Influence of 24-Epibrassinolide on the Energetic Parameters and Early Stages of Growth and Development in Seedlings of Two Maize (Zea mays L.) Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments, and Growth Conditions
2.2. Seed Germination and Determination of Morphometric Parameters of Seedlings
2.3. Electron Paramagnetic Resonance Spectroscopy
2.4. Thermodynamic Calculations
2.5. Statistical Evaluation
3. Results and Discussion
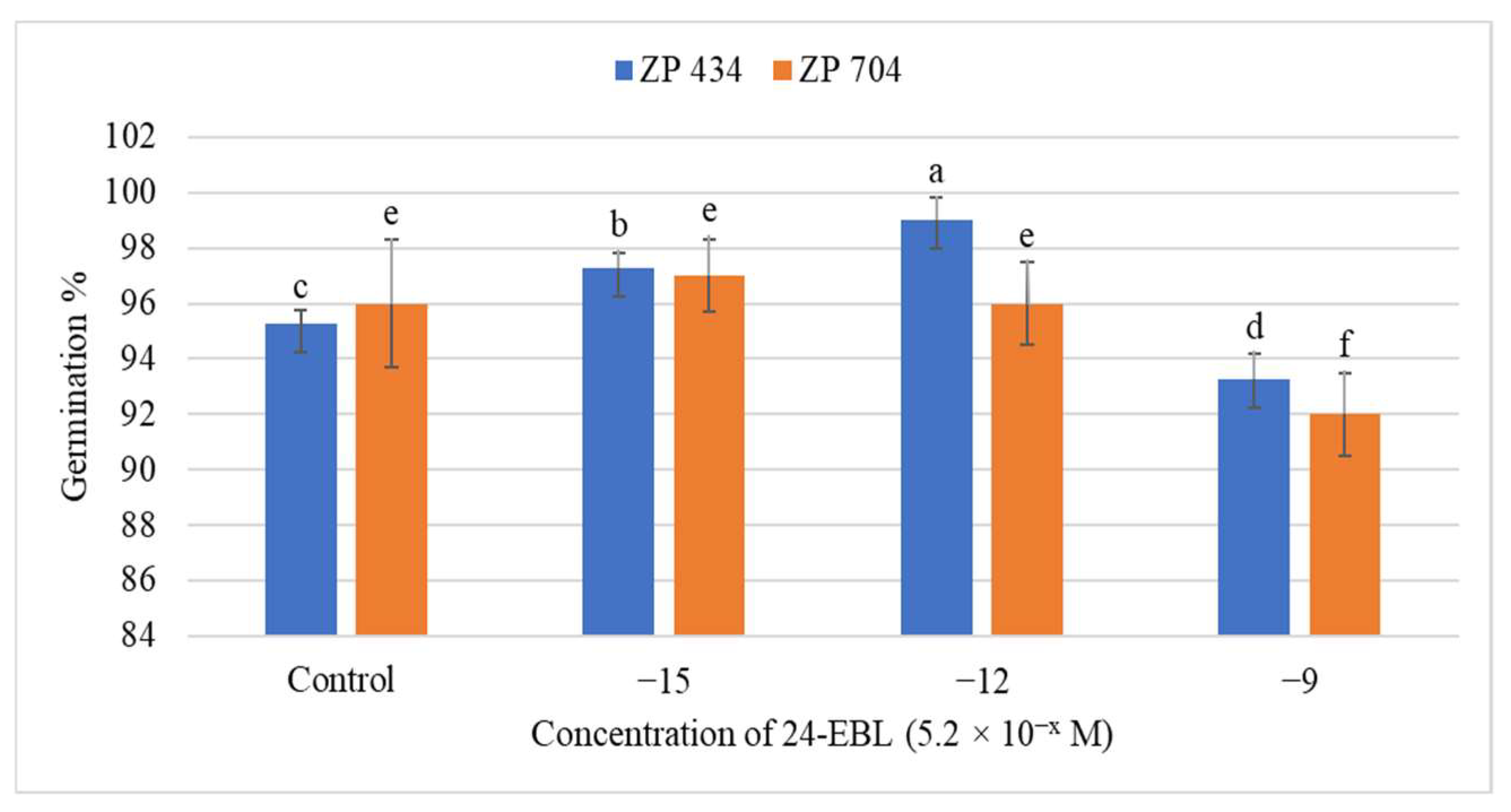
3.1. Influence of 24-EBL on Seed Germination
3.2. Determination of Morphometric Parameters of Treated Seedlings
3.3. Impact of 24-EBL on The total Redox Status
3.4. Influence of 24-EBL on Thermodynamic Changes in Maize Seedlings
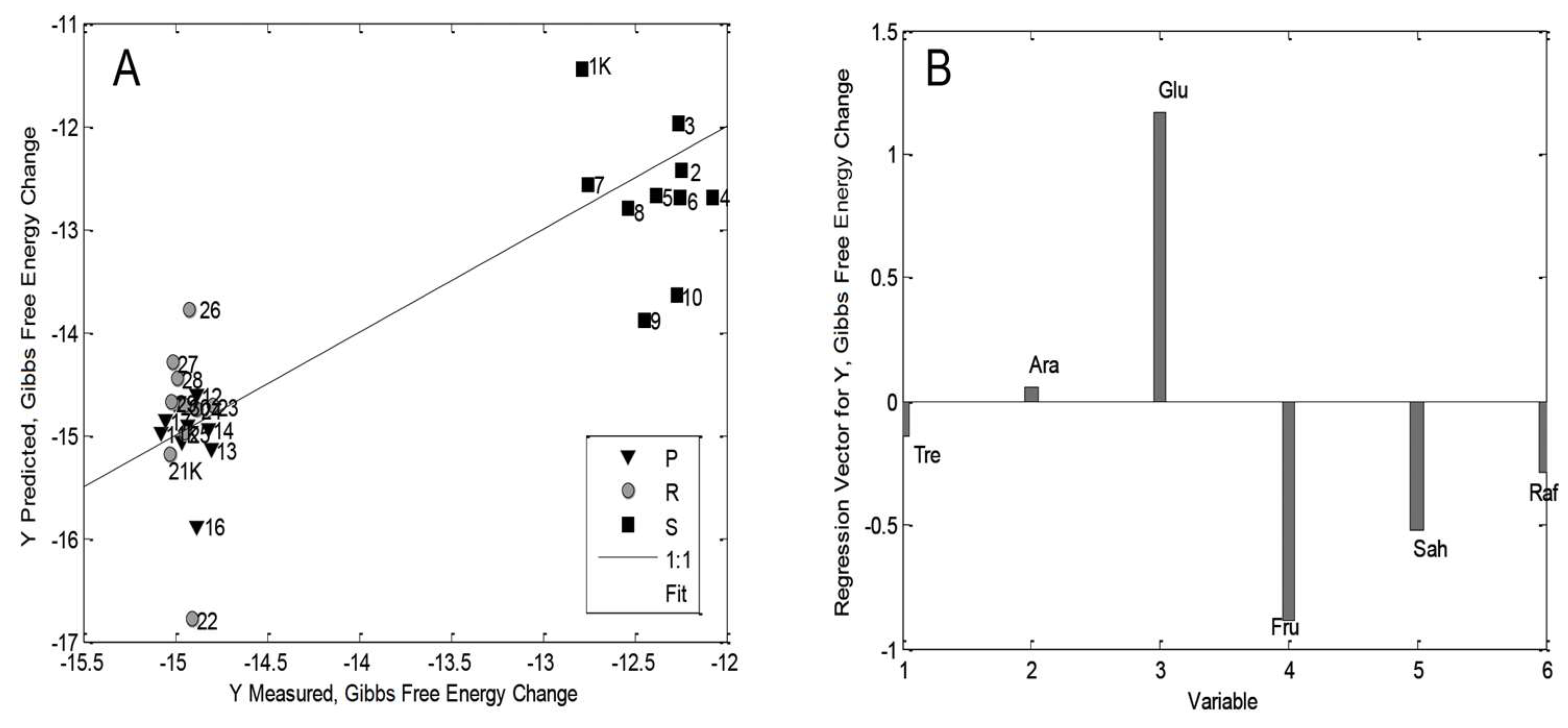
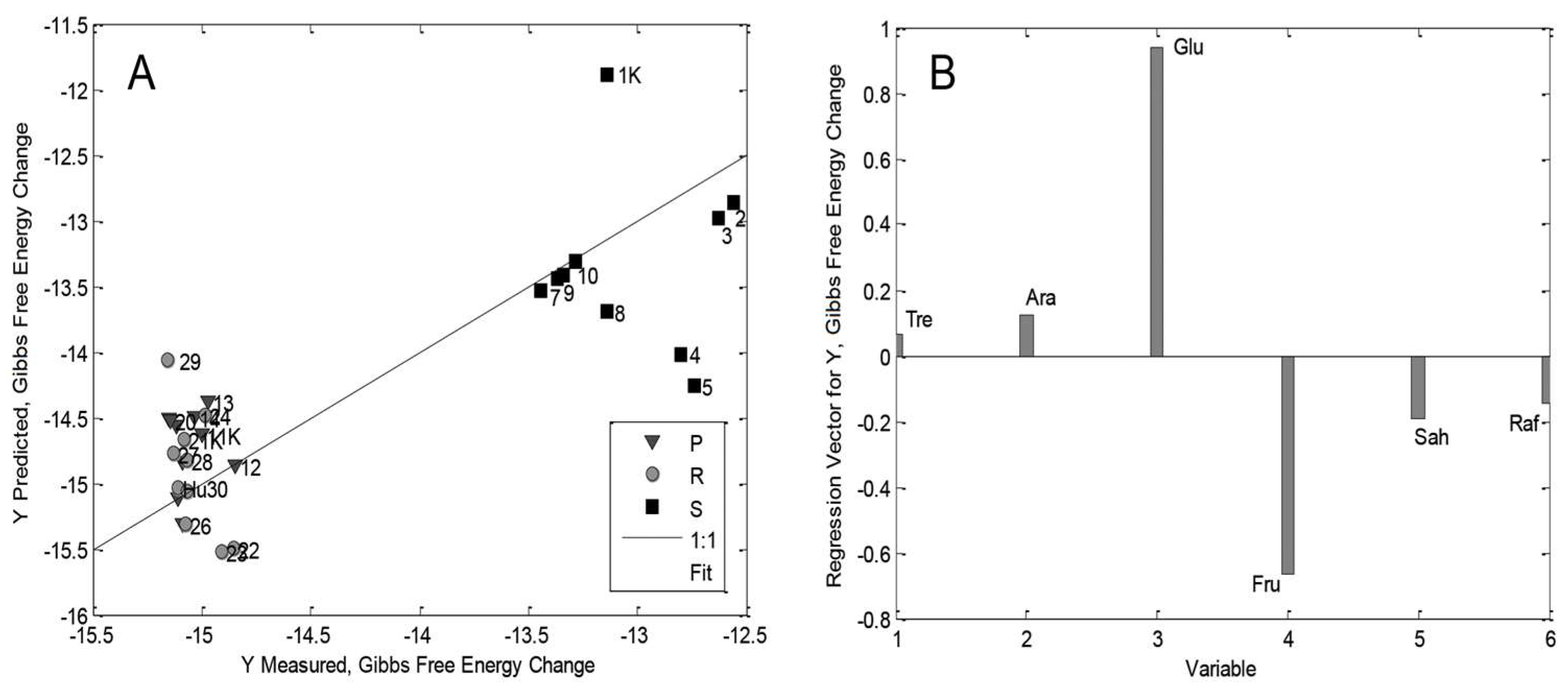
3.5. The multilinear Regression Model for Maize Seedlings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandava, N.B. Plant growth promoting brassinosteroids. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 23–52. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Lin, L.; Wang, F.; Sui, N. Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul. 2021, 93, 29–38. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid Signal Transduction: From Receptor Kinase Activation to Transcriptional Networks Regulating Plant Development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derevyanchuk, M.V.; Grabelnyh, O.I.; Litvinovskaya, R.P.; Voinikov, V.K.; Sauchuk, A.L.; Khripach, V.; Kravets, V. Influence of brassinosteroids on plant cell alternative respiration pathway and antioxidant systems activity under abiotic stress conditions. Biopolym. Cell 2014, 30, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Groszyk, J.; Szechyńska-Hebda, M. Effects of 24-Epibrassinolide, Bikinin, and Brassinazole on Barley Growth under Salinity Stress Are Genotype- and Dose-Dependent. Agronomy 2021, 11, 259. [Google Scholar] [CrossRef]
- Ahmad, A.; Tola, E.; Alshahrani, T.S.; Seleiman, M.F. Enhancement of Morphological and Physiological Performance of Zea mays L. under Saline Stress Using ZnO Nanoparticles and 24-Epibrassinolide Seed Priming. Agronomy 2023, 13, 771. [Google Scholar] [CrossRef]
