High Buffering Potential of Winter Wheat Composite Cross Populations to Rapidly Changing Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Field Site and Experimental Design
2.3. Assessments
2.4. Data Processing and Statistics
3. Results
3.1. Seed Size Effects
3.2. Comparison of the Cycling and Non-Cycling CCPs and Their Origins
3.3. Comparison of the CCP Entries
3.4. Comparison of the CCP Entries with the Reference Varieties
4. Discussion
5. Conclusions
- While the CCPs were capable of levelling out the effects of differing seed sizes on final yield and protein content through yield component parameters when environmental conditions were less stressful in 2013/14, this was not the case during the drought in 2014/15. Thus, for stringent comparisons of genotype-environment interactions, differences between the CCP entries and generations due to differing seed weight at sowing should be avoided, e.g., by a round of seed reproduction in a common environment before future experiments. This will ensure that only genetic differences between entries are reported.
- The similarity in performance of the cycling and non-cycling populations and the high trait profile plasticity in their response to environmental conditions demonstrate the value of well-designed heterogeneous populations in the face of unpredictable environmental conditions. The CCPs were more climate resilient and better able to buffer environmental stress than the pure-line reference varieties, resulting in a lower yield reduction under drought stress.
- Constantly changing environmental conditions did not confer obvious agronomic advantages or disadvantages to the cycling CCPs, highlighting the buffering capacity of intraspecific diversity and confirming the research by Patel et al. [31] that large environmental variance results in slow population evolution. This is of interest to farmers who want to cultivate EPs but do not want to produce their own seed, relying rather on regionally decentralised certified seed production, which should not affect EP performance.
- In contrast, continuously applying differential selection environments for only five to eight years can lead to significant changes. Thus, the increased number of awned ears in the Hungarian home population, as well as the slightly higher susceptibility to stripe rust, fit the overall more continental environmental conditions with colder winters and lower precipitation in Hungary.
- Overall, EPs show great potential in terms of agronomic performance, particularly under conditions where higher biotic and abiotic pressures exist. Decentralised development of such populations allows for the dynamic maintenance of intra-specific diversity with high plasticity. In addition to providing an interesting alternative to genetically homogeneous varieties, it can contribute to the development of future genetic resources.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Østergård, H.; Finckh, M.R.; Fontaine, L.; Goldringer, I.; Hoad, S.P.; Kristensen, K.; Lammerts van Bueren, E.T.; Mascher, F.; Munk, L.; Wolfe, M.S. Time for a shift in crop production: Embracing complexity through diversity at all levels. J. Sci. Food Agric. 2009, 89, 1439–1445. [Google Scholar] [CrossRef]
- Döring, T.F.; Knapp, S.; Kovacs, G.; Murphy, K.; Wolfe, M.S. Evolutionary plant breeding in cereals-into a new era. Sustainability 2011, 3, 1944–1971. [Google Scholar] [CrossRef]
- Bacanovic-Sisic, J.; Dennenmoser, D.; Finckh, M.R. (Eds.) Breeding for Diversification. In Symposium on Breeding for Diversity: A Joint Meeting of the EUCARPIA Section Organic and Low-Input Agriculture, ECP-PB, DIVERSify, INSUSFAR, HealthyMinorCereals, LIVESEED, ReMIX and Wheatamix; Kassel University Press: Witzenhausen, Germany, 2018; pp. 1–133. [Google Scholar]
- Bocci, R.; Bussi, B.; Petitti, M.; Franciolini, R.; Altavilla, V.; Galluzzi, G.; Di Luzio, P.; Migliorini, P.; Spagnolo, S.; Floriddia, R.; et al. Yield, yield stability and farmers’ preferences of evolutionary populations of bread wheat: A dynamic solution to climate change. Eur. J. Agron. 2020, 121, 126156. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Evolutionary Plant Breeding as a Response to the Complexity of Climate Change. iScience 2020, 23, 101815. [Google Scholar] [CrossRef] [PubMed]
- Merrick, L.F.; Lyon, S.R.; Balow, K.A.; Murphy, K.M.; Jones, S.S.; Carter, A.H. Utilization of evolutionary plant breeding increases stability and adaptation of winter wheat across diverse precipitation zones. Sustainability 2020, 12, 9728. [Google Scholar] [CrossRef]
- Suneson, C. An evolutionary plant breeding method. Agron. J. 1956, 143, 188–191. [Google Scholar]
- European Commission Commission implementing decision of 18 March 2014 on the organisation of a temporary experiment providing for certain derogations for the marketing of plant populations of the plant species wheat, barley, oats and maize pursuant to Council Directive 66/40. Off. J. Eur. Union 2014, L, 82/29–82/36.
- European Commission Commission implementing decision of of 9 October 2018 amending Implementing Decision 2014/150/EU on the organisation of a temporary experiment providing for certain derogations for the marketing of populations of the plant species wheat, barley, oats and. Off. J. Eur. Union 2018, L, 256/65–256/66.
