Different Concentrations of Chemical Topping Agents Affect Cotton Yield and Quality by Regulating Plant Architecture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Site
2.2. Experimental Design
2.3. Sampling and Determination
2.3.1. Yield Components and Cotton Fiber Quality
2.3.2. Plant Architecture Characteristics
2.3.3. Leaf Area Index
2.3.4. Net Photosynthetic Rate of a Single Leaf
2.4. Data Processing
3. Results
3.1. Seed Cotton Yield and Yield Components
3.2. Plant Architecture Characteristics
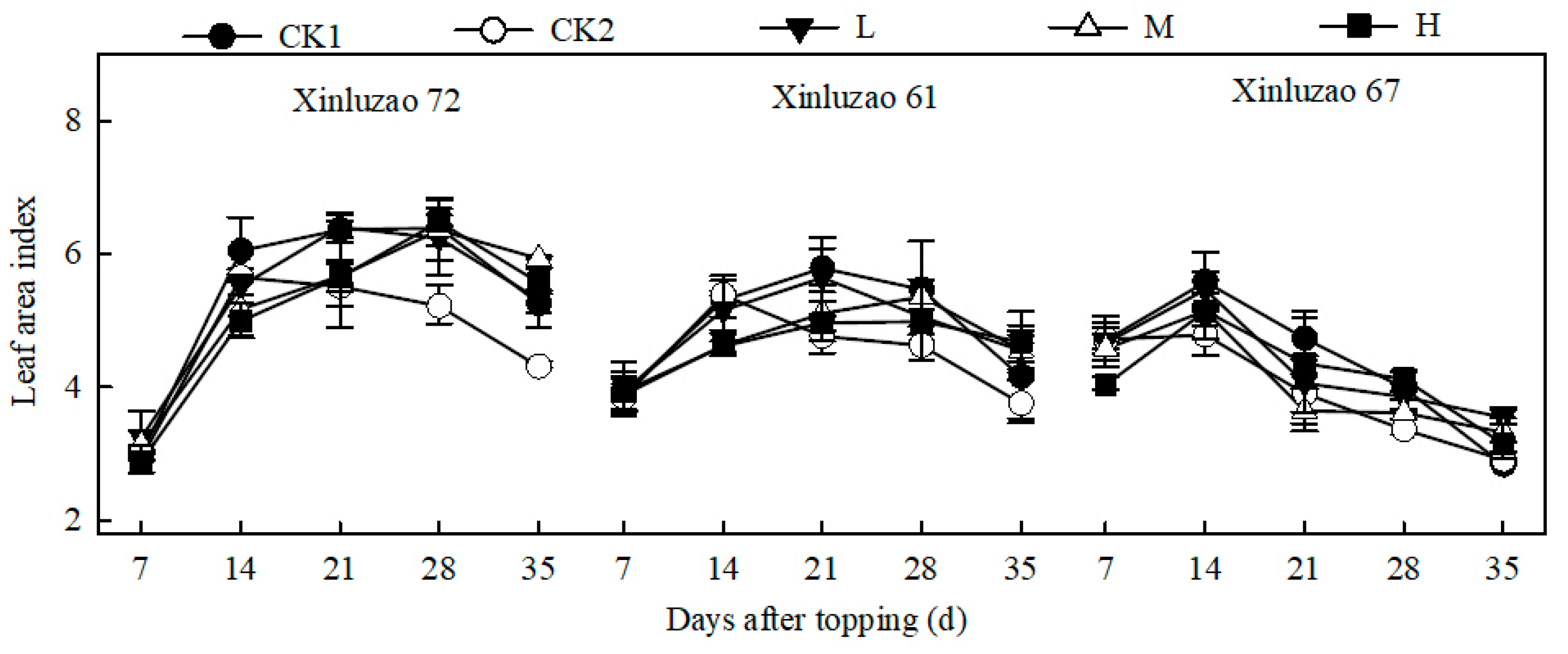
3.3. Dynamics of LAI
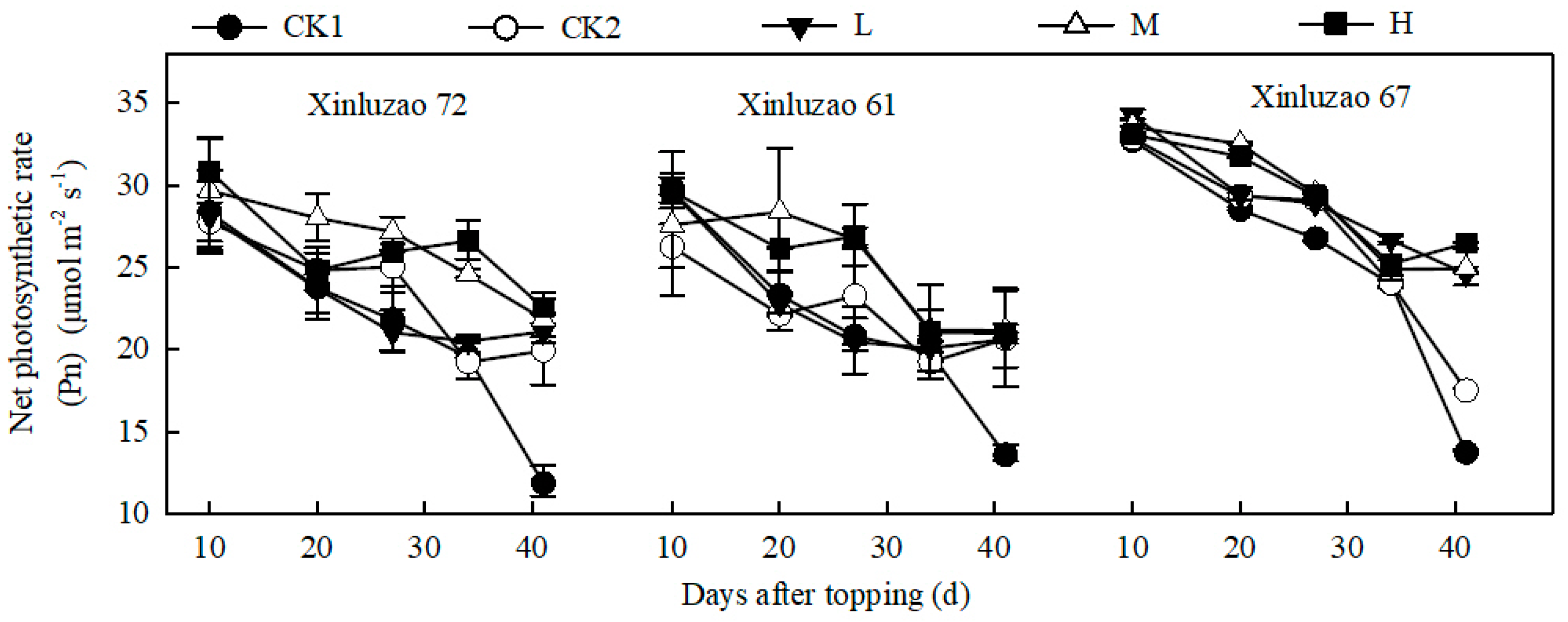
3.4. Net Photosynthetic Rate of Leaves
4. Discussion
4.1. Chemical Topping Has a Positive Regulatory Effect on Cotton Yield and Yield Components
4.2. Chemical Topping Creates a Compact Plant Architecture by Affecting Plant-Related Traits
4.3. Chemical Topping Maintains the Relative Stability of Yield by Regulating Horizontal and Vertical Growth
4.4. Chemical Topping Can Regulate Plant Architecture and Increase the Photosynthetic Area and Photosynthetic Function Period
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, J.L.; Li, W.J.; Zhang, D.M.; Tang, W.; Li, Z.H.; Lu, H.Q.; Kong, X.Q.; Luo, Z.; Xu, S.Z.; Xin, C.S.; et al. Competitive yield and economic benefits of cotton achieved through a combination of extensive pruning and a reduced nitrogen rate at high plant density. Field Crops Res. 2017, 209, 65–72. [Google Scholar] [CrossRef]
- Siebert, J.D.; Stewart, A.M. Influence of plant density on cotton response to mepiquat chloride application. Agron. J. 2006, 98, 1634–1639. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Oosterhuis, D.M.; Souzade, S.F. Cotton response to mepiquat chloride and temperature. Sci. Agric. 2013, 70, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.Z.; Zuo, W.Q.; Chen, M.Z.; Sui, L.L.; Dong, H.Y.; Jiu, X.L.; Zhang, W.F. Effect of Drip Irrigation Amount on the Agronomic Traits and Yield of Cotton Grown with a Chemical Topping in Northern Xinjiang, China. Cotton Sci. 2017, 29, 345–355. [Google Scholar]
- Zhao, Q.; Zhou, C.J.; Zhang, J.S.; Li, S.L.; Yun, Y.L.; Tian, X.L. Effect of Chemical Detopping on the Canopy and Yield of Cotton (Gossypium hirsutum) in South Xinjiang. Cotton Sci. 2011, 23, 329–333. [Google Scholar]
