Effect of N stabilizers on fertilizer-N fate in the soil-crop system: A meta-analysis. Agric. Ecosyst. Environ. 2020, 290, 106763. [Google Scholar] [CrossRef]
- Zhu, G.; Ju, X.; Zhang, J.; Müller, C.; Sylvester-Bradley, R. Effects of the nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on gross N transformation rates and N2O emissions. Biol. Fertil. Soils 2019, 55, 603–615. [Google Scholar] [CrossRef]
- Carmona, G.; Christianson, C.B.; Byrnes, B.H. Temperature and low concentration effects of the urease inhibitor N-(n-butyl) thiophosphoric triamide (n-BTPT) on ammonia volatilization from urea. Soil Biol. Biochem. 1990, 22, 933–937. [Google Scholar] [CrossRef]
- Moyo, C.; Kissel, D.; Cabrera, M. Temperature effects on soil urease activity. Soil Biol. Biochem. 1989, 21, 935–993. [Google Scholar] [CrossRef]
- McGeough, K.L.; Watson, C.J.; Müller, C.; Laughlin, R.J.; Chadwick, D.R. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol. Biochem. 2016, 94, 222–232. [Google Scholar] [CrossRef]
- Kelliher, F.; van Koten, C.; Kear, M.; Sprosen, M.; Ledgard, S.; de Klein, C.; Letica, S.; Luo, J.; Rys, G. Effect of temperature on dicyandiamide (DCD) longevity in pastoral soils under field conditions. Agric. Ecosyst. Environ. 2014, 186, 201–204. [Google Scholar] [CrossRef]
- Irigoyen, I.; Muro, J.; Azpilikueta, M.; Aparicio-Tejo, P.; Lamsfus, C. Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Aust. J. Soil Res. 2003, 41, 1177–1183. [Google Scholar] [CrossRef]
- Mahmood, T.; Rehmat, A.; Asma, L.; Muhammad, S. 4-Amino-1, 2, 4-triazole can be more effective than commercial nitrification inhibitors at high soil temperatures. Soil Res. 2017, 55, 715–722. [Google Scholar] [CrossRef]
- Marsden, K.A.; Jones, D.L.; Chadwick, D.R. DMPP is ineffective at mitigating N2O emissions from sheep urine patches in a UK grassland under summer conditions. Agric. Ecosyst. Environ. 2017, 246, 1–11. [Google Scholar] [CrossRef]
- Engel, R.; Williams, E.; Wallander, R.; Hilmer, J. Apparent persistence of N-(n-butyl) thiophosphoric triamide is greater in alkaline soils. Soil Sci. Soc. Am. J. 2013, 77, 1424–1429. [Google Scholar] [CrossRef]
- Engel, R.E.; Towey, B.D.; Gravens, E. Degradation of the urease inhibitor NBPT as affected by soil pH. Soil Sci. Soc. Am. J. 2015, 79, 1674–1683. [Google Scholar] [CrossRef]
- Tao, L.; Yuanliang, S.; Xuewen, L.; Guolin, L. Degradation and its affecting factors of NBPT in soil. Chin. J. Ecol. 2006, 25, 1082–1086. [Google Scholar]
- He, J.-Z.; Hu, H.-W.; Zhang, L.-M. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- He, J.-Z.; Shen, J.-P.; Zhang, L.-M.; Di, H.J. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar]
- Jia, Z.; Zhou, X.; Xia, W.; Fornara, D.; Wang, B.; Wasson, E.A.; Christie, P.; Polz, M.F.; Myrold, D.D. Evidence for niche differentiation of nitrifying communities in grassland soils after 44 years of different field fertilization scenarios. Pedosphere 2020, 30, 87–97. [Google Scholar] [CrossRef]
- Liu, R.; Hayden, H.L.; Hu, H.; He, J.; Suter, H.; Chen, D. Effects of the nitrification inhibitor acetylene on nitrous oxide emissions and ammonia-oxidizing microorganisms of different agricultural soils under laboratory incubation conditions. Appl. Soil Ecol. 2017, 119, 80–90. [Google Scholar] [CrossRef]
- Prosser, J.I.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous Oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Chang. Biol. 2020, 26, 103–118. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, L.; Bi, Q.; Dai, P.; Sun, D.; Sun, C.; Liu, W.; Lu, L.; Ni, W.; Lin, X. Comparative effects of 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) on ammonia-oxidizing bacteria and archaea in a vegetable soil. Appl. Microbiol. Biotechnol. 2015, 99, 477–487. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; De La Torre, J.R.; Stahl, D.A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Hu, H.-W.; Shen, J.-P.; He, J.-Z. Ammonia-Oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Shi, X.; Hu, H.; He, J.; Chen, D.; Suter, H. Effects of 3, 4-dimethylpyrazole phosphate (DMPP) on nitrification and the abundance and community composition of soil ammonia oxidizers in three land uses. Biol. Fertil. Soils 2016, 52, 927–939. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils A review. Chem. Erde-Geochem. 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Christianson, C.B.; Baethgen, W.E.; Carmona, G.; Howard, R.G. Microsite reactions of urea-nBTPT fertilizer on the soil surface. Soil Biol. Biochem. 1993, 25, 1107–1117. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wu, Z.J.; Zhou, Q.X. Dicyandiamide sorption-desorption behaviour on soils and peat humus. Pedosphere 2004, 14, 395–399. [Google Scholar]