- Goldringer, I.; Enjalbert, J.; David, J.; Paillard, S.; Pham, J.; Brabant, P. Dynamic management of genetic resources: A 13-year experiment on wheat. In Broadening the Genetic Base of Crop Production; Cooper, H., Spillane, C., Hodgkin, T., Eds.; CABI: Wallingford, UK, 2001; pp. 245–260. [Google Scholar]
- Phillips, S.L.; Wolfe, M.S. Evolutionary plant breeding for low input systems. J. Agric. Sci. 2005, 143, 245–254. [Google Scholar] [CrossRef]
- Finckh, M.R.; Wolfe, M.S. Biodiversity Enhancement. In Plant Diseases and their Management in Organic Agriculture; Finckh, M.R., van Bruggen, A.H.C., Tamm, L., Eds.; APS Press: St. Paul, MN, USA, 2015; pp. 153–174. [Google Scholar]
- Dwivedi, S.L.; Lammerts van Bueren, E.T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef]
- van Frank, G.; Rivière, P.; Pin, S.; Baltassat, R.; Berthellot, J.-F.; Caizergues, F.; Dalmasso, C.; Gascuel, J.-S.; Hyacinthe, A.; Mercier, F.; et al. Genetic Diversity and Stability of Performance of Wheat Population Varieties Developed by Participatory Breeding. Sustainability 2020, 12, 384. [Google Scholar] [CrossRef]
- Baresel, J.P.; Bülow, L.; Finckh, M.R.; Frese, L.; Knapp, S.; Schmidhalter, U.; Weedon, O.D. Performance and evolutionary adaptation of heterogeneous wheat populations. Euphytica 2022, 218, 137. [Google Scholar] [CrossRef]
- Allard, R. Relationship between genetic diversity and consistency of performance in different environments. Crop Sci. 1960, 1, 127–133. [Google Scholar]
- Soliman, K.M.; Allard, R.W. Grain Yield of Composite Cross Populations of Barley: Effects of Natural Selection. Crop Sci. 1991, 31, 705–708. [Google Scholar]
- Brumlop, S.; Pfeiffer, T.; Finckh, M. Evolutionary Effects on Morphology and Agronomic Performance of Three Winter Wheat Composite Cross Populations Maintained for Six Years under Organic and Conventional Conditions. Org. Farming 2017, 3, 34–50. [Google Scholar] [CrossRef]
- Raggi, L.; Ciancaleoni, S.; Torricelli, R.; Terzi, V.; Ceccarelli, S.; Negri, V. Evolutionary breeding for sustainable agriculture: Selection and multi-environmental evaluation of barley populations and lines. Field Crops Res. 2017, 204, 76–88. [Google Scholar] [CrossRef]
- Weedon, O.D.; Finckh, M.R. Heterogeneous Winter Wheat Populations Differ in Yield Stability Depending on their Genetic Background and Management System. Sustainability 2019, 11, 6172. [Google Scholar] [CrossRef]
- Weedon, O.D.; Finckh, M.R. Response of Wheat Composite Cross Populations to Disease and Climate Variation Over 13 Generations. Front. Agric. Sci. Eng. 2021, 8, 400–415. [Google Scholar] [CrossRef]
- Paillard, S.; Goldringer, I.; Enjalbert, J.; Doussinault, G.; de Vallavieille-Pope, C.; Brabant, P. Evolution of resistance against powdery mildew in winter wheat populations conducted under dynamic management. I—Is specific seedling resistance selected? Theor. Appl. Genet. 2000, 101, 449–456. [Google Scholar] [CrossRef]
- Paillard, S.; Goldringer, I.; Enjalbert, J.; Trottet, M.; David, J.; De Vallavieille-Pope, C.; Brabant, P. Evolution of resistance against powdery mildew in winter wheat populations conducted under dynamic management. II. Adult plant resistance. Theor. Appl. Genet. 2000, 101, 457–462. [Google Scholar] [CrossRef]
- Goldringer, I.; Prouin, C.; Rousset, M.; Galic, N.; Bonnin, I. Rapid differentiation of experimental populations of wheat for heading time in response to local climatic conditions. Ann. Bot. 2006, 98, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Rhoné, B.; Remoué, C.; Galic, N.; Goldringer, I.; Bonnin, I. Insight into the genetic bases of climatic adaptation in experimentally evolving wheat populations. Mol. Ecol. 2008, 17, 930–943. [Google Scholar] [CrossRef]
- Rhoné, B.; Vitalis, R.; Goldringer, I.; Bonnin, I. Evolution of flowering time in experimental wheat populations: A comprehensive approach to detect genetic signatures of natural selection. Evolution 2010, 64, 2110–2125. [Google Scholar] [CrossRef] [PubMed]
- Bertholdsson, N.O.; Weedon, O.; Brumlop, S.; Finckh, M.R. Evolutionary changes of weed competitive traits in winter wheat composite cross populations in organic and conventional farming systems. Eur. J. Agron. 2016, 79, 23–30. [Google Scholar] [CrossRef]
- Vijaya Bhaskar, A.V.; Baresel, J.P.; Weedon, O.D.; Finckh, M.R. Effects of ten years organic and conventional farming on early seedling traits of evolving winter wheat composite cross populations. Sci. Rep. 2019, 9, 9053. [Google Scholar] [CrossRef]
- Döring, T.F.; Annicchiarico, P.; Clarke, S.; Haigh, Z.; Jones, H.E.; Pearce, H.; Snape, J.; Zhan, J.; Wolfe, M.S. Comparative analysis of performance and stability among composite cross populations, variety mixtures and pure lines of winter wheat in organic and conventional cropping systems. Field Crops Res. 2015, 183, 235–245. [Google Scholar] [CrossRef]
- Weiner, J. Applying plant ecological knowledge to increase agricultural sustainability. J. Ecol. 2017, 105, 865–870. [Google Scholar] [CrossRef]
- Patel, J.D.; Reinbergs, E.; Mather, D.E.; Choo, T.M.; Sterling, J.D.E. Natural selection in a double-haploid mixture and a composite cross of barley. Crop Sci. 1987, 27, 474–479. [Google Scholar]
- Brumlop, S.; Weedon, O.; Link, W.; Finckh, M.R. Effective population size (Ne) of organically and conventionally grown composite cross winter wheat populations depending on generation. Eur. J. Agron. 2019, 109, 125922. [Google Scholar] [CrossRef]
- VDLUFA. Die Untersuchung von Böden. Methodenbuch Band 1; VDLUFA Verlag: Darmstadt, Germany, 1991. [Google Scholar]
- Lupton, F.G.H. Estimation of yield in wheat from measurements of photosynthesis and translocation in the field. Ann. Appl. Biol. 1969, 64, 363–374. [Google Scholar]
- Shaner, G.; Finney, R.E. The Effect of Nitrogen Fertilization on the Expression of Slow-Mildewing Resistance in Knox Wheat. Phytopathology 1977, 77, 1051–1056. [Google Scholar] [CrossRef]
- Fry, W.E. Quantification of General Resistance of Potato Cultivars and Fungicide Effects for Integrated Control of Potato Late Blight. Phytopathology 1978, 68, 1650–1655. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 23 May 2023).
