Arbuscular Mycorrhizal Fungi and Diazotrophic Diversity and Community Composition Responses to Soybean Genotypes from Different Maturity Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Soil Physical and Chemical Analysis
2.3. DNA Extraction, Library Preparation, and Sequencing Data Processing
2.4. Data Analysis
3. Results
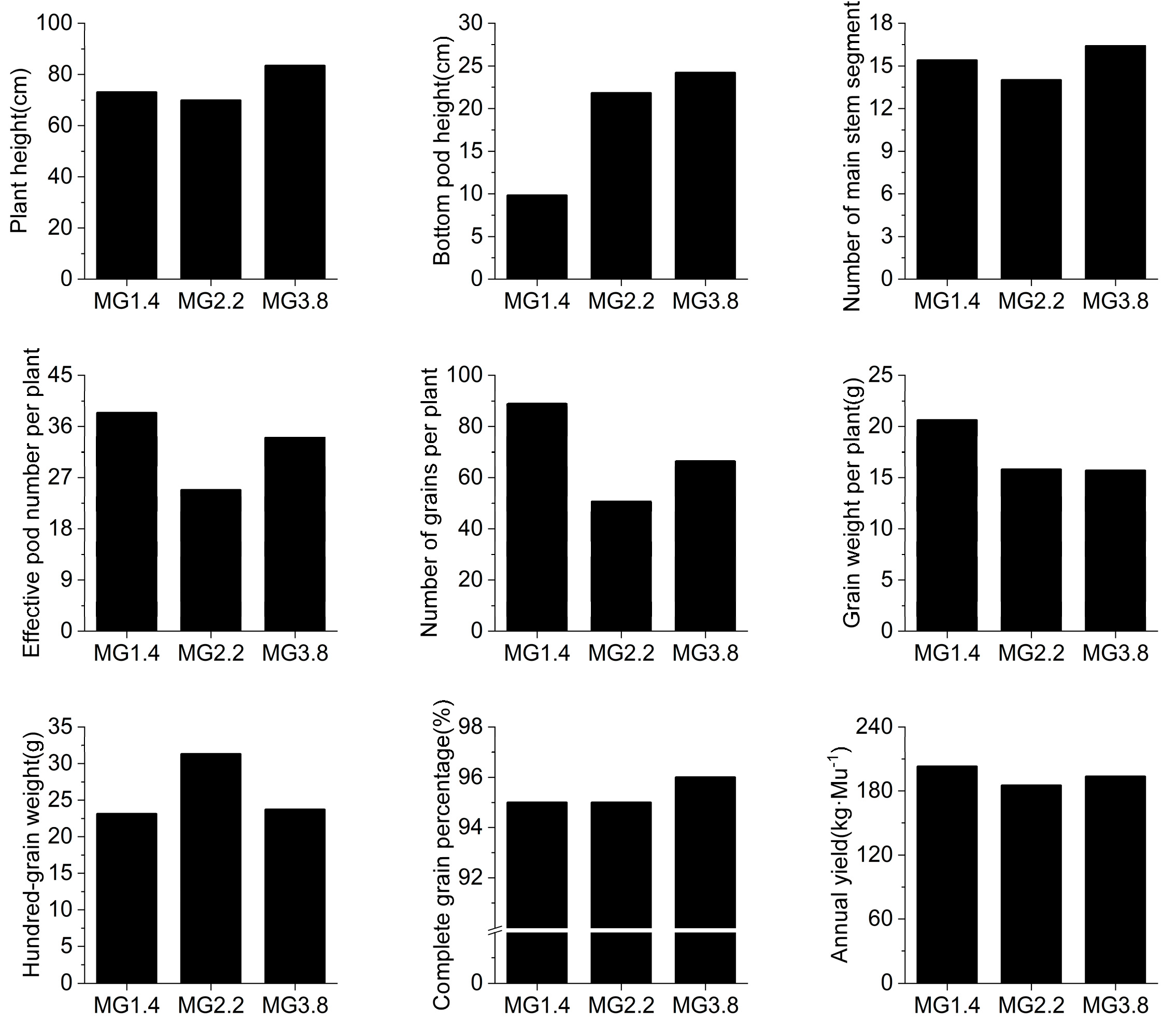
3.1. Physiological and Soil Chemical Indexes of Soybean
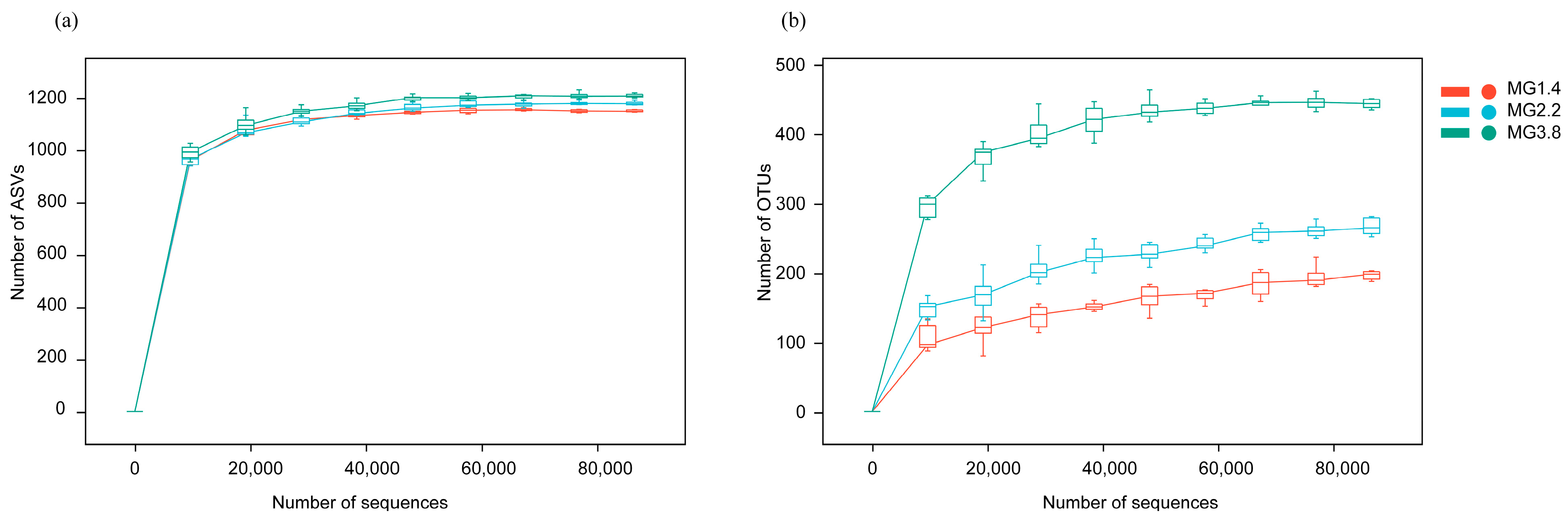
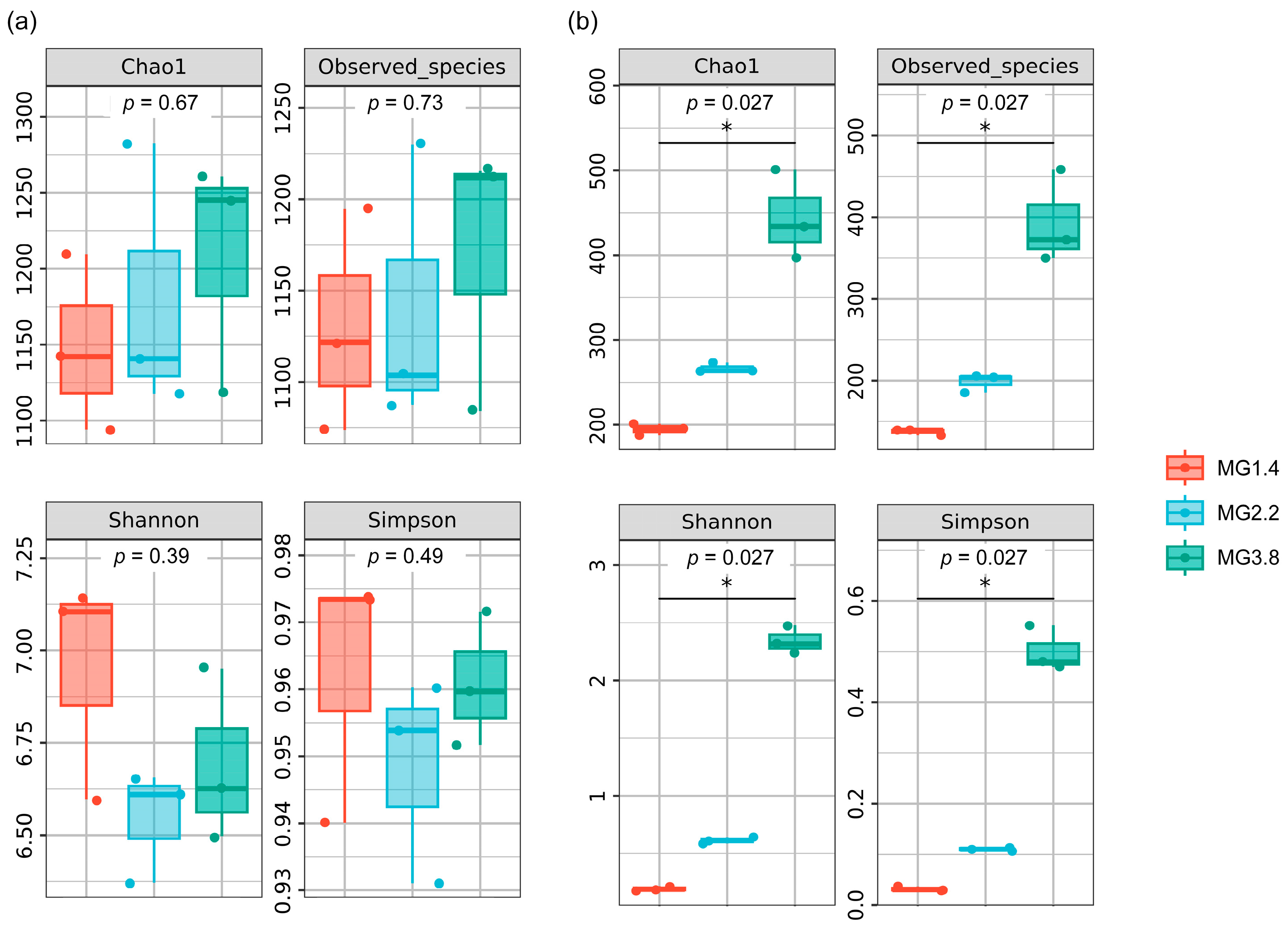
3.2. Diversity of AMF and Diazotrophs
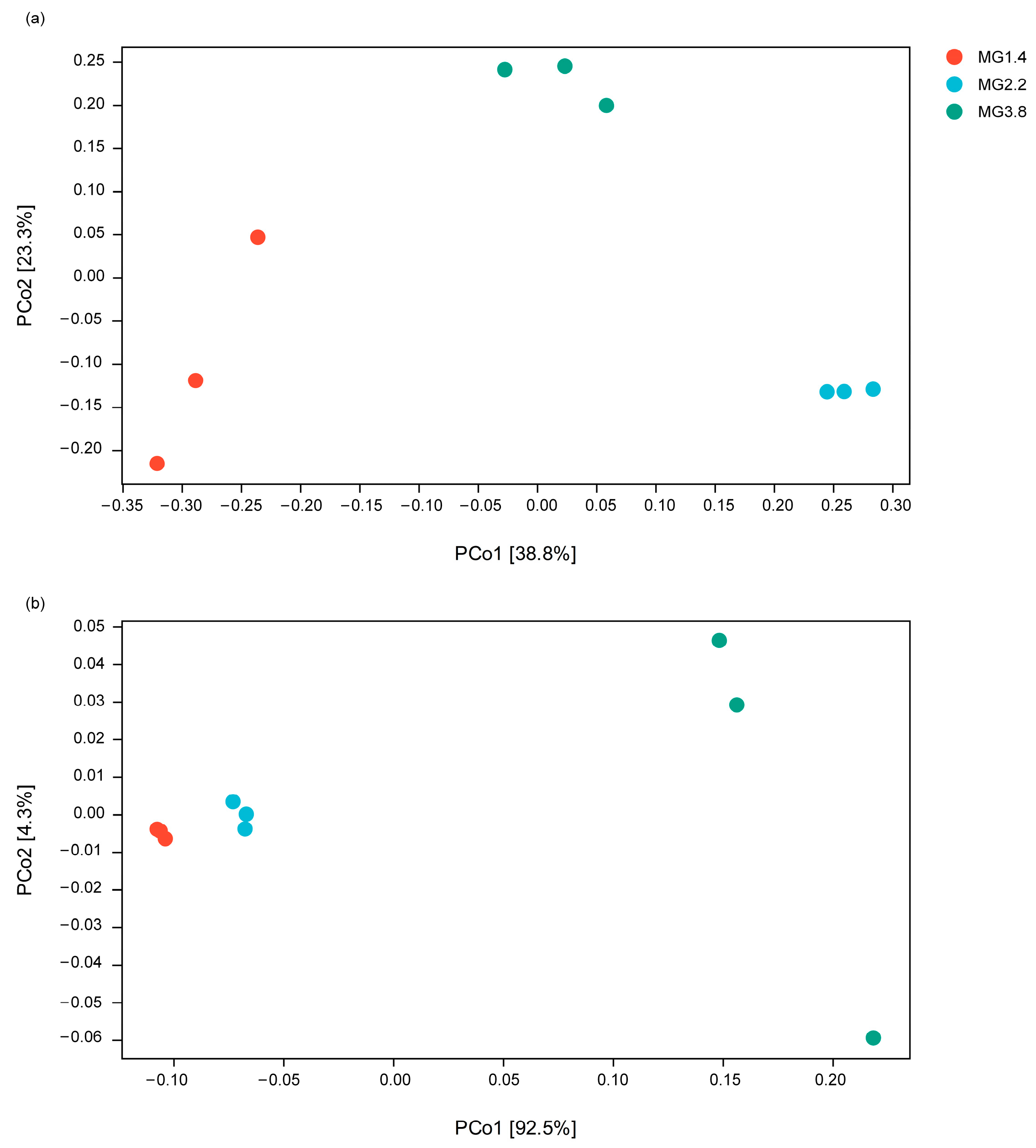
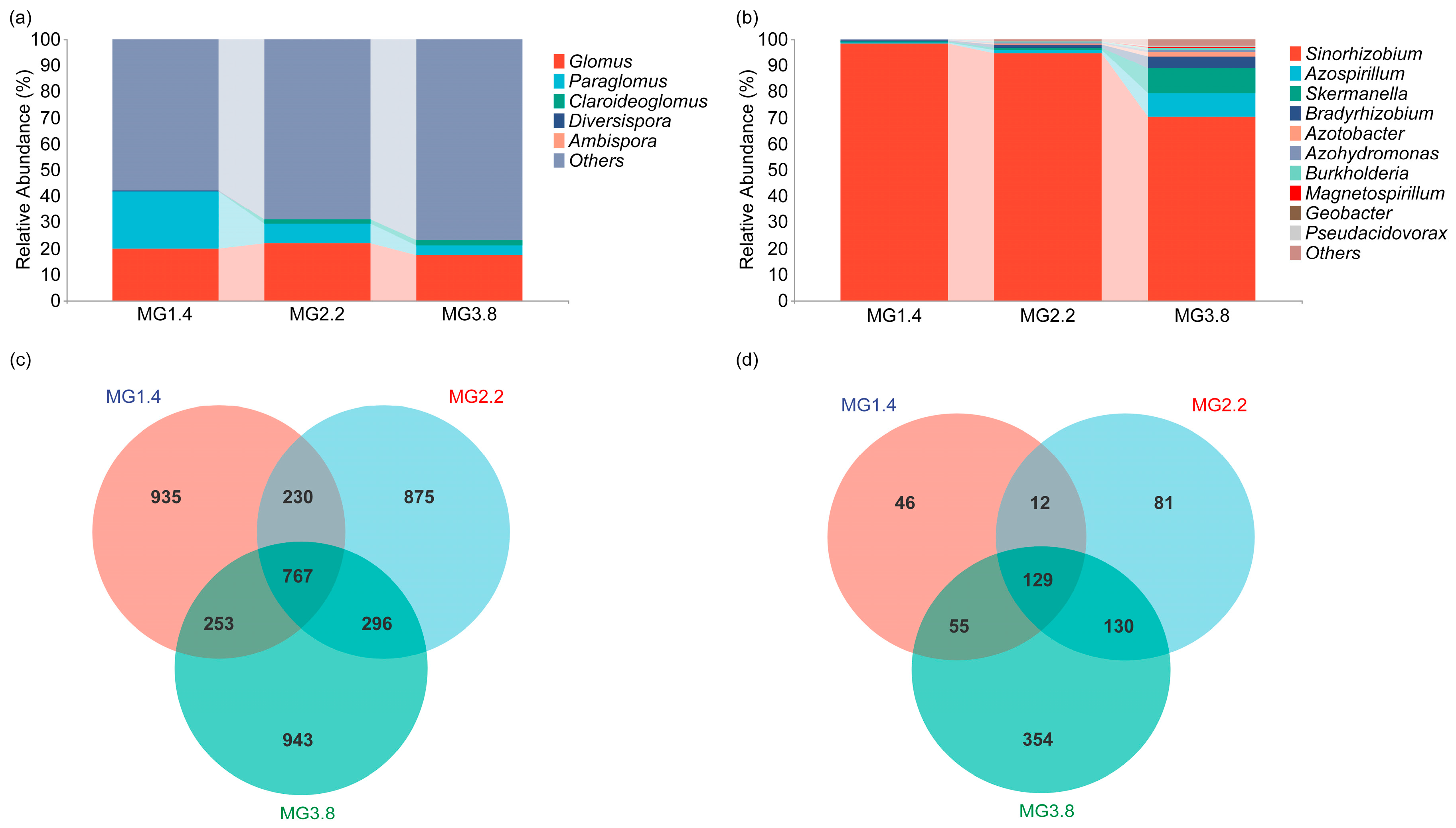
3.3. Composition and Structure of AMF and Diazotrophs
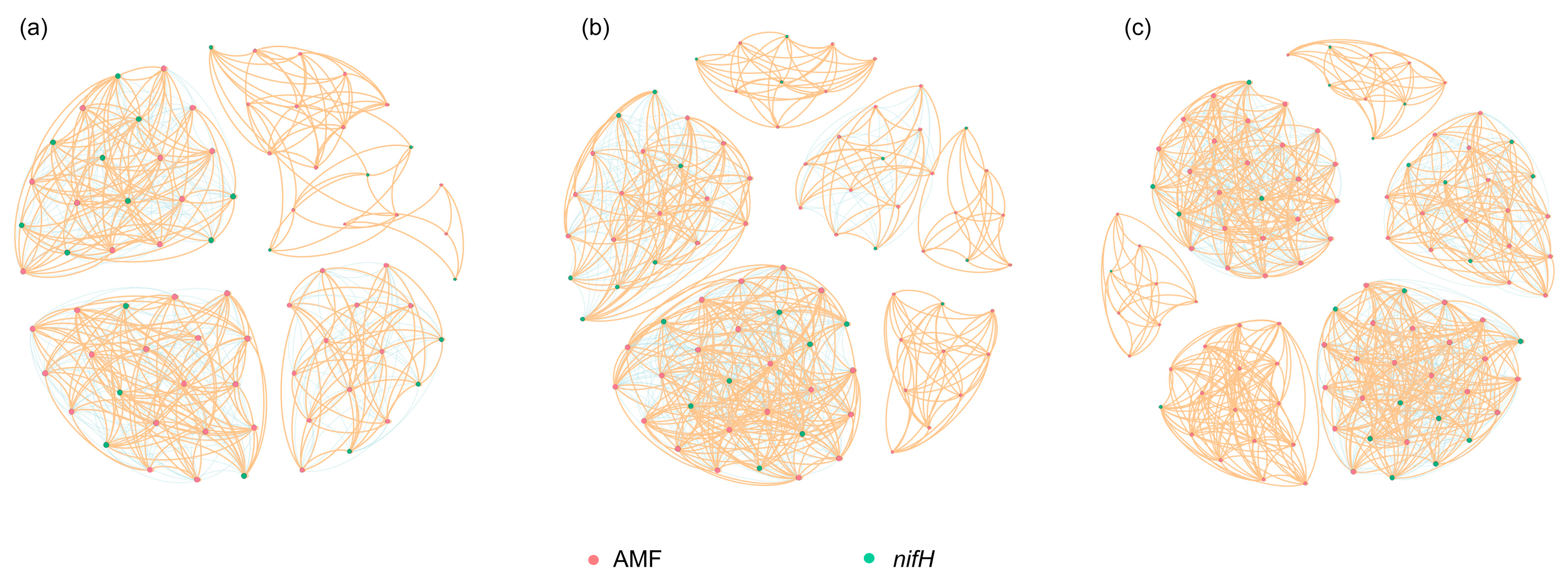
3.4. Co-Occurrence Patterns between AMF and the Diazotrophs
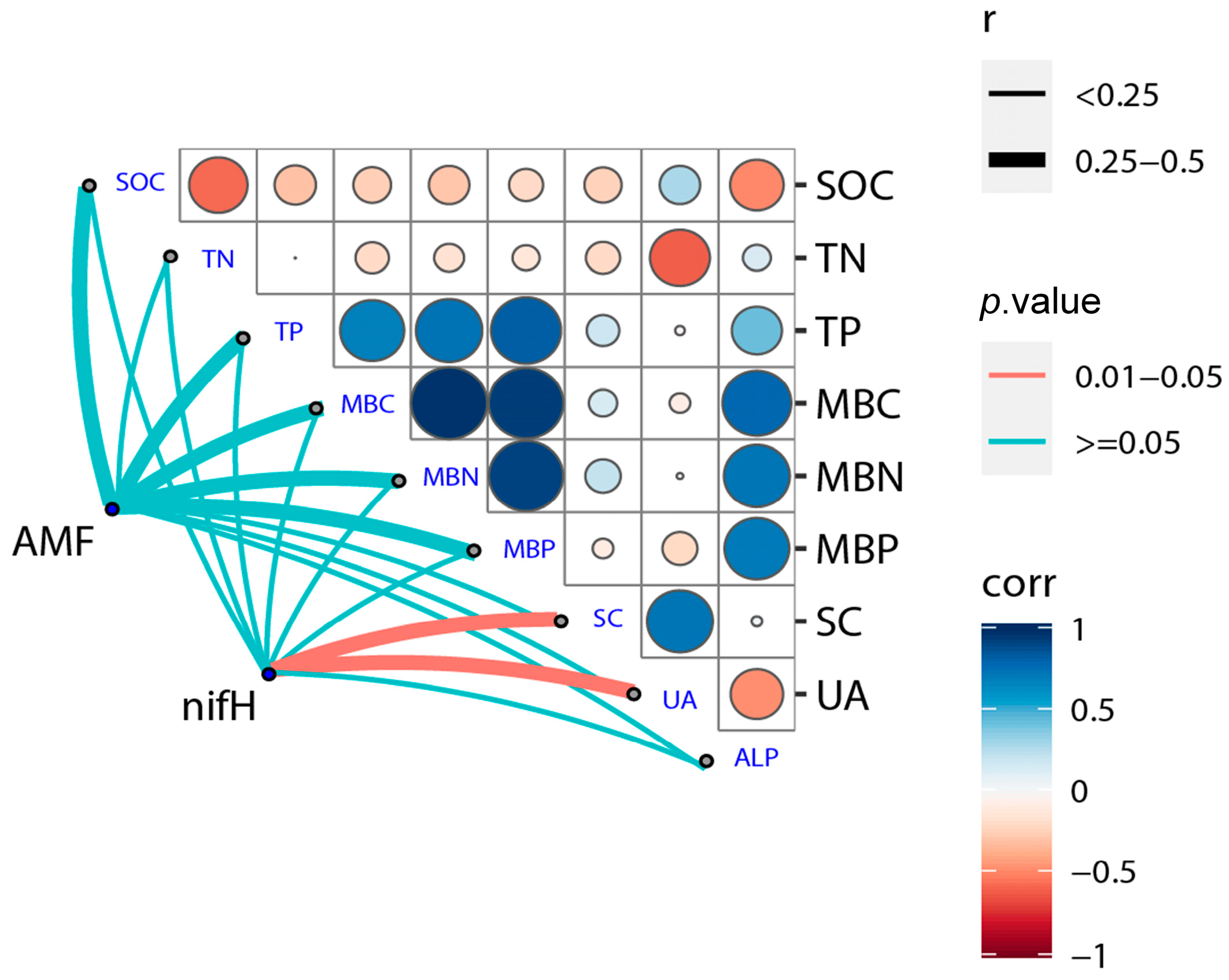
3.5. Correlation of Soil Physical-Chemical Properties and Microbial Communities
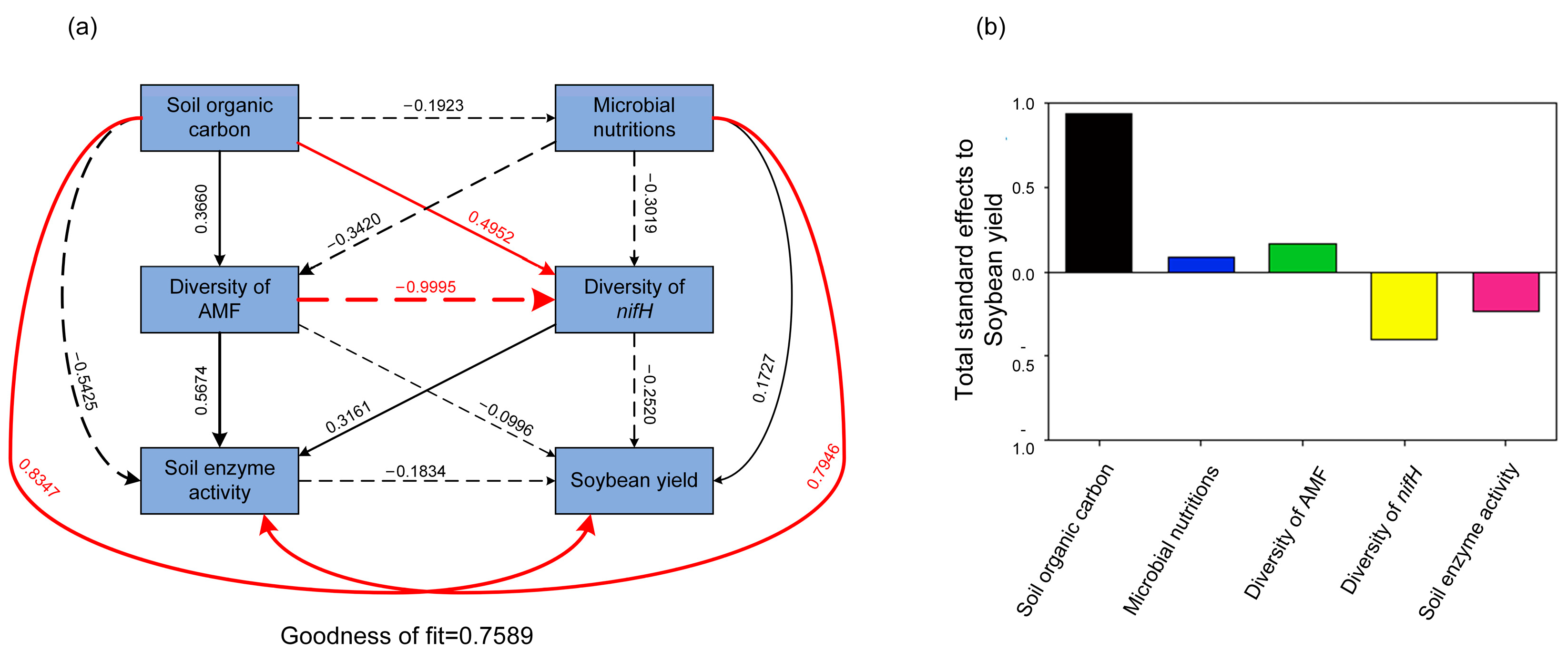
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Li, Y.; Yang, T.; Zhang, P.; Zou, A.; Peng, X.; Wang, L.; Yang, R.; Qi, J.; Yang, Y. Differential responses of the diazotrophic community to aluminum-tolerant and aluminum-sensitive soybean genotypes in acidic soil. Eur. J. Soil Biol. 2012, 53, 76–85. [Google Scholar] [CrossRef]
- Kafle, A.; Garcia, K.; Wang, X.; Pfeffer, P.E.; Strahan, G.D.; Bücking, H. Nutrient demand and fungal access to resources control the carbon allocation to the symbiotic partners in tripartite interactions of Medicago truncatula. Plant. Cell Environ. 2019, 42, 270–284. [Google Scholar] [CrossRef] [Green Version]
- Lotfabadi, Z.E.; Weisany, W.; Tahir, N.A.; Torkashvand, A.M. Arbuscular mycorrhizal fungi species improve the fatty acids profile and nutrients status of soybean cultivars grown under drought stress. J. Appl. Microbiol. 2021, 132, 2177–2188. [Google Scholar] [CrossRef]