- McLean, E.H.; Ludwig, M.; Rierson, P.F. Root hydraulic conductance and aquaporin abundance respond rapidly to partial root-zone drying events in a riparian Melaleuca species. New Phytol. 2011, 192, 664–675. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Wang, X.R.; Du, M.W.; Zhou, C.J.; Yin, X.F.; Xu, D.Y.; Lu, H.Y.; Tian, X.L.; Li, Z.H. Cotton chemical topping with mepiquat chloride application in the north of Yellow River valley of China. Sci. Agric. Sin. 2016, 49, 2497–2510. [Google Scholar]
- Zhang, J.; Han, Y.C.; Li, Y.B.; Li, X.F.; Wang, G.P.; Wang, Z.B.; Du, W.L.; Feng, L. Inhibition of apical dominance affects boll spatial distribution, yield and fiber quality of field-grown cotton. Ind. Crops Prod. 2021, 173, 114098. [Google Scholar] [CrossRef]
- Dai, J.L.; Tian, L.W.; Zhang, Y.J.; Zhang, D.M.; Xu, S.Z.; Cui, Z.P.; Li, Z.H.; Li, W.J.; Zhan, L.J.; Li, C.D. Plant topping effects on growth, yield, and earliness of field-grown cotton as mediated by plant density and ecological conditions. Field Crops Res. 2022, 275, 108337. [Google Scholar] [CrossRef]
- Gwathmey, C.O.; Clement, J.D. Alteration of cotton source-sink relations with plant population density and mepiquat chloride. Field Crops Res. 2010, 116, 101–107. [Google Scholar] [CrossRef]
- Mao, L.L.; Li, Z.Z.; Sun, X.Z.; Wopke, V.D.W.; Evers, J.B.; Zhao, X.H.; Zhang, S.P.; Song, X.L.; Li, Z.H. Use of the beta growth function to quantitatively characterize the effects of plant density and a growth regulator on growth and biomass partitioning in cotton. Field Crops Res. 2018, 224, 28–36. [Google Scholar] [CrossRef]
- Yang, C.X.; Zhang, W.F.; Xu, S.Z.; Sui, L.L.; Liang, F.B.; Dong, H.Y. Effects of Spraying Chemical Topping Agents on Canopy Structure and Canopy Photosynthetic Production in Cotton. Sci. Agric. Sin. 2016, 49, 1672–1684. [Google Scholar]
- Tung, S.A.; Huang, Y.; Hafeez, A.; Ali, S.; Khan, A.; Souliyanonh, B.; Song, X.; Liu, A.; Yang, G. Mepiquat chloride effects on cotton yield and biomass accumulation under late sowing and high density. Field Crops Res. 2018, 215, 59–65. [Google Scholar] [CrossRef]
- Zhu, L.X.; Liu, L.T.; Zhang, Y.J.; Sun, H.C.; Zhang, K.; Bai, Z.Y.; Dong, H.Z.; Li, C.D. The regulation and evaluation indexes screening of chemical topping on cotton’s plant architecture. Sci. Agric. Sin. 2020, 53, 4152–4163. [Google Scholar]