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M.; Riebl, H. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.4.8. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 23 May 2023).
- Olivoto, T.; Lúcio, A.D. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Yan, W. Crop Variety Trials: Data Management and Analysis; Wiley-Blackwell: Chichester, UK, 2014; ISBN 978-1-118-68855-7. [Google Scholar]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to “ggplot2”. R Package Version 2.0.0. 2020. Available online: https://CRAN.R-project.org/package=GGally (accessed on 23 May 2023).
- Monaghan, J.M.; Snape, J.W.; Chojecki, A.J.S.; Kettlewell, P.S. The use of grain protein deviation for identifying wheat cultivars with high grain protein concentration and yield. Euphytica 2001, 122, 309–317. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; MacHet, J.M.; Jeuffroy, M.H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the grain protein concentration-grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.L. Seed Variation in Wild Radish: Effect of Seed Size on Components of Seedling and Adult Fitness. Ecology 1984, 65, 1105–1112. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Khan, I.; Sanaullah, M.; Farooq, M. Influence of seed size on the growth, productivity, and water use efficiency of bread wheat planted by different methods. Arch. Agron. Soil Sci. 2021, 67, 354–370. [Google Scholar] [CrossRef]
- Shoaib, M.; Nawaz, M.; Ilyas, M.; Shafique, M.; Khan, I.; Aslam, M.T.; Bazmi, M.S.A.; Arshad, M.; Ahmad, G.; Irfan, M.; et al. Effect of Different Seed Sizes and Seed Rates on the Growth and Productivity of Wheat Grown Under Semi-Arid Conditions. Pak. J. Agric. Res. 2022, 35, 122–130. [Google Scholar] [CrossRef]
- Jones, H.; Clarke, S.; Haigh, Z.; Pearce, H.; Wolfe, M. The effect of the year of wheat variety release on productivity and stability of performance on two organic and two non-organic farms. J. Agric. Sci. 2010, 148, 303–317. [Google Scholar] [CrossRef][Green Version]
- Danquah, E.Y.; Barrett, J.A. Grain yield in Composite Cross Five of barley: Effects of natural selection. J. Agric. Sci. 2002, 138, 171–176. [Google Scholar] [CrossRef]
- Allard, R.W.; Hansche, P.E. Some parameters of population variability and their impications in plant breeding. Adv. Agron. 1964, 16, 281–325. [Google Scholar] [CrossRef]
- Murphy, K.; Lammer, D.; Lyon, S.; Carter, B.; Jones, S.S. Breeding for organic and low-input farming systems: An evolutionary–participatory breeding method for inbred cereal grains. Renew. Agric. Food Syst. 2005, 20, 48–55. [Google Scholar] [CrossRef]