- Kim, T.-W.; Wang, Z.-Y. Brassinosteroid Signal Transduction from Receptor Kinases to Transcription Factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef] [Green Version]
- Pokotylo, I.V.; Kretynin, S.V.; Khripach, V.A.; Ruelland, E.; Blume, Y.B.; Kravets, V.S. Influence of 24-epibrassinolide on lipid signaling and metabolism in Brassica napus. Plant Growth Regul. 2014, 73, 9–17. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current Understanding of the Interplay between Phytohormones and Photosynthesis under Environmental Stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef] [Green Version]
- Gururani, M.A.; Ganesan, M.; Song, P.-S. Photo-biotechnology as a tool to improve agronomic traits in crops. Biotechnol. Adv. 2015, 33, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F.; Tola, E. Seed Priming with Nanoparticles and 24-Epibrassinolide Improved Seed Germination and Enzymatic Performance of Zea mays L. in Salt-Stressed Soil. Plants 2023, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Yu, J.; Wang, J.; Gao, Q.; Huang, L.; Chen, C.; Zhang, C.; Fan, X.; Zhao, D.; Liu, Q.Q.; et al. Brassinosteroids regulate rice seed germination through the BZR1-RAmy3D transcriptional module. Plant Physiol. 2022, 189, 402–418. [Google Scholar] [CrossRef]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Komor, E. Source physiology and assimilate transport: The interaction of sucrose metabolism, starch storage and phloem export in source leaves and the effects on sugar status in plants. Aust. J. Plant Physiol. 2000, 27, 497–505. [Google Scholar] [CrossRef]
- Roland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.-R.C.F.; de Melo Souza, A.C.; da Silva, M.D.; Benko-Iseppon, A.M.; Pandolfi, V.; da Costa, A.F.; Kido, E.K. The Transcriptional Modulation of Inositol and Raffinose Family Oligosaccharides Pathways in Plants—An (A)biotic Stress, Perspective. In Abiotic and Biotic Stress in Plants: Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; IntechOpen: London, UK, 2016; Volume 4, pp. 81–99. [Google Scholar]
- Carpita, N. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef]