- Liu, Y. Effects of Mepiquat Chloride and Pruning with Topping on Cotton Crop; Huazhong Agricultural University: Wuhan, China, 2013. [Google Scholar]
- Gwathmey, C.O.; Craig, C.C. Managing earliness in cotton with mepiquat-type growth regulators. Crop Manag. 2003, 2, 1–8. [Google Scholar] [CrossRef]
- Wilson, D.G.; York, A.C.; Edmisten, K.L. Narrow-row cotton response to mepiquat chloride. J. Cotton Sci. 2007, 11, 177–185. [Google Scholar]
- Zhou, M.D.; Li, M.Y.; Wu, L.N.; Guo, L.J.; Wang, Q.; Ma, M. Studies on Regulation of Plant Architecture by TaKLU Gene in Wheat. J. Triticeae Crops 2022, 42, 399–406. [Google Scholar]
- Wei, X.Y.; Yang, J.; Lei, D.; Feng, H.; Yang, Z.; Wen, G.; He, Z.; Zeng, W.; Zou, J. The SlTCP26 promoting lateral branches development in tomato. Plant Cell Rep. 2021, 40, 1115–1126. [Google Scholar] [CrossRef]
- Mathan, J.; Bhattacharya, J.; Ranjan, A. Enhancing crop yield by optimizing plant developmental features. Development 2016, 143, 3283–3294. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.Z.; Zhang, Q.F. Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 2010, 61, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Doust, A. Architectural evolution and its implications for domestication in grasses. Ann. Bot. 2007, 100, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef]
- Wang, R.L.; Stec, A.; Hey, J.; Lukens, L.; Doebley, J. The limits of selection during maize domestication. Nature 1999, 398, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Kaggwa, A.R.; Andrade, S.P.; Wang, G.Y. Plant architecture influences growth and yield response of upland cotton to population density. Field Crops Res. 2013, 145, 52–59. [Google Scholar] [CrossRef]
- Chen, M.Z.; Yang, Y.L.; Wang, Y.X.; Tian, J.S.; Xu, S.Z.; Liu, N.N.; Dang, K.; Zhang, W.F. Plant Type Characteristics and Evolution of Main Economic Characters in Early Maturing Upland Cotton Cultivar Replacement in Xinjiang. Sci. Agric. Sin. 2019, 52, 3279–3290. [Google Scholar]
- Wang, F.Y.; Han, H.Y.; Lin, H.; Chen, B.; Kong, X.H.; Ning, X.Z.; Wang, X.W.; Yu, Y.; Liu, J.D. Effects of planting patterns on yield, quality, and defoliation in machine-harvested cotton. J. Integr. Agric. 2019, 18, 2019–2028. [Google Scholar] [CrossRef]