- Meena, R.S.; Vijayakumar, V.; Yadav, G.S.; Mitran, T. Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant. Growth Regul. 2018, 84, 207–223. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z.; Xiao, Y. Distinct community assembly and co-existence of arbuscular mycorrhizal fungi and diazotrophs across large-scale soil fertility to improve functions in alfalfa cultivation systems. Environ. Microbiol. 2022, 24, 5277–5291. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, G.; Luo, G.; Kong, Y.; Guo, J.; Wang, M.; Guo, J.; Wang, M.; Guo, S.; Ling, N.; et al. N-fertilizer-driven association between the arbuscular mycorrhizal fungal community and diazotrophic community impacts wheat yield. Agric. Ecosyst. Environ. 2018, 254, 191–201. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Q.; Chen, F.; Yan, X.; Liao, H. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 2011, 21, 173–181. [Google Scholar] [CrossRef]
- Primieri, S.; Santos, J.C.P.; Antunes, P.M. Nodule-associated bacteria alter the mutualism between arbuscular mycorrhizal fungi and N2 fixing bacteria. Soil Biol. Biochem. 2021, 154, 108–149. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.S.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant. Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Van Versendaal, E.; Carcedo, A.J.; Adee, E.; Sassenrath, G.; Dooley, S.; Lingenfelser, J.; Ciampitti, I.A. Integrating Field Data and a Modeling Approach to Inform Optimum Planting Date × Maturity Group for Soybeans under Current and Future Weather Conditions in Kansas. Sustainability 2023, 15, 1081. [Google Scholar] [CrossRef]
