Effect of Selenium Application on Growth, Antioxidative Capacity, and Nutritional Quality in Purple Lettuce Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Culture Condition, and Se Treatment
2.2. Biomass, Root Length, and Water Content
2.3. Histochemical Analysis
2.3.1. Reactive Oxygen Species
2.3.2. Cytoplasmic Membrane Integrity
2.4. Malondialdehyde (MDA) Content
2.5. Antioxidant Enzyme Activity Assays
2.6. Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.7. Determination of Se and Other Compounds
2.7.1. Se Content
2.7.2. Total Soluble Protein Content
2.7.3. Total Soluble Sugar Content
2.7.4. Anthocyanin Content
2.7.5. Vitamin C Content
2.8. Statistical Analysis
3. Results
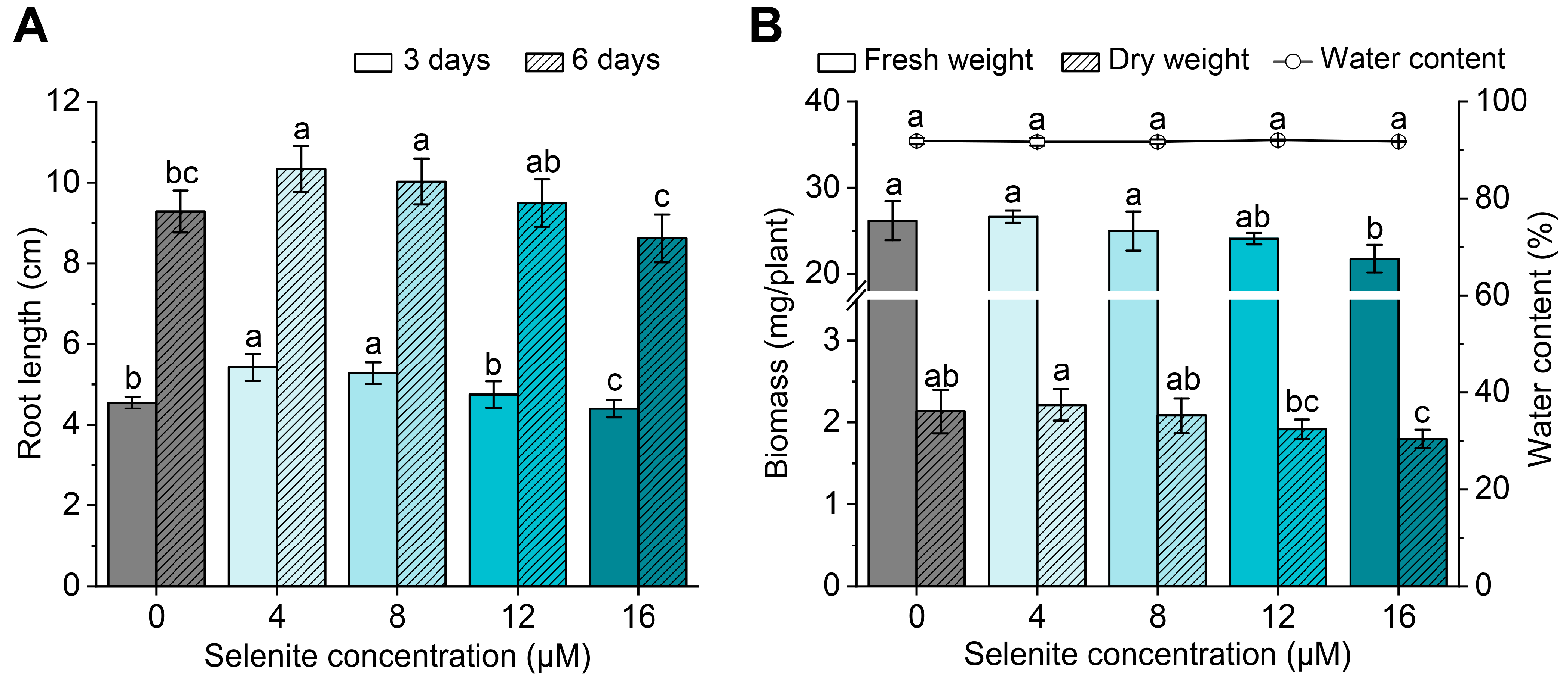
3.1. Effect of Exogenous Se Application on Seedling Growth
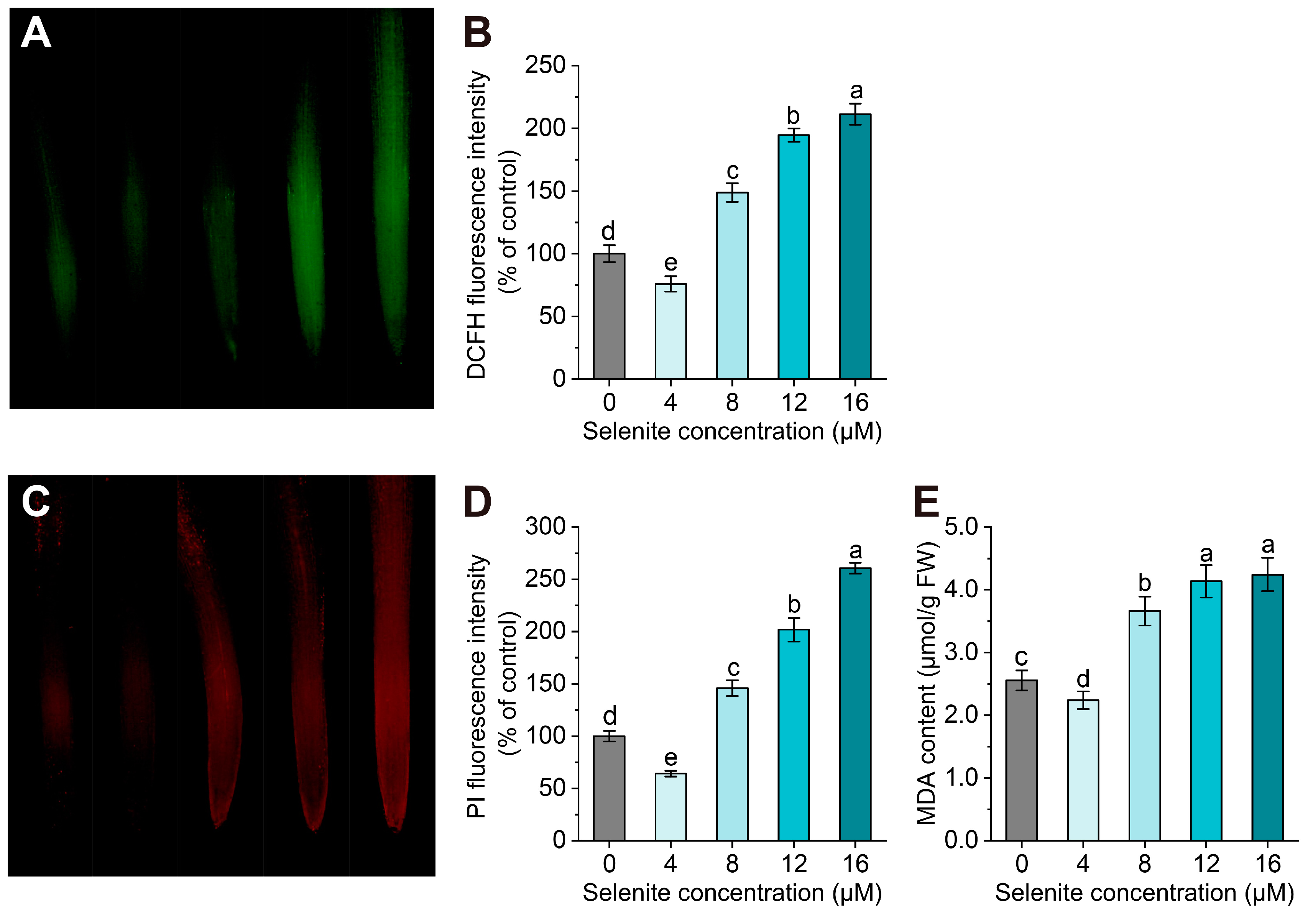
3.2. Oxidative Damage Induced by the High Se Application Concentration
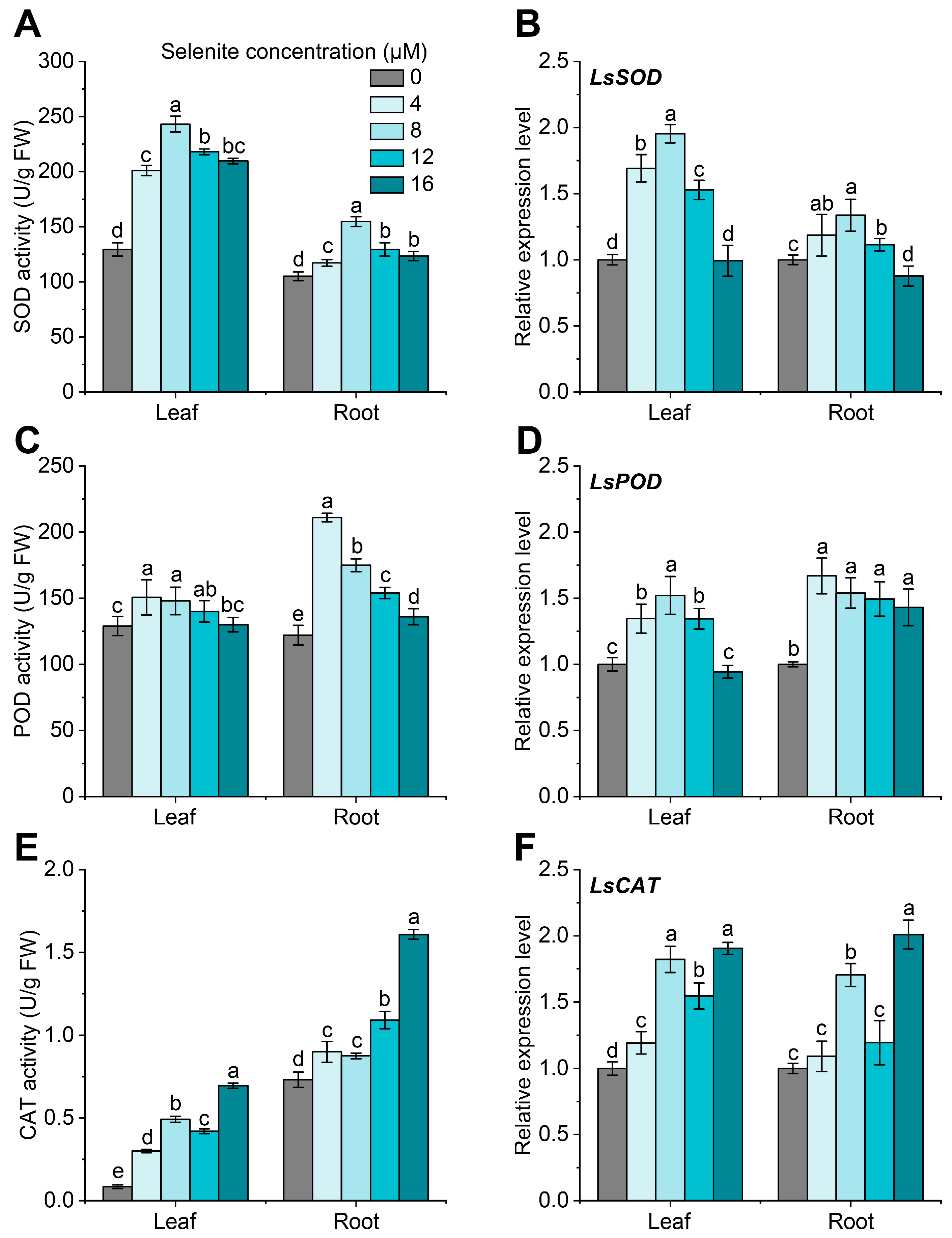
3.3. Effect of Exogenous Se Application on the Antioxidant Enzymes
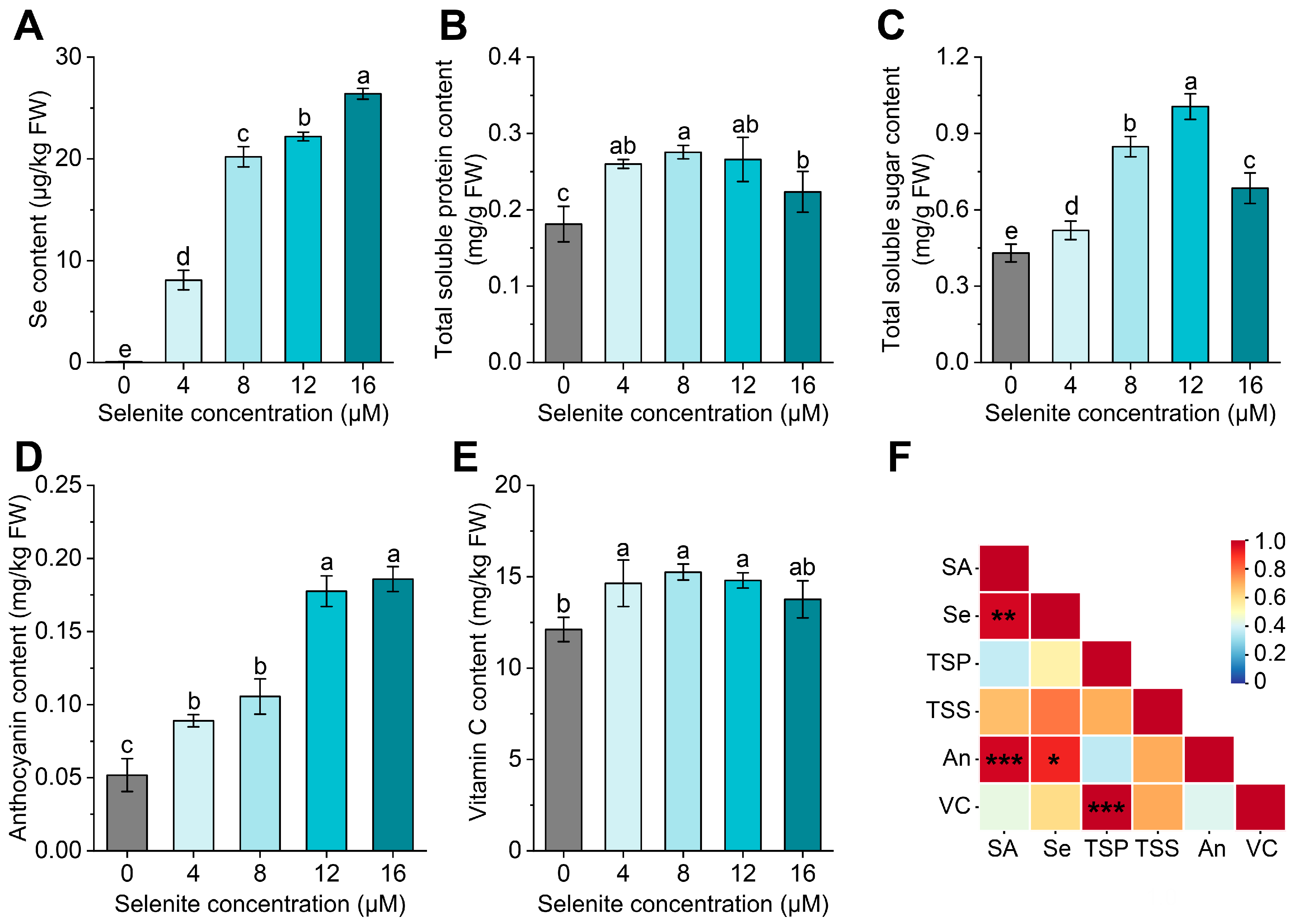
3.4. Effect of Exogenous Se Application on the Se and Nutrient Accumulation
3.5. The Anthocyanin Content was Positively Correlated with the Expression Level of LsCHS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y. Selenium transport mechanism via selenoprotein P-its physiological role and related diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and its supplementation in cardiovascular disease—What do we know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Reyes, R.; Suetens, C.; Mathieu, F.; Begaux, F.; Zhu, D.; Rivera, M.T.; Boelaert, M.; Nève, J.; Perlmutter, N.; Vanderpas, J. Kashin–Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N. Engl. J. Med. 1998, 339, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yu, F.; Shao, W.; Ding, D.; Yu, Z.; Chen, F.; Geng, D.; Tan, X.; Lammi, M.J.; Guo, X. Associations between Selenium content in hair and Kashin-Beck disease/Keshan disease in children in northwestern China: A prospective cohort study. Biol. Trace Elem. Res. 2018, 184, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W. Increased intakes of selenium-enriched foods may benefit human health. J. Sci. Food Agric. 2007, 87, 1620–1629. [Google Scholar] [CrossRef]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention—A review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Lanza, M.; Reis, A.R.D. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; Reis, A.R.D. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium biofortification: Strategies, progress and challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, C. Selenium uptake, transport, metabolism, reutilization, and biofortification in rice. Rice 2022, 15, 30. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium enrichment of horticultural crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compost. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef]
- Sager, M. Selenium in agriculture, food, and nutrition. Pure Appl. Chem. 2006, 78, 111–133. [Google Scholar] [CrossRef]
- Businelli, D.; D’Amato, R.; Onofri, A.; Tedeschini, E.; Tei, F. Se-enrichment of cucumber ( Cucumis sativus L.), lettuce (Lactuca sativa L.) and tomato (Solanum lycopersicum L. Karst) through fortification in pre-transplanting. Sci. Hortic. 2015, 197, 697–704. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, H.; Wang, Y.; Ying, Z.; Bian, Z.; Zhu, W.; Liu, W.; Yang, L.; Jiang, D. How exogenous selenium affects anthocyanin accumulation and biosynthesis-related gene expression in purple lettuce. Pol. J. Environ. Stud. 2017, 26, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci. Hortic. 