- Mežaka, I.; Ločmele, I.; Ruņģis, D.; Legzdiņa, L. Response of bi-parental spring barley populations to cultivation in organic and conventional farming systems. Zemdirbyste-Agriculture 2017, 104, 157–164. [Google Scholar] [CrossRef][Green Version]
- Kingsolver, J.G.; Hoekstra, H.E.; Hoekstra, J.M.; Berrigan, D.; Vignieri, S.N.; Hill, C.E.; Hoang, A.; Gilbert, P.; Beerli, P. The strength of phenotypic selection in natural populations. Am. Nat. 2001, 157, 245–261. [Google Scholar] [CrossRef]
- Goldringer, I.; Paillard, S.; Enjalbert, J.; David, J.L.; Brabant, P. Divergent evolution of wheat populations conducted under recurrent selection and dynamic management. Agronomie 1998, 18, 413–425. [Google Scholar] [CrossRef]
- Blum, A. The effects of heat stress on wheat leaf and ear photosynthesis. J. Exp. Bot. 1986, 37, 111–118. [Google Scholar]
- Frey, K.J. Mass selection for seed width in oat populations. Euphytica 1967, 16, 341–349. [Google Scholar]
- Döring, T.F.; Crowley, O.; Wolfe, M.S. Against the Grain. Org. Farming 2011, 107, 42–43. [Google Scholar]
- Duveiller, E.; Singh, R.P.; Nicol, J.M. The challenges of maintaining wheat productivity: Pests, diseases, and potential epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Le Boulc’h, V.; David, J.; Brabant, P.; de Vallavieille-Pope, C. Dynamic conservation of variability—Responses of wheat populations to different selective forces including powdery mildew. Genet. Sel. Evol. 1994, 26, 221–240. [Google Scholar] [CrossRef]
- Porcher, E.; Gouyon, P.H.; Lavigne, C. Dynamic management of genetic resources: Maintenance of outcrossing in experimental metapopulations of a predominantly inbreeding species. Conserv. Genet. 2004, 5, 259–269. [Google Scholar] [CrossRef]
- Jackson, L.; Kahler, A.; Webster, R.; Allard, R. Conservation of scald resistance in barley composite cross populations. Phytopathology 1978, 68, 645–650. [Google Scholar] [CrossRef]
- Webster, R.K.; Saghai Maroof, M.A.; Allard, R.W. Evolutionary Response of Barley Composite Cross II to Rhynchosporium secalis Analyzed by Pathogenic Complexity and by Gene-by-Race Relationships. Phytopathology 1986, 76, 661–668. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Barrett, J.A. Evolution of mildew resistance in a hybrid bulk population of barley. Heredity 1991, 67, 247–256. [Google Scholar] [CrossRef]
- Danquah, E.Y.; Barrett, J.A. Hordein variation and reaction to powdery mildew in composite cross XLII of barley. Genetica 2002, 114, 81–87. [Google Scholar] [CrossRef]
- Vaughton, G.; Ramsey, M. Sources and consequences of seed mass variation in Banksia marginata (Proteaceae). J. Ecol. 1998, 86, 563–573. [Google Scholar] [CrossRef]