- Cober, E.R.; Stewart, D.W.; Voldeng, H.D. Photoperiod and temperature responses in earlymaturing, near-isogenic soybean lines. Crop Sci. 2001, 41, 721–727. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Gaska, J.M.; Conley, S.P. Delineating optimal soybean maturity groups across the United States. Environments 2015, 1, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, Y.A.; Gesch, R.W.; Matthees, H.L.; Wells, S.S. Maturity selection but not sowing date enhances soybean productivity and land use in a winter camelina–soybean relay system. Food Energy Secur. 2022, 11, e346. [Google Scholar] [CrossRef]
- Buade, R.; Chourasiya, D.; Prakash, A.; Sharma, M.P. Changes in arbuscular mycorrhizal fungal community structure in soybean rhizosphere soil assessed at different growth stages of soybean. Agric. Res. 2020, 10, 32–43. [Google Scholar] [CrossRef]
- Faggioli, V.; Covacevich, F.; Grilli, G.; Lorenzón, C.; Aimetta, B.; Sagadin, M.; Langarica-Fuentes, A.; Cabello, M.N. Environmental response of arbuscular mycorrhizal fungi under soybean cultivation at a regional scale. Mycorrhiza 2022, 32, 425–438. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Kumar, A. Taxonomical and functional bacterial community profiling in disease-resistant and disease-susceptible soybean cultivars. Braz. J. Microbiol. 2022, 53, 1355–1370. [Google Scholar] [CrossRef]
- Jakobsen, I.; Rosendahl, L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New. Phytol. 1990, 115, 77–83. [Google Scholar] [CrossRef]
- Provorov, N.A.; Tikhonovich, I.A. Genetic resources for improving nitrogen fixation in legume-rhizobia symbiosis. Genet. Resour. Crop. Evol. 2003, 50, 89–99. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, A.; Venter, M.; Kossmann, J.; Valentine, A. The reallocation of carbon in P deficient lupins affects biological nitrogen fixation. J. Plant. Physiol. 2014, 171, 1619–1624. [Google Scholar] [CrossRef]
- Xiao, D.; Chen, Y.; He, X.; Xu, Z.; Bai, S.H.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Temperature and precipitation significantly influence the interactions between arbuscular mycorrhizal fungi and diazotrophs in karst ecosystems. For. Ecol. Manag. 2021, 497, 119464. [Google Scholar] [CrossRef]