- Wolf, S.; Mravec, J.; Greiner, S.; Mouille, G.; Höfte, H. Plant Cell Wall Homeostasis Is Mediated by Brassinosteroid Feedback Signaling. Curr. Biol. 2012, 22, 1732–1737. [Google Scholar] [CrossRef] [Green Version]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Reg. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Sadhukhan, S. Imperative role of trehalose metabolism and trehalose-6-phosphate signalling on salt stress responses in plants. Physiol. Plant. 2022, 174, e13647. [Google Scholar] [CrossRef] [PubMed]
- Iordachescu, M.; Imai, R. Trehalose and Abiotic Stress in Biological Systems. In Abiotic Stress in Plants—Mechanims and Adaptation; Shanker, A., Ed.; InTech: Rijeka, Croatia, 2011; Volume 10, pp. 215–234. [Google Scholar]
- Li, J.; Nam, K.H. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 2002, 295, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Q. Methods for the Study of Water Relations Under Desiccation Stress. In Desiccation and Survival in Plants. Drying Without Dying; Black, M., Pritchard, H.W., Eds.; CABI: Walingford, UK, 2002; Volume 2, pp. 47–91. [Google Scholar]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Flock, T.; Weatheritt, R.J.; Latysheva, N.S.; Babu, M.M. Controlling entropy to tune the functions of intrinsically disordered regions. Curr. Opin. Struct. Biol. 2014, 26, 62–72. [Google Scholar] [CrossRef]
- Popović, M.; Minceva, M. Standard Thermodynamic Properties, Biosynthesis rates, and the Driving Force of Growth of Five Agricultural Plants. Front. Plant Sci. 2021, 12, 671868. [Google Scholar] [CrossRef]
- Dragicevic, V.; Sredojevic, S. Thermodynamics of Seed and Plant Growth. In Thermodynamics—Systems in Equilibrium and Non-Equilibrium; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef] [Green Version]
- Waisi, H.; Nikolic, B.; Jankovic, B. Transformation of Matter and Energy in Crops Under the Influence of Brassinosteroids. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Sandler, S. Chemical and Engineering Thermodinamics, 3rd ed.; JohnWiley & Sons: Hoboken, NJ, USA, 1989. [Google Scholar]
- Pincus, S.M.; Singer, B.H. Randomness and degrees of irregularity. Proc. Natl. Acad. Sci. USA 1996, 93, 2083–2088. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, A. Developmental Algorithms for Multieellular Organisms: A Survey of L-Systemst. J. Theor. Biol. 1975, 54, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Prusinkiewicz, P.; Lindenmayer, A. The Algorithmic Beauty of Plants; Springer: New York, NY, USA, 1990; p. 228. [Google Scholar]