- Lehtilä, K.; Ehrlén, J. Seed size as an indicator of seed quality: A case study of Primula veris. Acta Oecol. 2005, 28, 207–212. [Google Scholar] [CrossRef]
- Goldringer, I.; Enjalbert, J.; Raquin, A.-L.; Brabant, P. Strong selection in wheat populations during ten generations of dynamic management. Genet. Sel. Evol. 2001, 33, 441–463. [Google Scholar] [CrossRef]
- Silvertown, J. The Paradox od Seed Size and Adaptation. Trends Ecol. Evol. 1989, 4, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Engbersen, N.; Stefan, L.; Schmid, B.; Sun, H.; Schöb, C. Diversity increases yield but reduces harvest index in crop mixtures. Nat. Plants 2021, 7, 893–898. [Google Scholar] [CrossRef] [PubMed]
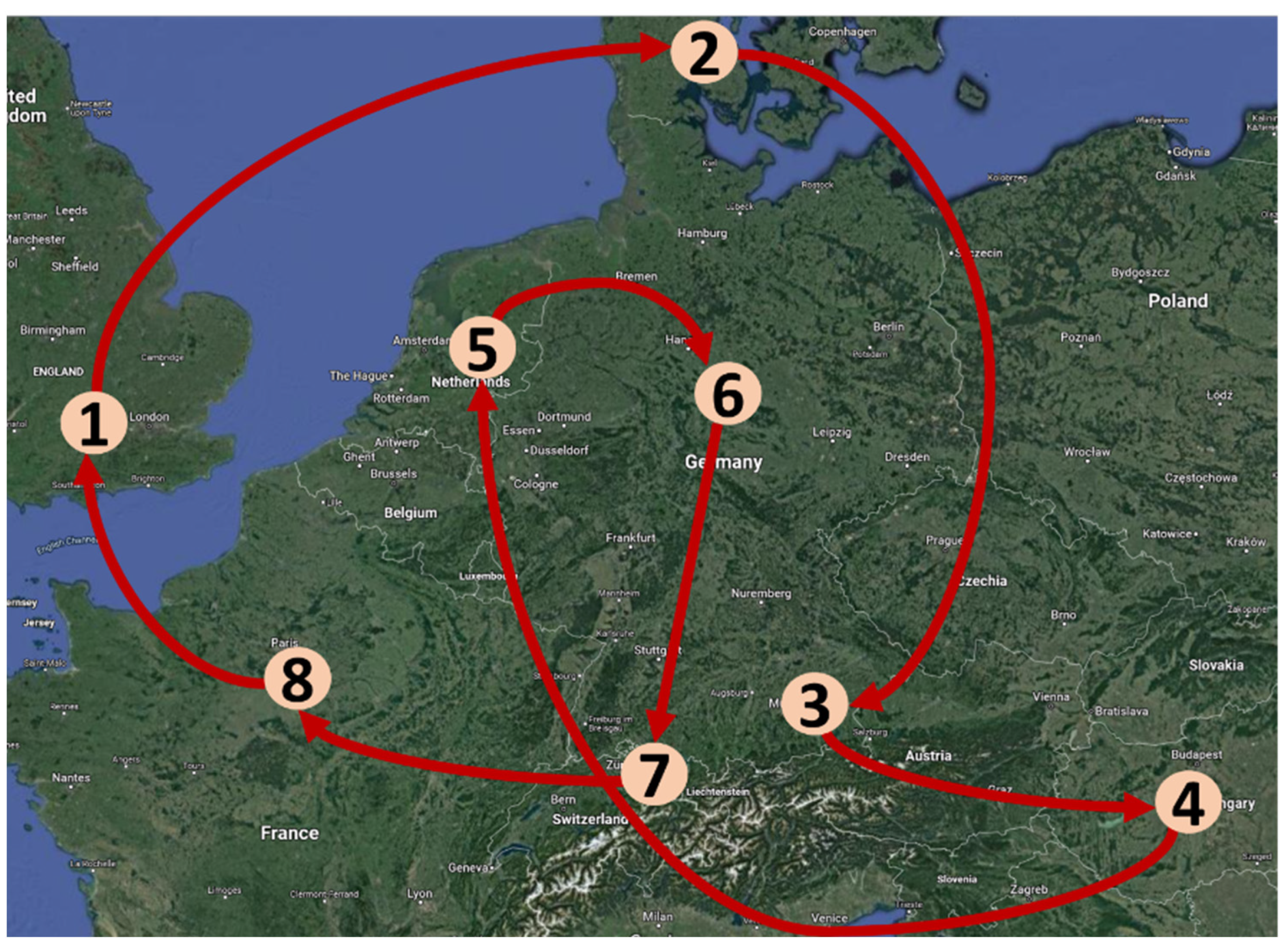

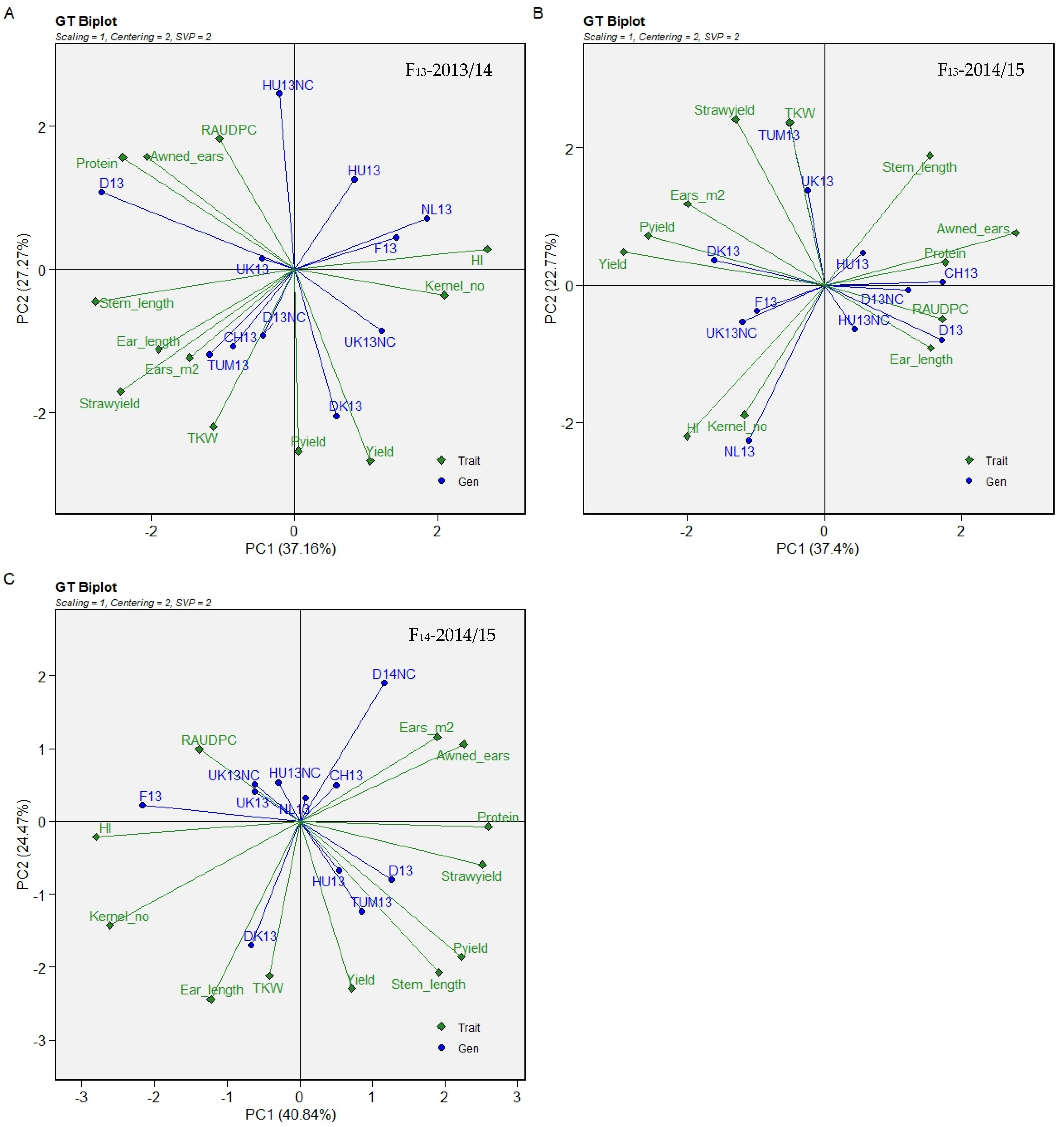
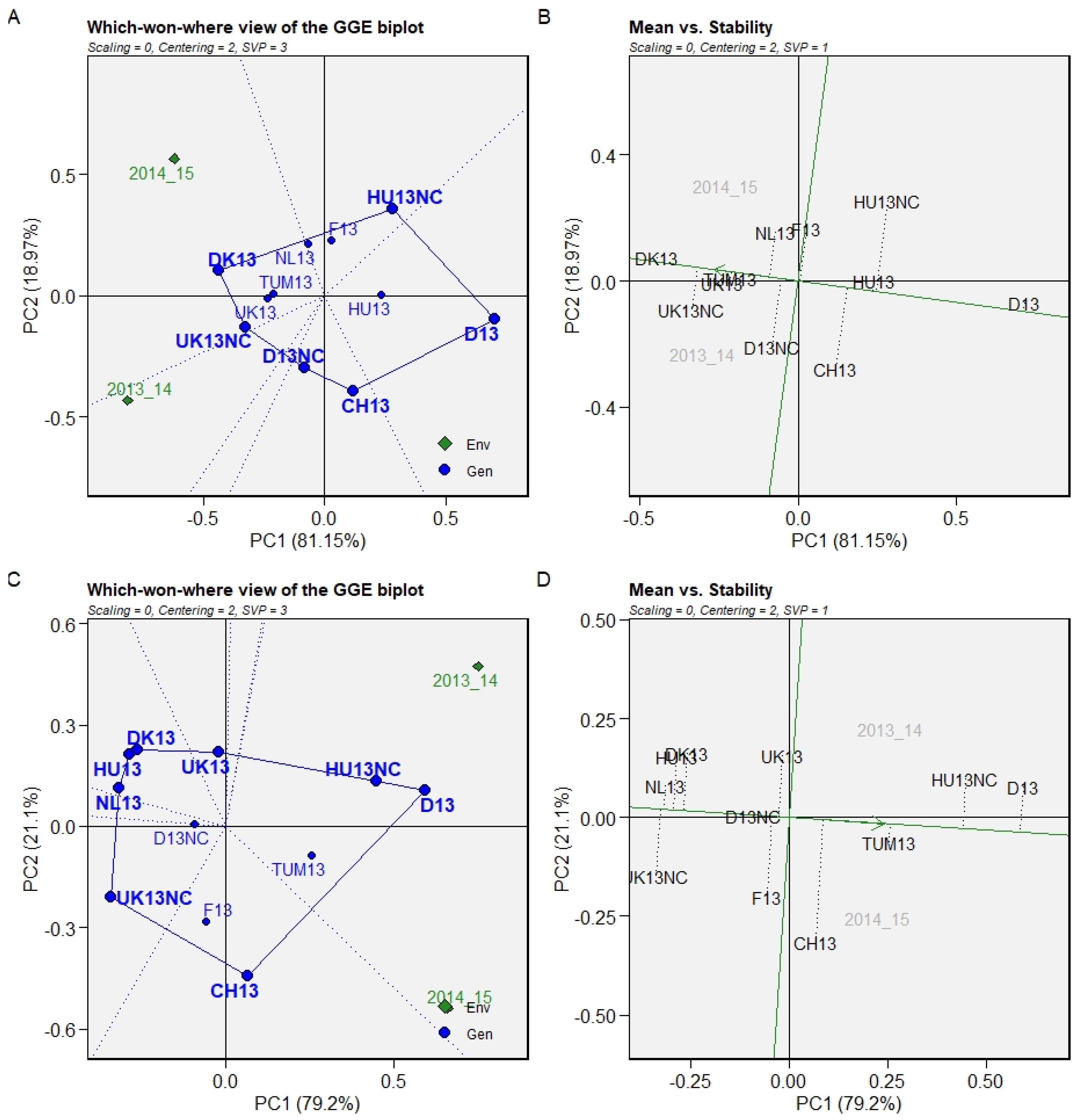
| Origin | 2008/09 | 2009/10 | 2010/11 | 2011/12 | 2012/13 | CCP Code Used in This Study | Cycling (C)/Non-Cycling (NC) |
|---|---|---|---|---|---|---|---|
 | F13/F14 ** | ||||||
| D | D(KS) * | D(KS) | D(KS) | D(KS) | D(KS) | D13NC/D14NC | NC |
| D(KS) | CH | F | UK | DK | DK13 | C | |
| CH | F | DK | D(TUM) | HU | HU13 | C | |
| CH | F | UK | DK | D(TUM) | TUM13 | C | |
| HU | HU | HU | HU | HU | HU | HU13NC | NC |
| HU | NL | D(KS) | CH | F | F13 | C | |
| NL | D(KS) | CH | F | UK | UK13 | C | |
| UK | UK | UK | UK | UK | UK | UK13NC | NC |
| D(TUM) | HU | NL | D(KS) | CH | CH13 | C | |
| DK | D(TUM) | HU | NL | D(KS) | D13 | C | |
| UK | DK | D(TUM) | HU | NL | NL13 | C | |
| 2013/14 | ||||||||
| Gen. | Yield | HI | AUDPC | TKW | Ears_m2 | Kernel no. | Protein | Stem length |
| F13 | 0.08 | −0.19 | 0.32 * | 0.36 * | −0.01 | −0.27 | 0.12 | 0.22 |
| 2014/15 | ||||||||
| F13 | −0.01 | −0.43 ** | 0.02 | 0.14 | 0.01 | −0.25 | −0.14 | 0.41 ** |
| F14 | −0.10 | −0.13 | 0.05 | 0.15 | 0.06 | −0.08 | −0.02 | 0.02 |
| Origin | Yield (t/ha) | HI | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | ||||||||||||||||||||
| F13 | F13 | F14 | F13 | F13 | F14 | ||||||||||||||||||
| NC | C | NC | C | NC | C | NC | C | NC | C | NC | C | ||||||||||||
| D | 4.92 | ab | 4.82 | 4.09 | 4.31 | b | 4.09 | 4.37 | 0.39 | 0.40 | 0.38 | 0.39 | 0.37 | a* | 0.39 | ab | |||||||
| HU | 4.34 | a | 4.76 | 4.24 | 4.33 | b | 4.19 | 4.11 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | b | 0.41 | b | |||||||
| UK | 5.04 | b* | 4.56 | 4.34 | 4.00 | a | 4.22 | 4.27 | 0.40 | 0.40 | 0.41 | 0.40 | 0.41 | b* | 0.38 | a | |||||||
| Mean | 4.77 | 4.71 | 4.22 | 4.22 | 4.17 | 4.25 | 0.40 | 0.40 | 0.40 | 0.39 | 0.39 | 0.39 | |||||||||||
| RAUDPC | TKW (g) | ||||||||||||||||||||||
| D | 0.06 | 0.05 | 0.14 | b* | 0.11 | 0.10 | a | 0.11 | 45.9 | b* | 44.1 | 45.7 | b | 45.2 | b | 44.2 | * | 45.6 | b | ||||