- Zhang, F.G.; Zhou, Z.B.; Xiao, Y. Abundant and rare bacteria possess different diversity and function in crop monoculture and rotation systems across regional farmland. Soil Biol. Biochem. 2022, 171, 108742. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.; Yu, X.; Gu, H.; Zheng, X.; Wang, C.; Xiao, F.; Shu, L.; et al. Co-symbiosis of arbuscular mycorrhizal fungi AMF and diazotrophs promote biological nitrogen fixation in mangrove ecosystems. Soil Biol. Biochem. 2021, 161, 108382. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, J.; Wang, Y.; Chen, K.; Lambers, H.; Wang, X. The relative contribution of indigenous and introduced arbuscular mycorrhizal fungi and rhizobia to plant nutrient acquisition in soybean/maize intercropping in unsterilized soils. Appl. Soil Ecol. 2021, 168, 104–124. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Q.; Chen, Y.; Tian, B.; Xu, L.; Bai, Y.; Chen, W.; Li, X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J. 2020, 14, 1915–1928. [Google Scholar] [CrossRef] [Green Version]
- Salmeron, M.; Gbur, E.E.; Bourland, F.M.; Buehring, N.W.; Earnest, L.; Fritschi, F.B.; Golden, B.R.; Hathcoat, D.; Lofton, J.; Miller, T.D.; et al. Soybean maturity group choices for early and late plantings in the Midsouth. Agron. J. 2014, 106, 1893–1901. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2008; pp. 16–27. [Google Scholar]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. QIIME 2: Reproducible interactive scalable and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v2. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with qiime 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar] [CrossRef]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bul. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [Green Version]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Heck, K.L., Jr.; van Belle, G.; Simberloff, D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 1975, 56, 1459–1461. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us. Fems Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]
- Barberán, A.; Ramirez, K.S.; Leff, J.W.; Bradford, M.A.; Wall, D.H.; Fierer, N. Why are some microbes more ubiquitous than others? Predicting the habitat breadth of soil bacteria. Ecol. Lett. 2014, 17, 794–802. [Google Scholar] [CrossRef]
- Sanchez, G. PLS Path Modeling with R. 2013. Available online: https://www.gastonsanchez.com/PLS_Path_Modeling_with_R.pdf (accessed on 18 September 2021).
- Wang, J.; Pan, F.; Soininen, J.; Heino, J.; Shen, J. Nutrient enrichment modifies temperature-biodiversity relationships in large-scalefield experiments. Nat. Commun. 2016, 7, 13960. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Shi, K.; Wang, Y.; Kong, D.; Liu, T.; Jiao, J.; Liu, M.; Li, H.; Hu, F. Soil quality assessment of wheat-maize cropping system with different productivities in China: Establishing a minimum data set. Soil Tillage Res. 2019, 190, 31–40. [Google Scholar] [CrossRef]
- Benintende, S.M.; Benintende, M.C.; Sterren, M.A.; De Battista, J.J. Soil microbiological indicators of soil quality in four rice rotations systems. Ecol. Indic. 2008, 8, 704–708. [Google Scholar] [CrossRef]
- Wang, D.; Shannon, M.C. Emergence and seedling growth of soybean cultivars and maturity groups under salinity. Plant Soil 1999, 214, 117–124. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Yang, F.; Wang, X.; Yong, T.; Liu, J.; Pu, T.; Yan, Y.; Yang, W. Dynamic of recovery growth of intercropped soybean after maize harvest in maize–soybean relay strip intercropping system. Food Energy Secur. 2022, 11, e350. [Google Scholar] [CrossRef]