- Prusinkiewicz, P.; Runions, A. Computational models of plant development and form. New Phytol. 2012, 193, 549–569. [Google Scholar] [CrossRef]
- Prusinkiewicz, P.; Cieslak, M.; Ferraro, P.; Hanan, J. Modeling plant development with L-systems. In Mathematical Modeling in Plant Biology; Morris, P., Ed.; Springer: New York, NY, USA, 2018; Volume 8, pp. 139–169. [Google Scholar]
- Allen, M.T.; Prusinkiewicz, P.; DeJong, T.M. Using L-systems for modeling source-sink interactions, architecture and physiology of growing trees: The L-PEACH model. New Phytol. 2005, 166, 869–880. [Google Scholar] [CrossRef] [Green Version]
- Graeff, M.; Rana, S.; Marhava, P.; Moret, B.; Hardtke, C.S. Local and Systemic Effects of Brassinosteroid Perception in Developing Phloem. Curr. Biol. 2020, 30, 1626–1638.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, M.V.; Nievas, F.; Zygadlo, J.; Giordano, W.; Banchio, E. Effects of growth regulators on biomass and the production of secondary metabolites in Peppermint (Mentha piperita) micropropagated in vitro. Am. J. Plant Sci. 2013, 4, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Ozga, J.; Dennis, M. Hormonal interactions in fruit development. J. Plant Growth Regul. 2003, 22, 73–81. [Google Scholar] [CrossRef]
- Divi, U.K.; Krishna, P. Brassinosteroid: A Biotechnological Target for Enhancing Crop Yield and Stress Tolerance. New Biotechnol. 2009, 26, 131–136. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, X.; Tang, W.; Oses-Prieto, J.A.; Suzuki, N.; Gendron, J.M.; Chen, H.; Guan, S.; Chalkley, R.J.; Peterman, T.K.; et al. A proteomics study of brassinosteroid response in Arabidopsis. Mol. Cell. Proteom. 2007, 6, 2058–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z. Brassinosteroids regulate plant growth, development, and stress responses via members of the GSK3/shaggy-like kinase family. Mol. Plant 2019, 12, 659–671. [Google Scholar]
- Steber, C.M.; McCourt, P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Waisi, H.; Janković, B.; Nikolić, B.; Dragičević, V.; Panić, I.; Tosti, T.; Trifković, J. Influence of various concentrations of 24-epibrassinolide on the kinetic parameters during isothermal dehydration of two maize hybrids. S. Afr. J. Bot. 2018, 119, 69–79. [Google Scholar] [CrossRef]