| HU | 0.07 | 0.07 | 0.17 | b* | 0.12 | 0.15 | b | 0.13 | 41.7 | a* | 44.1 | 43.3 | a* | 44.8 | b | 44.8 | 45.2 | ab | |||||
| UK | 0.04 | 0.05 | 0.10 | a | 0.12 | 0.12 | ab | 0.12 | 42.7 | a | 43.5 | 44.1 | ab | 43.4 | a | 44.2 | 44.5 | a | |||||
| Mean | 0.06 | 0.06 | 0.13 | B | 0.12 | A | 0.12 | 0.12 | 43.4 | 43.9 | 44.3 | 44.4 | 44.4 | 45.1 | |||||||||
| Ears_m2 | Kernel no. | ||||||||||||||||||||||
| D | 399 | 420 | ab | 368 | a* | 427 | 462 | 419 | 29 | 30 | 27 | 25 | a | 22 | a* | 26 | |||||||
| HU | 389 | 389 | a | 417 | ab | 433 | 418 | 381 | 30 | 30 | 26 | 26 | ab | 26 | b | 27 | |||||||
| UK | 408 | 441 | b | 436 | b | 403 | 442 | 419 | 30 | 30 | 27 | 27 | b | 27 | b | 25 | |||||||
| Mean | 399 | 417 | 407 | 421 | 441 | B | 407 | A | 30 | 30 | 27 | 26 | 25 | 26 | |||||||||
| Protein (%) | Awned ears (%) | ||||||||||||||||||||||
| D | 9.7 | ab | 9.7 | 9.7 | 9.7 | 9.8 | 9.7 | 11 | b | 7 | 11 | b | 7 | 10 | 7 | ab | |||||||
| HU | 10.1 | b | 9.7 | 10.0 | 9.8 | 9.7 | 9.6 | 14 | b* | 7 | 11 | b* | 6 | 11 | * | 6 | a | ||||||
| UK | 9.4 | a | 9.8 | 9.7 | 9.9 | 9.4 | 9.9 | 3 | a* | 10 | 3 | a* | 10 | 5 | * | 10 | b | ||||||
| Mean | 9.7 | 9.7 | 9.8 | 9.8 | 9.7 | 9.7 | 9 | 8 | 9 | 8 | 9 | 7 | |||||||||||
| Stem length (cm) | Ear length (cm) | ||||||||||||||||||||||
| D | 92.2 | 94.0 | 89.9 | 88.5 | 87.8 | 90.6 | 8.56 | 8.39 | 7.68 | 7.42 | 7.08 | * | 7.67 | ||||||||||
| HU | 94.5 | 92.7 | 86.6 | 87.9 | 88.0 | 87.5 | 8.44 | 8.36 | 7.51 | 7.53 | 7.61 | 7.61 | |||||||||||
| UK | 94.1 | 95.3 | 86.8 | 88.8 | 88.3 | 90.3 | 8.30 | 8.47 | 7.59 | 7.62 | 7.47 | 7.56 | |||||||||||
| Mean | 93.6 | 94.0 | 87.8 | 88.4 | 88.0 | 89.5 | 8.43 | 8.41 | 7.59 | 7.52 | 7.39 | A | 7.61 | B | |||||||||
| Origin | Entry | Yield (t/ha) | HI | AUDPC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | ||||||||||||||
| F13 | F13 | F14 | F13 | F13 | F14 | F13 | F13 | F14 | |||||||||||
| References | Achat | 4.9 | bc | 4.3 | 4.3 | 0.43 | c | 0.41 | bcd | 0.41 | bc | 98 | ab | 209 | a | 209 | ab | ||
| Akteur | 3.7 | a | 3.8 | 3.8 | 0.40 | abc | 0.38 | abc | 0.38 | abc | 175 | b | 338 | c | 338 | b | |||
| Capo | 5.6 | c | 4.2 | 4.2 | 0.41 | abc | 0.37 | a | 0.37 | ab | 43 | a | 176 | a | 176 | a | |||
| D | D13NC | 4.9 | bc | 4.1 | 4.1 | 0.39 | a | 0.38 | abc | 0.37 | a | 72 | a | 262 | abc | 190 | a | ||
| DK13 | 5.0 | bc | 4.5 | 4.4 | 0.41 | abc | 0.40 | abcd | 0.39 | abc | 52 | a | 182 | a | 197 | a | |||
| HU13 | 4.5 | ab | 4.1 | 4.4 | 0.40 | abc | 0.38 | ab | 0.39 | abc | 73 | ab | 229 | ab | 194 | a | |||
| TUM13 | 4.9 | bc | 4.3 | 4.4 | 0.39 | a | 0.38 | abc | 0.39 | abc | 53 | a | 211 | a | 209 | ab | |||
| HU | HU13NC | 4.3 | ab | 4.2 | 4.2 | 0.40 | abc | 0.40 | abcd | 0.40 | abc | 94 | ab | 318 | bc | 276 | ab | ||