- Osman, C.; Demirel, U.; Karakus, M. Effects of several plant growth regulators on the yield and fiber quality of cotton (Gossypium hirsutum L.). Not. Bot. Horti Agrobot. Cluj Napoca 2010, 38, 104–110. [Google Scholar]
- Yang, C.X. Effects of Chemical Topping on Plant Morphological, Canopy Structure, and Canopy Photosynthetic Production of Cotton; Shihezi University: Shihezi, China, 2016. [Google Scholar]
- Nichols, S.P.; Snipes, C.E.; Jones, M.A. Evaluation of row spacing and mepiquat chloride in cotton. J. Cotton Sci. 2003, 7, 148–155. [Google Scholar]
- Bogiani, J.C.; Rosolem, C.A. Sensibility of cotton cultivars to mepiquat chloride. Pesqui. Agropecuária Bras. 2009, 44, 1246–1253. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.S.; Wei, X.; Xu, X.X. Effects of Mepiquat Chloride on Physiological Indicators of Leaf Function and Characteristics of Yield of Hybrid Cotton in South Xinjiang. Cotton Sci. 2014, 26, 122–129. [Google Scholar]
- Malone, S.; Herbert, D.A.; Holshouser, D.L. Evaluation of the LAI-2000 plant canopy analyzer to estimate leaf area in manually defoliated soybean. Agron. J. 2002, 94, 1012–1019. [Google Scholar] [CrossRef]
- Deng, J. Introduction to grey system. J. Grey Syst. 1989, 1, 1–24. [Google Scholar]
- Ng, D.K.W.; Deng, J. Contrasting grey system theory to probability and fuzzy. ACM Sigice Bull. 1995, 20, 3–9. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Chen, J.Y.; Qin, Y.K.; Wang, Y.P.; Cheng, H.H.; Xia, S.N. Effects of chemical capping agent on direct cotton after rape. Hubei Agric. Sci. 2021, 60, 36–40. [Google Scholar]
- Li, X.; Zhu, C.H.; Xia, K.; Gan, L.J. Effects of Methyl Octanoate Methyl Decanoate and6-BA on Topping in Cotton. Cotton Sci. 2009, 21, 70–72. [Google Scholar]
- Li, J.F.; Wang, C.; Liang, F.B.; Chen, H.C.; Tian, J.S.; Kang, P.; Zhang, W.F. Row Spacing and Planting Density Affect Canopy Structure and Yield in Machine-Picked Cotton in Xinjiang. Cotton Sci. 2017, 29, 157–165. [Google Scholar]
- Yi, Z.B.; Cheng, Z.L.; Liu, H.Y. Study on the Application Effect of Chemical Topping and Pruning Agent on Cotton. China Agric. Technol. Ext. 2013, 29, 32–33. [Google Scholar]