2008, 116, 248–255. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating micronutrient deficiency and enhancing food functional quality through selenium fortification of select lettuce genotypes grown in a closed soilless system. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.J.; Blasco, B.; Cervilla, L.M.; Rosales, M.A.; Sanchez-Rodriguez, E.; Romero, L.; Ruiz, J.M. Production and detoxification of H2O2 in lettuce plants exposed to selenium. Ann. Appl. Biol. 2009, 154, 107–116. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Czernicka, M.; Halka, M.; Keska, K.; Sady, W. Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata). Front. Plant Sci. 2016, 7, 1553. [Google Scholar] [CrossRef] [Green Version]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Shalaby, T.; Bayoumi, Y.; Alshaal, T.; Elhawat, N.; Sztrik, A.; El-Ramady, H. Selenium fortification induces growth, antioxidant activity, yield and nutritional quality of lettuce in salt-affected soil using foliar and soil applications. Plant Soil 2017, 421, 245–258. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Wick, J.E.; Famuyide, I.M.; McGaw, L.J.; Mühling, K.H. Selenium enrichment of green and red lettuce and the induction of radical scavenging potential. Horticulturae 2021, 7, 488. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Yang, K.; Shi, Z.; Wang, N.; Yang, L.; Chen, J. Characterization, expression, and functional analysis of polyamine oxidases and their role in selenium-induced hydrogen peroxide production in Brassica rapa. J. Sci. Food Agric. 2019, 99, 4082–4093. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, C.; He, X.; Sheng, Y.; Ma, T.; Sun, Z.; Liu, X.; Liu, C.; Fan, S.; Xu, W.; et al. Purple lettuce (Lactuca sativa L.) attenuates metabolic disorders in diet induced obesity. J. Funct. Foods 2018, 45, 462–470. [Google Scholar] [CrossRef]