- Vidya Vardhini, B.; Seeta Ram Rao, S. Amelioration of osmotic stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. Plant Growth Regul. 2003, 41, 25–31. [Google Scholar] [CrossRef]
- ISTA, International Seed Testing Association. Seed Testing International (1996). International rules for seed testing. Seed Sci. Technol. 1996, 21, 1–288. [Google Scholar]
- Waisi, H.K.; Petković, A.Z.; Nikolić, B.R.; Janković, B.Ž.; Raičević, V.B.; Lalević, B.T.; Giba, Z.S. Influence of 24-epibrassinolide on seedling growth and distribution of mineral elements in two maize hybrids. Chem. Ind. 2017, 71, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Waisi, H. The Influence of Brassinosteroid 24-Epibrassinolide on Germination and Early Stages of Growth and Development of Different Maize Hybrids (Zea mays L.). Ph.D. Thesis, University of Belgrade, Faculty of Biology, Belgrade, Serbia, 2016. [Google Scholar]
- Matusmoto, T.; Yamada, K.; Yoshizawa, Y.; Oh, K. Comparison of effect of brassinosteroid and gibberellin biosynthesis inhibitors on growth of rice seedlings. Rice Sci. 2016, 23, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhao, J.; Li, X.; Dai, H.; Lei, J. Seed morphology and germination of native Tulipa species. Agriculture 2023, 13, 466. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14 (Suppl. S1), S61–S80. [Google Scholar] [CrossRef] [Green Version]
- Piazza, F.; Colella, M.; Cinelli, G.; Lopez, F.; Donati, I.; Sacco, P. Effect of α-Amylase on the Structure of Chia Seed Mucilage. Biomimetics 2022, 7, 141. [Google Scholar] [CrossRef]
- Yadav, R.K.; Devi, L.L.; Singh, A.P. Brassinosteroids in plant growth and development. In Plant Hormones in Crop Improvement; Academic Press: Cambridge, MA, USA, 2023; pp. 185–203. [Google Scholar]
- Bajguz, A.; Orczyk, W.; Gołębiewska, A.; Chmur, M.; Piotrowska-Niczyporuk, A. Occurrence of brassinosteroids and influence of 24-epibrassinolide with brassinazole on their content in the leaves and roots of Hordeum vulgare L. cv. Golden Promise. Planta 2019, 249, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [Green Version]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [Green Version]