- Ortel, C.C.; Roberts, L.T.; Hoegenauer, A.K.; Purcell, L.C.; Slaton, N.A.; Gbur, E.E. Soybean maturity group and planting date influence grain yield and nitrogen dynamics. Agrosystems Geosci. Environ. 2020, 3, e20077. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D.; Hobbie, S. Linkages between plant functional composition, fine root processes and potential soil N mineralization rates. J. Ecol. 2009, 97, 48–56. [Google Scholar] [CrossRef]
- Hazra, K.K.; Nath, C.P.; Singh, U.; Praharaj, C.S.; Kumar, N.; Singh, S.S.; Singh, N.P. Diversification of maize-wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma 2019, 353, 308–319. [Google Scholar] [CrossRef]
- Stemmer, M.; Gerzabek, M.H.; Kandeler, E. Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol. Biochem. 1998, 30, 9–17. [Google Scholar] [CrossRef]
- Jin, K.; Sleutel, S.; Buchan, D.; Neve, S.D.; Cai, D.X.; Gabriels, D.; Jin, J. Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Tillage Res. 2009, 104, 115–120. [Google Scholar] [CrossRef]
- Kumagai, E.; Sameshima, R. Genotypic differences in soybean yield responses to increasing temperature in a cool climate are related to maturity group. Agric. For. Meteorol. 2014, 198, 265–272. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Chen, B.; Rilling, M.C. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol. Biochem. 2012, 46, 53–62. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. Impact of long-term N additions upon coupling between soil microbial community structure and activity.; and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.G.; Wang, J.T.; Singh, B.K.; Han, L.L.; Shen, J.; Li, P.; Wang, G.; Wu, C.; Ge, A.; et al. Host selection shapes crop microbiome assembly and network complexity. New. Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Olanrewaju, O.S.; Babalola, O.O. Plant growth stage drives the temporal and spatial dynamics of the bacterial microbiome in the rhizosphere of vgna subterranea. Front. Microbiol. 2022, 13, 825377. [Google Scholar] [CrossRef]