| F13 | 4.6 | b | 4.3 | 4.2 | 0.40 | abc | 0.41 | bcd | 0.42 | c | 65 | a | 240 | abc | 238 | ab | |||
| UK13 | 4.9 | bc | 4.4 | 4.1 | 0.39 | ab | 0.39 | abc | 0.39 | abc | 109 | ab | 225 | ab | 249 | ab | |||
| UK | UK13NC | 5.0 | bc | 4.3 | 4.2 | 0.40 | abc | 0.41 | cd | 0.41 | bc | 56 | a | 182 | a | 220 | ab | ||
| CH13 | 4.8 | bc | 3.9 | 4.2 | 0.39 | ab | 0.39 | abcd | 0.38 | ab | 67 | a | 238 | abc | 240 | ab | |||
| D13 | 4.2 | ab | 3.7 | 4.2 | 0.38 | a | 0.39 | abcd | 0.37 | a | 79 | ab | 230 | ab | 208 | ab | |||
| NL13 | 4.7 | bc | 4.4 | 4.4 | 0.42 | bc | 0.42 | d | 0.40 | bc | 58 | a | 215 | a | 243 | ab | |||
| Reference mean | 4.7 | 4.1 | 4.1 | 0.41 | B | 0.39 | 0.39 | 105 | 241 | 241 | |||||||||
| CCP mean | 4.7 | 4.2 | 4.2 | 0.39 | A | 0.40 | 0.39 | 71 | 230 | 224 | |||||||||
| Origin | Entry | TKW (g) | Ears_m2 | Kernel no. per ear | |||||||||||||||
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | ||||||||||||||
| F13 | F13 | F14 | F13 | F13 | F14 | F13 | F13 | F14 | |||||||||||
| References | Achat | 48.1 | e | 48.0 | d | 48.0 | c | 389 | ab | 409 | ab | 410 | ab | 31 | ab | 27 | bc | 27 | |
| Akteur | 46.6 | de | 42.9 | a | 42.9 | a | 324 | a | 336 | a | 336 | a | 27 | a | 26 | abc | 26 | ||
| Capo | 46.2 | de | 46.0 | cd | 46.0 | bc | 393 | ab | 378 | ab | 379 | ab | 30 | ab | 26 | abc | 26 | ||
| D | D13NC | 45.9 | cde | 45.7 | bcd | 44.2 | ab | 399 | ab | 367 | ab | 462 | b | 29 | ab | 27 | abc | 22 | |
| DK13 | 44.9 | bcd | 45.0 | abc | 45.9 | bc | 442 | b | 428 | b | 388 | ab | 31 | ab | 27 | bc | 27 | ||
| HU13 | 43.3 | abc | 44.4 | abc | 45.6 | b | 396 | ab | 412 | ab | 427 | ab | 29 | ab | 23 | a | 25 | ||
| TUM13 | 44.1 | abcd | 46.1 | cd | 45.4 | b | 420 | b | 439 | b | 441 | b | 29 | ab | 25 | abc | 25 | ||
| HU | HU13NC | 41.7 | a | 43.3 | ab | 44.8 | ab | 389 | ab | 417 | ab | 418 | ab | 30 | ab | 26 | abc | 26 | |
| F13 | 43.3 | ab | 45.0 | abc | 44.7 | ab | 379 | ab | 434 | b | 374 | ab | 32 | b | 25 | abc | 28 | ||
| UK13 | 45.0 | bcd | 44.7 | abc | 45.6 | b | 398 | ab | 431 | b | 388 | ab | 28 | ab | 26 | abc | 26 | ||
| UK | UK13NC | 42.7 | ab | 44.1 | abc | 44.2 | ab | 408 | b | 435 | b | 441 | b | 30 | ab | 27 | bc | 27 | |
| CH13 | 45.2 | bcd | 44.7 | abc | 44.6 | ab | 442 | b | 392 | ab | 407 | ab | 30 | ab | 25 | abc | 24 | ||
| D13 | 43.4 | abc | 42.5 | a | 44.5 | ab | 453 | b | 414 | ab | 402 | ab | 28 | ab | 26 | abc | 25 | ||
| NL13 | 42.0 | a | 42.9 | a | 44.2 | ab | 425 | b | 401 | ab | 449 | b | 30 | ab | 29 | c | 26 | ||
| Reference mean | 46.9 | B | 45.6 | B | 45.6 | 369 | A | 375 | A | 375 | A | 29 | 26 | 26 | |||||
| CCP mean | 43.8 | A | 44.4 | A | 44.9 | 414 | B | 415 | B | 417 | B | 30 | 26 | 26 | |||||