- Waisi, H.; Kosović, A.; Krstić, Đ.; Milojković-Opsenica, D.; Nikolić, B.; Dragićević, V.; Trifković, J. Polyphenolic profile of maize seedlings treated with 24-epibrassinolide. J. Chem. 2015, 2015, 976971. [Google Scholar] [CrossRef] [Green Version]
- Buitink, J.; Hoekstra, F.A.; Leprince, O. Biochemistry and Biophysics of Tolerance system. In Desiccation and Survival in Plants. Drying Without Dying; Black, M., Pritchard, H.W., Eds.; CABI: Walingford, UK, 2002; Volume 10, pp. 293–318. [Google Scholar]
- Noctor, G.; Foyer, C.H. A re-evaluation of the ATP: NADPH budget dur-ing C3 photosynthesis. A contribution from nitrate assimilation and its associated respiratory activity? J. Exp. Bot. 1998, 49, 1895–1908. [Google Scholar] [CrossRef]
- Ho, L.C. Metabolism and Compartmentation of Imported Sugars in Sink Organs in Relation to Sink Strength. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Turgeon, R. The Sink-Source Transition in Leaves. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 119–138. [Google Scholar] [CrossRef]
- Kumar, R.; Bishop, E.; Bridges, W.C.; Tharayil, N.; Sekhon, R.S. Sugar partitioning and source–sink interaction are key determinants of leaf senescence in maize. Plant Cell Environ. 2019, 42, 2597–2611. [Google Scholar] [CrossRef]
- Ren, H.; Qi, H.; Zhao, M.; Zhou, W.; Wang, X.; Gong, X.; Jiang, Y.; Li, C. Characterization of source–sink traits and carbon translocation in maize hybrids under high plant density. Agronomy 2022, 12, 961. [Google Scholar] [CrossRef]
- Van Esse, G.W.; van Mourik, S.; Stigler, H.; ten Hove, C.A.; Molenaar, J.; de Vries, S.C. A Mathematical Model fo BRASSINOSTEROID INSENSITIVE1-Mediated Signaling in Root Growth and Hypocotil Elongation. Plant Physiol. 2012, 160, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symons, G.M.; Ross, J.J.; Jager, C.E.; Reid, J.B. Brassinosteroid transport. J. Exp. Bot. 2008, 59, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Leyser, O. Auxin, Self-Organisation, and the Colonial Nature of Plants. Curr. Biol. 2011, 21, R331–R337. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennet, M.; Traas, J.; Friml, J.; Kuhlmeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef]