- Wu, S.; Wu, L.; Shi, Q.; Wang, Z.; Chen, X.; Li, Y. Effects of a new nitrification inhibitor 3, 4–dimethylpyrazole phosphate (DMPP) on nitrate and potassium leaching in two soils. J. Environ. Sci. 2007, 19, 841–847. [Google Scholar] [CrossRef]
- Barth, G.; Von Tucher, S.; Schmidhalter, U. Effectiveness of 3, 4-dimethylpyrazole phosphate as nitrification inhibitor in soil as influenced by inhibitor content, application form, and soil matric potential. Pedosphere 2008, 18, 378–385. [Google Scholar] [CrossRef]
- Chen, D.; Suter, H.C.; Islam, A.; Edis, R. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol. Biochem. 2010, 42, 660–664. [Google Scholar] [CrossRef]
- International Fertilizer Industry Association. Sustainable Management of the Nitrogen Cycle in Agriculture and Mitigation of Reactive Nitrogen Side Effects; IFIA: Paris, France, 2007. [Google Scholar]
- Suter, H.C.; Sultana, H.; Davies, R.; Walker, C.; Chen, D. Influence of enhanced efficiency fertilisation techniques on nitrous oxide emissions and productivity response from urea in a temperate Australian ryegrass pasture. Soil Res. 2016, 54, 523–532. [Google Scholar] [CrossRef]
- Fan, X.; Yin, C.; Yan, G.; Cui, P.; Shen, Q.; Wang, Q.; Chen, H.; Zhang, N.; Ye, M.; Zhao, Y.; et al. The contrasting effects of N-(n-butyl) thiophosphoric triamide (NBPT) on N2O emissions in arable soils differing in pH are underlain by complex microbial mechanisms. Sci. Total Environ. 2018, 642, 155–167. [Google Scholar] [CrossRef]
- Lam, S.K.; Suter, H.; Bai, M.; Walker, C.; Davies, R.; Mosier, A.R.; Chen, D. Using urease and nitrification inhibitors to decrease ammonia and nitrous oxide emissions and improve productivity in a subtropical pasture. Sci. Total Environ. 2018, 644, 1531–1535. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; González-López, J.; Vallejo, A.; Bedmar, E.J. Effect of urease and nitrification inhibitors on ammonia volatilization and abundance of N-cycling genes in an agricultural soil. J. Plant Nutr. Soil Sci. USA 2019, 183, 99–109. [Google Scholar] [CrossRef]
- Frame, W. Ammonia volatilization from urea treated with NBPT and two nitrification inhibitors. Agron. J. 2017, 109, 378–387. [Google Scholar] [CrossRef]
- Pan, B.; Lam, S.K.; Mosier, A.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; de Campos Menegale, M.L. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.; de Brito, S. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J.; et al. Effects of nitrification inhibitors on soil nitrification and ammonia volatilization in three soils with different pH. Agronomy 2021, 11, 1674. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.; Reynolds, W.D.; Calder, W.; Oloya, T.O.; Woodley, A. combining urease and nitrification inhibitors with incorporation reduces ammonia and nitrous oxide emissions and increases corn yields. J. Environ. Qual. 2017, 46, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Bailey, L.D. Effect of seed-placed urea fertilizer and N-(n-butyl) thiophosphoric triamide (NBPT) on emergence and grain yield of barley. Can. J. Plant Sci. 1999, 79, 491–496. [Google Scholar] [CrossRef]
- Grant, C.A.; Derksen, D.A.; McLaren, D.; Irvine, R.B. Nitrogen fertilizer and urease inhibitor effects on canola seed quality in a one-pass seeding and fertilizing system. Field Crop. Res. 2011, 121, 201–208. [Google Scholar] [CrossRef]
- Xiaobin, W.; Jingfeng, X.; Grant, C.A.; Bailey, L.D. Effects of placement of urea with a urease inhibitor on seedling emergence, N uptake and dry matter yield of wheat. Can. J. Plant Sci. 1995, 75, 449–452. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Zamboni, A.; Varanini, Z.; Pinton, R. The urease inhibitor NBPT negatively affects DUR3-mediated uptake and assimilation of urea in maize roots. Front. Plant Sci. 2015, 6, 1007. [Google Scholar] [CrossRef] [PubMed]