- Drossard, C.; Alexy, U.; Bolzenius, K.; Kunz, C.; Kersting, M. Anthocyanins in the diet of infants and toddlers: Intake, sources and trends. Eur. J. Nutr. 2011, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Piberger, H.; Oehme, A.; Hofmann, C.; Dreiseitel, A.; Sand, P.G.; Obermeier, F.; Schoelmerich, J.; Schreier, P.; Krammer, G.; Rogler, G. Bilberries and their anthocyanins ameliorate experimental colitis. Mol. Nutr. Food Res. 2011, 55, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. Biofactors 2017, 43, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005, 8, 272–279. [Google Scholar] [CrossRef]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pietiainen, M.; Laitinen, R.A.E.; Albert, V.A.; Valkonen, J.P.T.; Elomaa, P.; Teeri, T.H. Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol. 2014, 201, 1469–1483. [Google Scholar] [CrossRef]
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. Anthocyanins as Natural Pigments in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–428. [Google Scholar]
- Zhang, Y.; Cheng, Y.; Ya, H.; Xu, S.; Han, J. Transcriptome sequencing of purple petal spot region in tree peony reveals differentially expressed anthocyanin structural genes. Front. Plant Sci. 2015, 6, 964. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of selenium application differentially modulate plant growth, selenium accumulation and speciation, protein, anthocyanins and concentrations of mineral elements in purple-grained wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Pu, Z.; Wei, G.; Liu, Z.; Chen, L.; Guo, H.; Li, Y.; Li, Y.; Dai, S.; Wang, J.; Li, W.; et al. Selenium and anthocyanins share the same transcription factors R2R3MYB and bHLH in wheat. Food Chem. 2021, 356, 129699. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Wang, H.; Zhan, J.; Yuan, X.; Cui, J.; Su, N. Selenium treatment promotes anthocyanin accumulation in radish sprouts (Raphanus sativus L.) by its regulation of photosynthesis and sucrose transport. Food Res. Int. 2023, 165, 112551. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of phenolic compounds, essential oil content and antioxidant properties of sweet basil (Ocimum basilicum L.) depending on type and concentration of selenium application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenchanmane Raju, S.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. 2018, 276, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.L.; Pedersen, M.F. Growth, photosynthesis and nutrient content of seedlings and mature plants of Cymodocea nodosa—The importance of clonal integration. Aquatic Botany 2000, 68, 265–271. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Zhang, Y.; Li, K.; Jin, B.; Lu, L.; Jin, C.; Lin, X. Reduced nitrogen supply enhances the cellular antioxidant potential of phenolic extracts through alteration of the phenolic composition in lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2019, 99, 4761–4771. [Google Scholar] [CrossRef]
- Iakimova, E.T.; Woltering, E.J. The wound response in fresh-cut lettuce involves programmed cell death events. Protoplasma 2018, 255, 1225–1238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Sun, S.W.; Shi, H.L.; Zhao, K.; Wang, J.; Liu, Y.; Liu, X.H.; Wang, W. Physiological and biochemical mechanisms mediated by allelochemical isoliquiritigenin on the growth of lettuce seedlings. Plants 2020, 9, 245. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef]