- Gosling, P.; Proctor, M.; Jones, J.; Bending, G.D. Distribution and diversity of ParaGlomus spp. in tilled agricultural soils. Mycorrhiza 2014, 24, 1–11. [Google Scholar] [CrossRef]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Cornwell, W.K.; Rillig, M.C. Bridging reproductive and microbial ecology: A case study in arbuscular mycorrhizal fungi. ISME 2018, 13, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.D.; Dhanapal, A.P.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Boykin, D.; Cregan, P.B.; Song, Q.; et al. Genome-wide association study of ureide concentration in diverse maturity group IV soybean [Glycine max (L.) Merr.] accessions. G3 Genes Genomes Genet. 2015, 5, 2391–2403. [Google Scholar] [CrossRef] [Green Version]
- Jugea, C.; Prévost, D.; Bertrand, A.; Bipfubusa, M.; Chalifour, F.P. Growth and biochemical responses of soybean to double and triple microbial associations with Bradyrhizobium.; Azospirillum and arbuscular mycorrhizae. Appl. Soil Ecol. 2012, 61, 147–157. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Hu, A.Y.; Zhu, C.Q.; Shen, R.F. Diazotroph abundance and community composition in an acidic soil in response to aluminum-tolerant and aluminum-sensitive maize Zea mays L. cultivars under two nitrogen fertilizer forms. Plant Soil 2018, 424, 463–478. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics.; biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
| Variables | Different MG Genotypes | ||
|---|---|---|---|
| MG1.4 | MG2.2 | MG3.8 | |
| SOC (g kg−1) | 16.48 ± 0.40 a | 13.57 ± 0.28 c | 15.51 ± 0.25 b |
| TN (g kg−1) | 0.68 ± 0.45 b | 0.82 ± 0.31 a | 0.60 ± 0.39 b |
