Synthesis of Benzoxazinones Sulphur Analogs and Their Application as Bioherbicides: 1.4-Benzothiazinones and 1.4-Benzoxathianones for Weed Control
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Synthesis
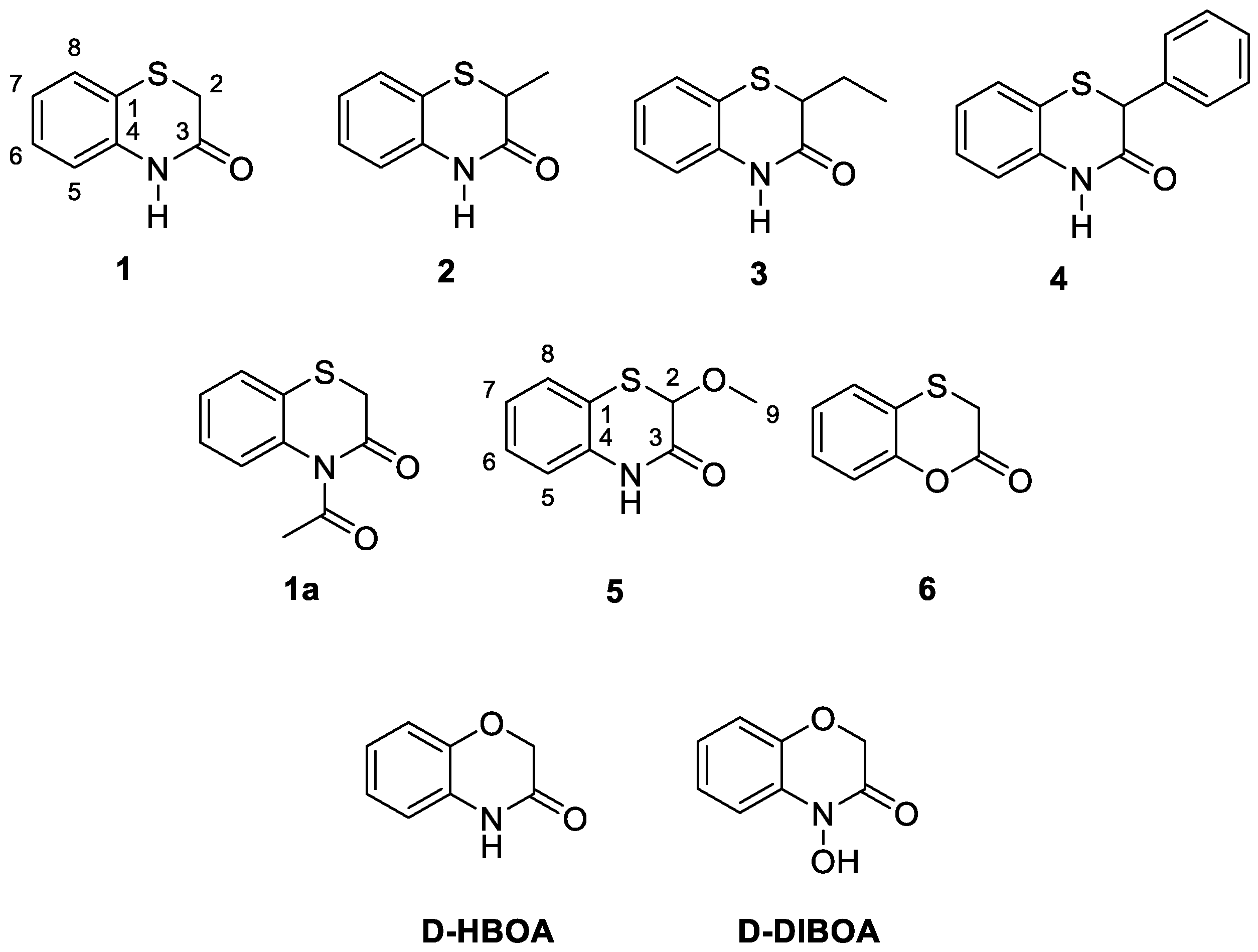
2.2.1. General Methods for the Synthesis of 1.4-benzothiazinones and 1.4-benzoxathianone (1–6)
2.2.2. Synthesis of 4-acetyl-2H-benzo[b][1,4]thiazin-3(4H)-one (1a)
2.3. In silico Experiments
2.3.1. Docking Experiments with HDA2 and HDA6
2.3.2. Molecular Dynamics Experiments with HDA
2.4. In Vitro Experiments
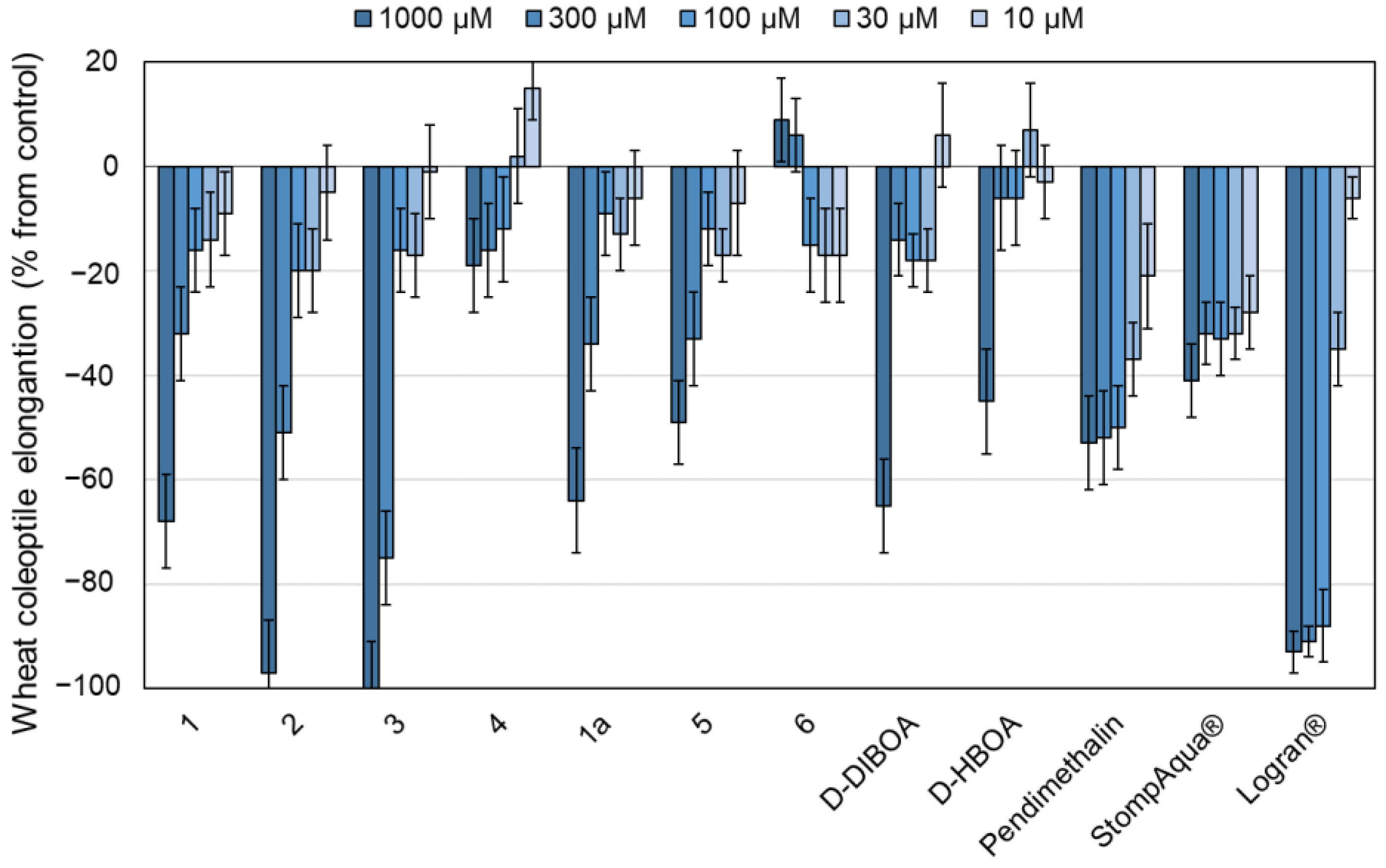
2.4.1. Etiolated Wheat Coleoptile Bioassay
2.4.2. Phytotoxicity Bioassay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friebe, A. Role of Benzoxazinones in Cereals. J. Crop. Prod. 2008, 4, 379–400. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliveros-Bastidas, A.; Marín, D.; Chinchilla, N.; Castellano, D.; Molinillo, J.M.G. Evidence for an Allelopathic Interaction between Rye and Wild Oats. J. Agric. Food Chem. 2014, 62, 9450–9457. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Chinchilla, N.; Varela, R.M.; Molinillo, J.M.G.; Marín, D.; De Siqueira, J.M. Modified Benzoxazinones in the System Oryza sativa-Echinochloa crus-galli: An Approach To the Development of Biorational Herbicide Models. J. Agric. Food Chem. 2008, 56, 9941–9948. [Google Scholar] [CrossRef]
- Copaja, S.V.; Barria, B.N.; Niemeyer, H.M. Hydroxamic Acid Content of Perennial Triticeae. Phytochemistry 1991, 30, 1531–1534. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Structure-Activity Relationships (SAR) Studies of Benzoxazinones, Their Degradation Products and Analogues. Phytotoxicity on Standard Target Species (STS). J. Agric. Food Chem. 2005, 53, 538–548. [Google Scholar] [CrossRef]
- Yin, J.; Straub, M.R.; Liao, J.D.; Birman, V.B. Acylative Kinetic Resolution of Cyclic Hydroxamic Acids. Org. Lett. 2022, 24, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Bakulina, O.; Bannykh, A.; Dar’in, D.; Krasavin, M. Cyclic Hydroxamic Acid Analogues of Bacterial Siderophores as Iron-Complexing Agents Prepared through the Castagnoli-Cushman Reaction of Unprotected Oximes. Chemistry 2017, 23, 17667–17673. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Belz, R.G.; Kämper, A.; Berger, A.; von Horn, K.; Wegner, A.; Böcker, A.; Zabulon, G.; Langenecker, T.; Kohlbacher, O.; et al. Plants Release Precursors of Histone Deacetylase Inhibitors to Suppress Growth of Competitors. Plant. Cell. 2015, 27, 3175–3189. [Google Scholar] [CrossRef]