- Smith, R.S.; Guyomarc’h, S.; Mandel, T.; Reinhardt, D.; Kuhlmeier, C.; Prusinkiewic, P. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA 2006, 103, 1301–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, S.; Shinoharabinose, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar, M.; Osmont, K.S.; Rolcik, J.; Gujas, B.; Tarkowska, D.; Strnad, M.; Xenarios, I.; Hardtke, C.S. A qualitative continuous model of cellular auxin and brassinosteroid signaling and their crosstalk. Bioinformatics 2011, 27, 1404–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, T.; Wang, Z.-Y. The molecular circuit of steroid signaling in plants. Essays Biochem. 2015, 58, 71–82. [Google Scholar] [PubMed]


| Thermodynamic Parameter | Seedling Parts | Model Parameters | Regression Coefficients in Model * |
|---|---|---|---|
| Entropy | Plumule (P) | Low statistical significance model | |
| Radicle (R) | RMSEC: 0.0020 RMSECV: 0.0046 R2Cal: 0.7561 R2CV: 0.2554 | Tre (+), Sah (+), Ara (+) Glu (−), Fru (−) Raf (0) | |
| Rest of seed (RoS/S) | Low statistical significance model | ||
| Whole seedling | Low statistical significance model | ||
| Enthalpy | Plumule (P) | Low statistical significance model | |
| Radicle (R) | RMSEC: 0.3711 RMSECV: 0.8215 R2Cal: 0.7563 R2CV: 0.2823 | Fru (+), Glu (+) Ara (−), Sah (−), Tre (−) Raf (0) | |
| Rest of seed (RoS/S) | Low statistical significance model | ||
| Whole seedling | Low statistical significance model | ||
| Gibbs Free Energy | Plumule (P) | RMSEC: 0.0152 RMSECV: 0.0539 R2Cal: 0.9714 R2CV: 0.8479 | Raf (+), Tre (+), Fru (+), Glu (+) Ara (−), Sah (−) |
| Radicle (R) | RMSEC: 0.0357 RMSECV: 0.0454 R2Cal: 0.8517 R2CV: 0.7779 | Glu (+), Fru (+), Tre (+), Sah (+), Raf (+), Ara (+) | |
| Rest of seed (RoS/S) | RMSEC: 0.1866 RMSECV: 0.2947 R2Cal: 0.6471 R2CV: 0.3319 | Sah (+), Raf (+) Ara (−), Glu (−), Fru (−), Tre (−) | |
| Whole seedling | RMSEC: 0.5123 RMSECV: 0.5931 R2Cal: 0.7188 R2CV: 0.6328 | Glu (+), Ara (+), Tre (+) Raf (−), Sah (−), Fru (−) |
| Thermodynamic Parameter | Seedling Parts | Model Parameters | Regression Coefficients in Model * |
|---|---|---|---|
| Entropy | Plumule (P) | RMSEC: 0.0072 RMSECV: 0.0098 R2Cal: 0.7339 R2CV: 0.5425 | Raf (+) Glu (−), Sah (−), Ara (−), Tre (−) Fru (0) |
| Radicle (R) | RMSEC: 0.0087 RMSECV: 0.0097 R2Cal: 0.6509 R2CV: 0.6180 | Raf (+), Fru (+), Glu (+), Sah (+) Ara (−), Tre (−) | |