| Origin | Entry | Protein (%) | Water (%) | Gluten (%) | Sedi. (Zeleny) | HFN (sec.) | Baking vol. (mL/100g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Achat | 10.0 | ab | 60.5 | 21.9 | abc | 31.8 | cd | 370 | d | 306.0 |
| Akteur | 10.7 | b | 62.3 | 24.3 | c | 35.8 | d | 373 | d | 318.4 | |
| Capo | 9.7 | a | 62.4 | 21.4 | abc | 31.0 | cd | 313 | c | 278.2 | |
| D | D14NC | 9.8 | ab | 60.5 | 21.7 | abc | 25.8 | ab | 277 | abc | 303.0 |
| DK13 | 9.5 | a | 59.7 | 19.8 | ab | 24.3 | ab | 304 | bc | 297.1 | |
| HU13 | 9.6 | a | 60.0 | 20.2 | ab | 22.8 | a | 260 | ab | 300.0 | |
| TUM13 | 9.9 | ab | 59.8 | 21.3 | abc | 22.0 | a | 281 | abc | 281.0 | |
| HU | HU13NC | 9.7 | a | 59.9 | 20.5 | ab | 25.8 | ab | 261 | ab | 295.0 |
| F13 | 9.3 | a | 59.3 | 18.6 | a | 21.5 | a | 249 | a | 279.0 | |
| UK13 | 9.8 | ab | 60.5 | 20.9 | abc | 25.0 | ab | 271 | abc | 301.0 | |
| UK | UK13NC | 9.4 | a | 60.0 | 20.0 | ab | 21.5 | a | 283 | abc | 294.4 |
| CH13 | 9.8 | ab | 62.0 | 20.8 | abc | 25.3 | ab | 287 | abc | 307.0 | |
| D13 | 10.1 | ab | 60.6 | 22.3 | bc | 28.0 | bc | 288 | abc | 310.0 | |
| NL13 | 9.7 | a | 60.1 | 20.1 | ab | 22.8 | a | 290 | abc | 283.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weedon, O.D.; Brumlop, S.; Haak, A.; Baresel, J.P.; Borgen, A.; Döring, T.; Goldringer, I.; Lammerts van Bueren, E.; Messmer, M.M.; Mikó, P.; et al. High Buffering Potential of Winter Wheat Composite Cross Populations to Rapidly Changing Environmental Conditions. Agronomy 2023, 13, 1662. https://doi.org/10.3390/agronomy13061662
Weedon OD, Brumlop S, Haak A, Baresel JP, Borgen A, Döring T, Goldringer I, Lammerts van Bueren E, Messmer MM, Mikó P, et al. High Buffering Potential of Winter Wheat Composite Cross Populations to Rapidly Changing Environmental Conditions. Agronomy. 2023; 13(6):1662. https://doi.org/10.3390/agronomy13061662
Chicago/Turabian StyleWeedon, Odette D., Sarah Brumlop, Annette Haak, Jörg Peter Baresel, Anders Borgen, Thomas Döring, Isabelle Goldringer, Edith Lammerts van Bueren, Monika M. Messmer, Péter Mikó, and et al. 2023. "High Buffering Potential of Winter Wheat Composite Cross Populations to Rapidly Changing Environmental Conditions" Agronomy 13, no. 6: 1662. https://doi.org/10.3390/agronomy13061662
APA StyleWeedon, O. D., Brumlop, S., Haak, A., Baresel, J. P., Borgen, A., Döring, T., Goldringer, I., Lammerts van Bueren, E., Messmer, M. M., Mikó, P., Nuijten, E., Pearce, B., Wolfe, M., & Finckh, M. R. (2023). High Buffering Potential of Winter Wheat Composite Cross Populations to Rapidly Changing Environmental Conditions. Agronomy, 13(6), 1662. https://doi.org/10.3390/agronomy13061662