- Kamila, S.; Koh, B.; Khan, O.; Zhang, H.; Biehl, E.R. Regioselective One Pot Synthesis of 2-Alkyl/Aryl-4h-Benzo[1,4] Thiazine-3-One via Microwave Irradiation. J. Heterocycl. Chem. 2006, 43, 1641–1646. [Google Scholar] [CrossRef]
- Rutkauskas, K.; Beresnevicius, Z.I. Reaction of 2-Aminothiophenol with Acrylic Acid and Conversion of the Resultant Adducts. Chem. Heterocycl. Compd. 2006, 42, 227–232. [Google Scholar] [CrossRef]
- Sakanashi, T.; Inagi, S.; Fuchigami, T. Highly Regioselective Anodic Monofluorination of 3H-1,4-Benzoxathian-2-Ones in Et4NF·4HF/MeCN. Electrochemistry 2008, 76, 896–899. [Google Scholar] [CrossRef]
- Greenwood, D.; Stevenson, H.A. Benz-1:3-Oxathioles, Benz-1:4-Oxathien, and Aw-Bisarylthioalkanes. J. Chem. Soc. 1953, 1, 1514. [Google Scholar] [CrossRef]
- Rathore, B.S.; Kumar, M. Design and Synthesis of 4H-1,4-Benzothiazines Containing Thiazole Ring System for Use as Potential Biopharmaceuticals. Res. Chem. Intermed. 2006, 32, 647–651. [Google Scholar] [CrossRef]
- Chinchilla, N.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G.; Macías, F.A. Soil Biodegradation of a Benzoxazinone Analog Proposed as a Natural Products-Based Herbicide. Plant. Soil. 2015, 393, 207–214. [Google Scholar] [CrossRef]
- Macías, F.A.; Chinchilla, N.; Arroyo, E.; Varela, R.M.; Molinillo, J.M.G.; Marín, D. Multifunctionalised Benzoxazinones in the Systems Oryza sativa-Echinochloa crus-galli and Triticum aestivum-Avena fatua as Natural-Product-Based Herbicide Leads. Pest. Manag. Sci. 2010, 66, 1137–1147. [Google Scholar] [CrossRef]
- Escobar, C.A.; Sicker, D.; Niemeyer, H.M. Evaluation of DIMBOA Analogs as Antifeedants and Antibiotics towards the Aphid Sitobion Avenae in Artificial Diets. J. Chem. Ecol. 1999, 25, 1543–1554. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Chinchilla, D.; Simonet, A.M.; Molinillo, J.M.G. Isolation and Synthesis of Allelochemicals from Gramineae: Benzoxazinones and Related Compounds. J. Agric. Food Chem. 2006, 54, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; Van Gunsteren, W.F. Definition and Testing of the GROMOS Force-Field Versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, D.M.