| Rest of seed (RoS/S) | RMSEC: 0.0077 RMSECV: 0.0102 R2Cal: 0.8687 R2CV: 0.8117 | Glu (+), Ara (+), Sah (+), Raf (+), Fru (+) Tre (−) | |
| Whole seedling | Low statistical significance model | ||
| Enthalpy | Plumule (P) | RMSEC: 1.3083 RMSECV: 1.7839 R2Cal: 0.7340 R2CV: 0.5433 | Tre (+), Ara (+), Sah (+), Glu (+), Raf (−) Fru (0) |
| Radicle (R) | RMSEC: 1.5849 RMSECV: 1.7754 R2Cal: 0.6508 R2CV: 0.5632 | Tre (+), Ara (+) Sah (−), Glu (−), Fru (−), Raf (−) | |
| Rest of seed (RoS/S) | RMSEC: 1.4122 RMSECV: 2.0551 R2Cal: 0.8687 R2CV: 0.7782 | Tre (+) Fru (−), Sah (−), Raf (−), Glu (−), Ara (−) | |
| Whole seedling | Low statistical significance model | ||
| Gibbs Free Energy | Plumule (P) | RMSEC: 0.0709 RMSECV: 0.1692 R2Cal: 0.3456 R2CV: 0.4042 | Tre (+), Raf (+), Fru (+), Ara (+), Glu (+) Sah (−) |
| Radicle (R) | Low statistical significance model | ||
| Rest of seed (RoS/S) | RMSEC: 0.0904 RMSECV: 0.2579 R2Cal: 0.8282 R2CV: 0.4600 | Sah (+), Ara (+), Fru (+), Tre (+) Glu (−), Raf (−) | |
| Whole seedling | RMSEC: 0.5291 RMSECV: 0.6804 R2Cal: 0.8075 R2CV: 0.7112 | Glu (+), Ara (+), Tre (−), Raf (−), Sah (−), Fru (−) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božilović, B.; Nikolić, B.; Waisi, H.; Trifković, J.; Dodevski, V.; Janković, B.; Krstić, S.; Mojović, M. Influence of 24-Epibrassinolide on the Energetic Parameters and Early Stages of Growth and Development in Seedlings of Two Maize (Zea mays L.) Genotypes. Agronomy 2023, 13, 1673. https://doi.org/10.3390/agronomy13071673
Božilović B, Nikolić B, Waisi H, Trifković J, Dodevski V, Janković B, Krstić S, Mojović M. Influence of 24-Epibrassinolide on the Energetic Parameters and Early Stages of Growth and Development in Seedlings of Two Maize (Zea mays L.) Genotypes. Agronomy. 2023; 13(7):1673. https://doi.org/10.3390/agronomy13071673
Chicago/Turabian StyleBožilović, Bojana, Bogdan Nikolić, Hadi Waisi, Jelena Trifković, Vladimir Dodevski, Bojan Janković, Sanja Krstić, and Miloš Mojović. 2023. "Influence of 24-Epibrassinolide on the Energetic Parameters and Early Stages of Growth and Development in Seedlings of Two Maize (Zea mays L.) Genotypes" Agronomy 13, no. 7: 1673. https://doi.org/10.3390/agronomy13071673