- Gunes, A.; Turan, M.; Kitir, N.; Tufenkci, M.S.; Cimrin, K.M.; Yildirim, E.; Ercisli, S. Effects of bio-bor fertilizer applications on fruit yield, antioxidant enzyme activity and freeze injury of strawberry. Erwerbsobstbau 2016, 58, 177–184. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Hiremani, N.S.; Gawande, S.P.; Sain, S.K.; Nagrale, D.T.; Narkhedkar, N.G.; Prasad, Y.G. Modulation of plant growth and antioxidative defense system through endophyte biopriming in cotton (Gossypium spp.) and non-host crops. Heliyon 2022, 8, e09487. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queirós, A.; Costa, M.S.; Trindade, H. Comparison of five agro-industrial waste-based composts as growing media for lettuce: Effect on yield, phenolic compounds and vitamin C. Food Chem. 2016, 209, 293–301. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, M.Y.; Phey, C.P.; Yang, H. Efficacy of low concentration acidic electrolysed water and levulinic acid combination on fresh organic lettuce (Lactuca sativa Var. Crispa L.) and its antimicrobial mechanism. Food Control 2019, 101, 241–250. [Google Scholar] [CrossRef]
- Moretti, C.; Bocchini, M.; Quaglia, M.; Businelli, D.; Orfei, B.; Buonaurio, R. Sodium selenate: An environmental-friendly means to control tomato bacterial speck disease. Agronomy 2022, 12, 1351. [Google Scholar] [CrossRef]
- Materska, M.; Olszówka, K.; Chilczuk, B.; Stochmal, A.; Pecio, Ł.; Pacholczyk-Sienicka, B.; Piacente, S.; Pizza, C.; Masullo, M. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Euro. Food Res. Technol. 2018, 245, 733–744. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low temperature promotes anthocyanin biosynthesis and related gene expression in the seedlings of purple head Chinese cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Li, X.; Wu, Q.; Lu, P.; Kang, Q.; Zhao, M.; Wang, A.; Dong, Q.; Sun, M.; Yang, Z.; et al. Selenium application enhances the accumulation of flavones and anthocyanins in bread wheat (Triticum aestivum L.). Grains. J. Agric. Food Chem. 2022, 70, 13431–13444. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Changes in anthocyanin content as indicator of maize sensitivity to selenium. J. Plant Nutr. 2008, 31, 1232–1242. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, S.Z.; Cheng, Y.W.; Ya, H.Y.; Han, J.M. Transcriptome analysis and anthocyanin-related genes in red leaf lettuce. Genet. Mol. Res. 2016, 15, 15017023. [Google Scholar] [CrossRef] [PubMed]
- Sanjari, S.; Shobbar, Z.S.; Ebrahimi, M.; Hasanloo, T.; Sadat-Noori, S.A.; Tirnaz, S. Chalcone synthase genes from milk thistle (Silybum marianum): Isolation and expression analysis. J. Genet. 2015, 94, 611–617. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Ying, Z.; Chen, J.; Yang, Y.; Zhang, J.; Yang, L.; Liu, M. Effect of Selenium Application on Growth, Antioxidative Capacity, and Nutritional Quality in Purple Lettuce Seedlings. Agronomy 2023, 13, 1664. https://doi.org/10.3390/agronomy13071664
Huang S, Ying Z, Chen J, Yang Y, Zhang J, Yang L, Liu M. Effect of Selenium Application on Growth, Antioxidative Capacity, and Nutritional Quality in Purple Lettuce Seedlings. Agronomy. 2023; 13(7):1664. https://doi.org/10.3390/agronomy13071664
Chicago/Turabian StyleHuang, Sijie, Zhengzheng Ying, Jian Chen, Yuwen Yang, Jibing Zhang, Lifei Yang, and Mingqing Liu. 2023. "Effect of Selenium Application on Growth, Antioxidative Capacity, and Nutritional Quality in Purple Lettuce Seedlings" Agronomy 13, no. 7: 1664. https://doi.org/10.3390/agronomy13071664