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; MacÍas, F.A. Synthesis of Pertyolides A, B, and C: A Synthetic Procedure to C17-Sesquiterpenoids and a Study of Their Phytotoxic Activity. J. Nat. Prod. 2021, 84, 2295–2302. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; Fernández, I.P.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Trasobares, S.; Macías, F.A. Encapsulation of Cynara cardunculus Guaiane-Type Lactones in Fully Organic Nanotubes Enhances Their Phytotoxic Properties. J. Agric. Food Chem. 2022, 70, 3644–3653. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; Carrasco, Á.; Durán, A.G.; Molinillo, J.M.G.; Macías, F.A.; Chinchilla, N. On the Formulation of Disulfide Herbicides Based on Aminophenoxazinones: Polymeric Nanoparticle Formulation and Cyclodextrin Complexation to Combat Crop Yield Losses. Pest. Manag. Sci. 2023, 79, 1547–1556. [Google Scholar] [CrossRef]
- Sicker, D.; Hartenstein, H.; Mouats, C.; Hazard, R.; Tallec, A. Electrochemical Reduction of O-Nitrophenylthioacetic Derivatives. Production of 2H-1,4-Benzothiazines. Electrochim. Acta 1995, 40, 1669–1674. [Google Scholar] [CrossRef]
- Konno, A.; Naito, W.; Fuchigami, T.; Peters, D.G.; Motevalli, M.; Murase, H.; Shono, T.; Toftlund, H. Electrolytic Partial Fluorination of Organic Compounds. 33. Regioselective Anodic Monofluorination of Alpha-Phenylsulfenyl Lactams and Sulfur-Containing Nitrogen Heterocycles. Acta Chem. Scand. 1999, 53, 887–891. [Google Scholar] [CrossRef]
- Pradhan, T.K.; Mukherjee, C.; Kamila, S.; De, A. Application of Directed Metalation in Synthesis. Part 6: A Novel Anionic Rearrangement under Directed Metalation Conditions Leading to Heteroannulation. Tetrahedron 2004, 60, 5215–5224. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; Durán, A.G.; Chinchilla, N.; Varela, R.M.; Álvarez, J.A.; Molinillo, J.M.G.; García-Cozar, F.; Macías, F.A. In Silico Evaluation of Sesquiterpenes and Benzoxazinoids Phytotoxins against Mpro, RNA Replicase and Spike Protein of SARS-CoV-2 by Molecular Dynamics. Inspired by Nature. Toxins 2022, 14, 599. [Google Scholar] [CrossRef]