- Artola, E.; Cruchaga, S.; Ariz, I.; Moran, J.F.; Garnica, M.; Houdusse, F.; Garcia-Mina, J.M.; Ignacio, I.; Lasa, B.; Aparicio-Tejo, P.M. Effect of N-(n-butyl) thiophosphorictriamide on urea metabolism and the assimilation of ammonium by Triticum aestivum L. Plant Growth Regul. 2011, 63, 73–79. [Google Scholar] [CrossRef]
- Cruchaga, S.; Lasa, B.; Jauregui, I.; González-Murua, C.; Aparicio-Tejo, P.M.; Ariz, I. Inhibition of endogenous urease activity by NBPT application reveals differential N metabolism responses to ammonium or nitrate nutrition in pea plants: A physiological study. Plant Soil 2013, 373, 813–827. [Google Scholar] [CrossRef]
- Cruchaga, S.; Artola, E.; Lasa, B.; Ariz, I.; Irigoyen, I.; Moran, J.F.; Aparicio-Tejo, P.M. Short term physiological implications of NBPT application on the N metabolism of Pisum sativum and Spinacea oleracea. J. Plant Physiol. 2011, 168, 329–336. [Google Scholar] [CrossRef]
- Krogmeier, M.J.; McCarty, G.W.; Bremner, J.M. Potential phytotoxicity associated with the use of soil urease inhibitors. Proc. Natl. Acad. Sci. USA 1989, 86, 1110–1112. [Google Scholar] [CrossRef]
- Watson, C.J.; Miller, H. Short-term effects of urea amended with the urease inhibitor N-(n-butyl) thiophosphoric triamide on perennial ryegrass. Plant Soil 1996, 184, 33–45. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; IFA, International Fertilizer Industry Association: Berlin, Germany, 2010. [Google Scholar]
- Rodrigues, J.M.; Lasa, B.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 2018, 624, 1180–1186. [Google Scholar] [CrossRef]
- Kallio, H.; Linko, R.R.; Tikanmäki, E.; Puntari, I. Effect of nitrapyrin on nitrapyrin residues and nitrate content in red beet roots fertilised with urea. J. Sci. Food Agric. 1980, 31, 701–708. [Google Scholar] [CrossRef]
- Dawar, K.; Rahman, U.; Alam, S.S.; Tariq, M.; Khan, A.; Fahad, S.; Datta, R.; Danish, S.; Saud, S.; Noor, M. Nitrification inhibitor and plant growth regulators improve wheat yield and nitrogen use efficiency. J. Plant Growth Regul. 2022, 41, 216–226. [Google Scholar] [CrossRef]
- Dawar, K.; Sardar, K.; Zaman, M.; Mueller, C.; Alberto, S.C.; Aamir, K.; Borzouei, A.; Perez-Castillo, A.G. Effects of the nitrification inhibitor nitrapyrin and the plant growth regulator gibberellic acid on yield-scale nitrous oxide emission in maize fields under hot climatic conditions. Pedosphere 2021, 31, 323–331. [Google Scholar] [CrossRef]
- Linquist, B.A.; Liu, L.J.; van Kessel, C.; van Groenigen, K.J. Enhanced efficiency nitrogen fertilizers for rice systems: Meta-analysis of yield and nitrogen uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 22075. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Recio, J.; Montoya, M.; Álvarez, J.M.; Vallejo, A. Inhibitor-coated enhanced-efficiency N fertilizers for mitigating NOX and N2O emissions in a high-temperature irrigated agroecosystem. Agric. For. Meteorol. 2020, 292, 108110. [Google Scholar] [CrossRef]
- Werneck, C.G.; Haim, P.G.; Breda, F.A.D.F.; Monte, M.B.D.M.; Bernardi, A.C.D.C.; Mazur, N.; Polidoro, J.C. Ammonia volatilization and agronomical efficiency of a mixture of urea with natural zeolite for rose fertilization. Pesqui. Agropecu. Bras. 2022, 56, 01449. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, J.J.; Wei, Z.; Dodla, S.K.; Fultz, L.M.; Gaston, L.A.; Xiao, R.; Park, J.-h.; Scaglia, G. Nitrification inhibitors reduce nitrogen losses and improve soil health in a subtropical pastureland. Geoderma 2021, 388, 114947. [Google Scholar] [CrossRef]
- Weiske, A.; Benckiser, G.; Herbert, T.; Ottow, J. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol. Fertil. Soils 2001, 34, 109–117. [Google Scholar]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrero, M.; Howitt, R.E.; Janssen, S.; et al. Brief history of agricultural systems modeling. Agric. Syst. 2017, 155, 240–254. [Google Scholar] [CrossRef]
- Else, H. Need to Make a Molecule? Ask This AI for Instructions. 2018. Available online: https://www.nature.com/articles/d41586-018-03977-w (accessed on 4 February 2023).