- Chen, R.F.; Fan, Y.G.; Zhou, H.W.; Mo, S.P.; Zhou, Z.F.; Yan, H.F.; Luo, T.; Huang, X.; Weng, M.L.; Lakshmanan, P.; et al. Global transcriptome changes of elongating internode of sugarcane in response to mepiquat chloride. BMC Genom. 2021, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, F.; Zhou, C.J.; Tian, X.-L.; Li, Z.-H. Morpho-physiological Responses of Cotton Shoot Apex to the Chemical Topping with Fortified Mepiquat Chloride. Crop J. 2018, 44, 1837–1843. [Google Scholar]
- Song, C.; Guan, Y.; Zhang, D.; Tang, X.; Chang, Y. Integrated mRNA and miRNA Transcriptome Analysis Suggests a Regulatory Network for UV-B-Controlled Terpenoid Synthesis in Fragrant Woodfern (Dryopteris fragrans). Int. J. Mol. Sci. 2022, 23, 5708. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Jing, X.; Wang, M.; Zhao, M.; Yu, J.; Qiu, Z.; Li, Y.F. Profiling of MicroRNAs Involved in Mepiquat Chloride-Mediated Inhibition of Internode Elongation in Cotton (Gossypium hirsutum L.) Seedlings. Front. Plant Sci. 2021, 12, 643213. [Google Scholar] [CrossRef]
- Gérardeaux, E.; Loison, R.; Palaï, O.; Sultan, B. Adaptation strategies to climate change using cotton (Gossypium hirsutum L.) ideotypes in rainfed tropical cropping systems in Sub-Saharan Africa. A modeling approach. Field Crops Res. 2018, 226, 38–47. [Google Scholar] [CrossRef]
- Wang, Z.J. Identification and Function Analysis of the Mepiquat Chloride Sensitive Genes of Cotton by RNA-Seq and BSA-Seq; Shihezi University: Shihezi, China, 2022. [Google Scholar]
- Wang, L.; Yin, Y.; Wang, L.F.; Wang, M.; Zhao, M.; Tian, Y.; Li, Y.F. Transcriptome Profiling of the Elongating Internode of Cotton (Gossypium hirsutum L.) Seedlings in Response to Mepiquat Chloride. Front. Plant Sci. 2020, 10, 1751. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, Y.J.; Zhu, Q.H.; Tian, L.W.; Liu, F.; Zhang, X.Y.; Sun, J. Transcriptome Profiling Provides New Insights into the Molecular Mechanism Underlying the Sensitivity of Cotton Varieties to Mepiquat Chloride. Int. J. Mol. Sci. 2022, 23, 5043. [Google Scholar] [CrossRef]