| TP (g kg−1) | 0.75 ± 0.67 a | 0.79 ± 0.01 a | 0.79 ± 0.61 a |
| MBC (mg kg−1) | 241.78 ± 2.14 a | 242.64 ± 1.08 a | 242.90 ± 0.84 a |
| MBN (mg kg−1) | 51.39 ± 2.35 a | 52.27 ± 0.63 a | 52.28 ± 0.81 a |
| MBP (mg kg−1) | 3.14 ± 0.16 a | 3.16 ± 0.75 a | 3.15 ± 0.08 a |
| SC Activity (g mg−1/24 h) | 22.81 ± 4.50 b | 31.57 ± 4.89 a | 40.28 ± 2.59 a |
| UA Activity (g mg−1/24 h) | 131.80 ± 1.61 ab | 126.47 ± 9.16 b | 149.33 ± 3.14 a |
| ALP Activity (g mg−1/24 h) | 0.46 ± 0.02 a | 0.49 ± 0.20 a | 0.47 ± 0.13 a |
| Network Properties | Different MG Genotypes | ||
|---|---|---|---|
| MG1.4 | MG2.2 | MG3.8 | |
| Number of nodes | 75 | 86 | 107 |
| Number of links | 637 | 822 | 1184 |
| Number of positive links | 382 | 495 | 725 |
| Percentage of positive links (%) | 60.0 | 60.0 | 61.2 |
| Average degree | 16.99 | 19.12 | 22.13 |
| Average clustering coefficient | 0.98 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Li, S.; Bai, J.; Wang, K.; Qu, Y.; Long, M.; Yang, P.; Hu, T.; He, S. Arbuscular Mycorrhizal Fungi and Diazotrophic Diversity and Community Composition Responses to Soybean Genotypes from Different Maturity Groups. Agronomy 2023, 13, 1713. https://doi.org/10.3390/agronomy13071713
Wu M, Li S, Bai J, Wang K, Qu Y, Long M, Yang P, Hu T, He S. Arbuscular Mycorrhizal Fungi and Diazotrophic Diversity and Community Composition Responses to Soybean Genotypes from Different Maturity Groups. Agronomy. 2023; 13(7):1713. https://doi.org/10.3390/agronomy13071713
Chicago/Turabian StyleWu, Mandi, Shengzhican Li, Jie Bai, Kezhen Wang, Yang Qu, Mingxiu Long, Peizhi Yang, Tianming Hu, and Shubin He. 2023. "Arbuscular Mycorrhizal Fungi and Diazotrophic Diversity and Community Composition Responses to Soybean Genotypes from Different Maturity Groups" Agronomy 13, no. 7: 1713. https://doi.org/10.3390/agronomy13071713