- Miljković, F.; Rodríguez-Pérez, R.; Bajorath, J. Impact of artificial intelligence on compound discovery, design, and synthesis. ACS Omega 2021, 6, 33293–33299. [Google Scholar] [CrossRef]
- Segler, M.H.S.; Preuss, M.; Waller, M.P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 2018, 555, 604–610. [Google Scholar] [CrossRef]
- Musiani, F.; Arnofi, E.; Casadio, R.; Ciurli, S. Structure-based computational study of the catalytic and inhibition mechanisms of urease. J. Biol. Inorg. Chem. 2001, 6, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Hyman, M.R.; Arp, D.J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J. Biol. Chem. 1992, 267, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Slangen, J.; Kerkhoff, P. Nitrification inhibitors in agriculture and horticulture: A literature review. J. Fert. Res. 1984, 5, 1–76. [Google Scholar] [CrossRef]
- Suwanchaikasem, P.; Idnurm, A.; Selby-Pham, J.; Walker, R.; Boughton, B.A. Root-TRAPR: A modular plant growth device to visualize root development and monitor growth parameters, as applied to an elicitor response of Cannabis sativa. Plant Methods 2022, 18, 46. [Google Scholar] [CrossRef]
- Sasse, J.; Kant, J.; Cole, B.J.; Klein, A.P.; Arsova, B.; Schlaepfer, P.; Gao, J.; Lewald, K.; Zhalnina, K.; Kosina, S.; et al. Multilab EcoFAB study shows highly reproducible physiology and depletion of soil metabolites by a model grass. New Phytol. 2019, 222, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Curl, E.; Truelove, B. Root exudates. In The Rhizosphere; Springer: Berlin/Heidelberg, Germany, 1986; pp. 55–92. [Google Scholar]
- Marschner, H. Mechanisms of manganese acquisition by roots from soils. In Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ held at the Waite Agricultural Research Institute; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; The University of Adelaide, Glen Osmond, South Australia; August 22–26; as an Australian Bicentennial Even; Springer: Dordrecht, Netherlands, 1988; pp. 191–204. [Google Scholar]
- Halvorson, A.D.; Del Grosso, S.J.; Reule, C.A. Nitrogen, tillage, and crop rotation effects on nitrous oxide emissions from irrigated cropping systems. J. Environ. Qual. 2008, 37, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Rozas, H.R.; Echeverría, H.E.; Picone, L.I. Denitrification in maize under no–tillage: Effect of nitrogen rate and application time. Soil Sci. Soc. Am. J. 2001, 65, 1314–1323. [Google Scholar] [CrossRef]
- Burton, D.L.; Zebarth, B.J.; Gillam, K.M.; Macleod, J.A. Effect of split application of fertilizer nitrogen on N2O emissions from potatoes. Can. J. Soil Sci. 2008, 88, 229–239. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Zhang, Y.; Shi, Y. Split nitrogen fertilizer application improved grain yield in winter wheat (Triticum aestivum L.) via modulating antioxidant capacity and 13C photosynthate mobilization under water-saving irrigation conditions. Ecol. Process. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Guardia, G.; Gonzalez-Murua, C.; Fuertes-Mendizabal, T.; Vallejo, A. The scarcity and distribution of rainfall drove the performance (i.e., mitigation of N oxide emissions, crop yield and quality) of calcium ammonium nitrate management in a wheat crop under rainfed semiarid conditions. Arch. Agron. Soil Sci. 2020, 66, 1827–1844. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Yildirim, S.; Andrikopoulos, B.; Wille, U.; Roessner, U. Deciphering the Interactions in the Root–Soil Nexus Caused by Urease and Nitrification Inhibitors: A Review. Agronomy 2023, 13, 1603. https://doi.org/10.3390/agronomy13061603
Gupta S, Yildirim S, Andrikopoulos B, Wille U, Roessner U. Deciphering the Interactions in the Root–Soil Nexus Caused by Urease and Nitrification Inhibitors: A Review. Agronomy. 2023; 13(6):1603. https://doi.org/10.3390/agronomy13061603
Chicago/Turabian StyleGupta, Sneha, Sibel Yildirim, Benjamin Andrikopoulos, Uta Wille, and Ute Roessner. 2023. "Deciphering the Interactions in the Root–Soil Nexus Caused by Urease and Nitrification Inhibitors: A Review" Agronomy 13, no. 6: 1603. https://doi.org/10.3390/agronomy13061603
APA StyleGupta, S., Yildirim, S., Andrikopoulos, B., Wille, U., & Roessner, U. (2023). Deciphering the Interactions in the Root–Soil Nexus Caused by Urease and Nitrification Inhibitors: A Review. Agronomy, 13(6), 1603. https://doi.org/10.3390/agronomy13061603