- Kotov, A.A.; Kotova, L.M.; Romanov, G.A. Signaling network regulating plant branching: Recent advances and new challenges. Plant Sci. 2021, 307, 110880. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [Green Version]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An Update on the Signals Controlling Shoot Branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pěnčík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins Are Initial Targets of Light in the Control of Bud Outgrowth. Plant Physiol. 2016, 172, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Kotov, A.A.; Kotova, L.M. Auxin-cytokinin interactions in the regulation of correlative inhibition in two-branched pea seedlings. J. Exp. Bot. 2018, 69, 2967–2978. [Google Scholar] [CrossRef] [Green Version]
- Bertheloot, J.; Barbier, F.; Boudon, F.; Perez-Garcia, M.D.; Péron, T.; Citerne, S.; Dun, E.; Beveridge, C.; Godin, C.; Sakr, S. Sugar availability suppresses the auxin-induced strigolactone pathway to promote bud outgrowth. New Phytol. 2020, 225, 866–879. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.X.; Li, C.D.; Sun, H.C.; Zhang, H.N.; Jia, L. Distribution of 14C-assimilates in boll-leaf system at full- blooming stage of three cotton genotypes with different boll weight. Plant Nutr. Fertil. Sci. 2007, 13, 714–718. [Google Scholar]
- Ren, X.M.; Zhang, L.L.; Du, M.W.; Evers, J.B.; Werf, W.V.; Tian, X.L.; Li, Z.H. Managing mepiquat chloride and plant density for optimal yield and quality of cotton. Field Crops Res. 2013, 149, 1–10. [Google Scholar] [CrossRef]
- Han, H.Y.; Wang, F.Y.; Chen, B.; Zhang, W.F.; Li, B.C.; Tian, X.L.; Li, Z.H. Effects of drip irrigation water amount on the regulation of cotton growth and yield by fortified 1,1-dimethyl-piperidinium chloride in northern Xinjiang. Cotton Sci. 2017, 29, 70–78. [Google Scholar]
- Nie, J.J.; Li, Z.H.; Zhang, Y.J.; Zhang, D.M.; Xu, S.Z.; He, N.; Zhan, Z.H.; Dai, J.L.; Li, C.D.; Li, W.J.; et al. Plant pruning affects photosynthesis and photoassimilate partitioning in relation to the yield formation of field-grown cotton. Ind. Crops Prod. 2021, 173, 114087. [Google Scholar] [CrossRef]
- Feng, G.Y.; Yao, Y.D.; Du, M.W.; Tian, J.S.; Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Dimethyl Piperidinium Chloride (DPC) Regulation of Canopy Architecture and Photosynthesis in a Cotton Hybrid in an Arid Region. Cotton Sci. 2012, 24, 44–51. [Google Scholar]