- Tabaglio, V.; Gavazzi, C.; Schulz, M.; Marocco, A. Alternative Weed Control Using the Allelopathic Effect of Natural Benzoxazinoids from Rye Mulch. Agron. Sustain. Dev. 2008, 28, 397–401. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Structure-Activity Relationship (SAR) Studies of Benzoxazinones, Their Degradation Products, and Analogues. Phytotoxicity on Problematic Weeds Avena fatua L. and Lolium rigidum Gaud. J. Agric. Food Chem. 2006, 54, 1040–1048. [Google Scholar] [CrossRef]
- Temman, H.; Sakamoto, T.; Ueda, M.; Sugimoto, K.; Migihashi, M.; Yamamoto, K.; Tsujimoto-Inui, Y.; Sato, H.; Shibuta, M.K.; Nishino, N.; et al. Histone Deacetylation Regulates de Novo Shoot Regeneration. PNAS Nexus 2023, 2, pgad002. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lv, S.; Zhang, C.; Yang, C. Histone Deacetylases and Their Functions in Plants. Plant. Cell. Rep. 2013, 32, 465–478. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejías, F.J.R.; Schwaiger, S.; Varela, R.M.; Molinillo, J.M.G.; Chinchilla, N.; Macías, F.A. Synthesis of Benzoxazinones Sulphur Analogs and Their Application as Bioherbicides: 1.4-Benzothiazinones and 1.4-Benzoxathianones for Weed Control. Agronomy 2023, 13, 1694. https://doi.org/10.3390/agronomy13071694
Mejías FJR, Schwaiger S, Varela RM, Molinillo JMG, Chinchilla N, Macías FA. Synthesis of Benzoxazinones Sulphur Analogs and Their Application as Bioherbicides: 1.4-Benzothiazinones and 1.4-Benzoxathianones for Weed Control. Agronomy. 2023; 13(7):1694. https://doi.org/10.3390/agronomy13071694
Chicago/Turabian StyleMejías, Francisco J. R., Stefan Schwaiger, Rosa M. Varela, José M. G. Molinillo, Nuria Chinchilla, and Francisco A. Macías. 2023. "Synthesis of Benzoxazinones Sulphur Analogs and Their Application as Bioherbicides: 1.4-Benzothiazinones and 1.4-Benzoxathianones for Weed Control" Agronomy 13, no. 7: 1694. https://doi.org/10.3390/agronomy13071694
APA StyleMejías, F. J. R., Schwaiger, S., Varela, R. M., Molinillo, J. M. G., Chinchilla, N., & Macías, F. A. (2023). Synthesis of Benzoxazinones Sulphur Analogs and Their Application as Bioherbicides: 1.4-Benzothiazinones and 1.4-Benzoxathianones for Weed Control. Agronomy, 13(7), 1694. https://doi.org/10.3390/agronomy13071694