- Hu, Y.Y.; Zhang, Y.L.; Luo, H.H.; Li, W.; Oguchi, R.; Fan, D.Y.; Chow, W.S.; Zhang, W.F. Important photosynthetic contribution from the non-foliar green organs in cotton at the late growth stage. Planta 2012, 235, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, S.A.; Huang, Y.; Hafeez, A.; Ali, S.; Liu, A.D.; Chattha, M.S.; Ahmad, S.; Yang, G.Z. Morpho-physiological Effects and Molecular Mode of Action of Mepiquat Chloride Application in Cotton: A Review. J. Soil Sci. Plant Nutr. 2020, 20, 2073–2086. [Google Scholar] [CrossRef]
| Varieties | Factors | Plant No. (104·hm−2) | Boll No. per Plant (No.) | Total Boll No. (104·hm−2) | Boll Weight (g) | Seed Cotton Yield (kg·hm−2) |
|---|---|---|---|---|---|---|
| Xinluzao72 | CK1 | 20.18 ± 0.96 a | 6.93 ± 1.22 b | 110.38 ± 4.02 c | 5.14 ± 0.40 b | 5515 ± 131 c |
| CK2 | 21.35 ± 0.72 a | 7.80 ± 1.01 a | 136.40 ± 5.40 b | 5.70 ± 0.56 a | 6632 ± 151 b | |
| L | 20.61 ± 0.92 a | 8.33 ± 0.62 a | 141.84 ± 6.29 ab | 5.56 ± 0.41 ab | 7055 ± 104 a | |
| M | 20.61 ± 1.07 a | 8.47 ± 0.52 a | 144.74 ± 4.23 a | 5.66 ± 0.56 a | 7134 ± 126 a | |
| H | 20.18 ± 1.11 a | 8.40 ± 0.83 a | 140.64 ± 4.92 ab | 5.60 ± 0.44 ab | 6877 ± 253 ab | |
| Xinluzao61 | CK1 | 20.76 ± 1.20 a | 7.00 ± 1.13 b | 114.91 ± 5.08 c | 4.82 ± 0.42 b | 5433 ± 141 b |
| CK2 | 21.20 ± 1.03 a | 7.53 ± 1.19 b | 129.68 ± 6.78 b | 5.31 ± 0.37 a | 6475 ± 270 a | |
| L | 20.03 ± 0.66 a | 8.40 ± 0.83 a | 139.77 ± 5.57 a | 5.08 ± 0.44 ab | 6877 ± 429 a | |
| M | 20.18 ± 0.78 a | 8.47 ± 0.64 a | 140.79 ± 4.95 a | 4.96 ± 0.41 ab | 6745 ± 372 a | |
| H | 20.03 ± 1.03 a | 8.60 ± 0.63 a | 141.96 ± 2.38 a | 5.02 ± 0.29 ab | 7120 ± 128 a | |
| Xinluzao67 | CK1 | 19.74 ± 0.76 a | 7.1 ± 0.57 b | 112.57 ± 3.65 b | 4.79 ± 0.56 b | 5427 ± 241 b |
| CK2 | 19.88 ± 0.67 a | 8.2 ± 1.03 a | 133.48 ± 3.08 ab | 5.25 ± 0.37 a | 6657 ± 56 a | |
| L | 19.59 ± 0.51 a | 8.6 ± 0.84 a | 138.89 ± 3.29 ab | 5.04 ± 0.34 ab | 6484 ± 304 a | |
| M | 19.74 ± 0.88 a | 8.5 ± 0.85 a | 138.3 ± 4.85 ab | 5.02 ± 0.27 ab | 6747 ± 200 a | |
| H | 19.88 ± 0.67 a | 8.8 ± 1.40 a | 142.84 ± 4.65 a | 5.12 ± 0.39 ab | 6743 ± 167 a | |
| Variety (V) | ns | ns | <0.0001 | <0.0001 | ns | |
| Source of variance | Plant topping (PT) | ns | 0.0001 | <0.0001 | 0.0008 | <0.0001 |
| V × PT | ns | ns | ns | ns | ns |
| Varieties | Plant Topping | Boll Weight (g) | Lint Percentage (%) | Boll Spatial Distribution (Number) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upper | Middle | Lower | Upper | Middle | Lower | Upper | Middle | Lower | ||
| Xinluzao72 | CK1 | 4.81 b | 5.27 b | 5.35 a | 39.93 a | 40.55 a | 39.08 a | 1.47 b | 2.8 a | 2.67 a |
| CK2 | 6.09 a | 6.03 ab | 4.99 a | 41.38 a | 41.08 a | 39.55 a | 2.20 a | 3.13 a | 2.47 a | |
| L | 5.53 ab | 5.85 ab | 5.31 a | 41.33 a | 40.95 a | 39.18 a | 2.47 a | 3.07 a | 2.8 a | |
| M | 5.47 ab | 6.24 a | 5.28 a | 41.26 a | 40.91 a | 37.78 a | 2.53 a | 3.07 a | 2.87 a | |
| H | 5.56 ab | 6.00 b | 5.24 a | 40.48 a | 37.44 a | 43.56 a | 2.60 a | 2.87 a | 2.93 a | |
| Xinluzao61 | CK1 | 4.41 b | 5.28 a | 4.77 a | 40.08 a | 40.96 a | 40.92 a | 1.60 b | 2.87 a | 2.53 a |
| CK2 | 5.65 a | 5.39 a | 4.88 a | 41.23 a | 41.41 a | 41.74 a | 2.13 ab | 2.93 a | 2.47 a | |
| L | 4.68 b | 5.43 a | 5.13 a | 40.40 a | 39.79 a | 40.48 a | 2.60 a | 3.00 a | 2.80 a | |
| M | 4.76 b | 5.34 a | 4.79 a | 40.80 a | 41.26 a | 41.03 a | 2.47 a | 3.07 a | 2.93 a | |
| H | 4.78 b | 5.33 a | 4.94 a | 40.51 a | 40.91 a | 40.91 a | 2.53 a | 3.00 a | 3.07 a | |
| Xinluzao67 | CK1 | 4.35 c | 5.38 a | 4.65 a | 44.9 a | 49.7 a | 48.44 a | 1.30 c | 3.00 a | 2.80 a |
| CK2 | 5.53 a | 5.39 a | 4.82 a | 45.04 a | 49.88 a | 48.98 a | 2.30 bc | 3.10 a | 2.80 a | |
| L | 4.74 b | 5.39 a | 4.98 a | 44.6 a | 49.67 a | 48.05 a | 2.70 ab | 3.00 a | 2.90 a | |
| M | 4.81 b | 5.33 a | 4.92 a | 45.36 a | 50.8 a | 48.61 a | 2.60 ab | 3.00 a | 2.90 a | |
| H | 4.73 b | 5.39 a | 5.24 a | 45.9 a | 49.43 a | 48.03 a | 2.90 a | 3.00 a | 2.90 a | |
| Varieties | Plant Topping | Upper Half Mean Length (mm) | Uniformity Index (%) | Fiber Strength (cN/tex) | Micronaire | Elongation (%) |
|---|---|---|---|---|---|---|
| Xinluzao 72 | CK1 | 31.37 ± 0.85 a | 86.59 ± 0.54 a | 31.13 ± 0.83 b | 3.39 ± 0.32 a | 6.62 ± 0.58 a |
| CK2 | 31.39 ± 1.34 a | 86.46 ± 1.21 a | 32.09 ± 0.73 a | 3.57 ± 0.32 a | 6.51 ± 0.79 a | |
| L | 31.69 ± 1.16 a | 86.76 ± 1.08 a | 31.63 ± 0.91 ab | 3.52 ± 0.4 a | 6.54 ± 0.42 a | |
| M | 31.86 ± 0.74 a | 86.78 ± 0.88 a | 31.57 ± 1.14 ab | 3.73 ± 0.53 a | 6.43 ± 0.45 a | |
| H | 32.00 ± 1.05 a | 86.67 ± 1.12 a | 32.23 ± 0.7 a | 3.58 ± 0.47 a | 6.54 ± 0.36 a | |
| Xinluzao 61 | CK1 | 30.58 ± 1.24 a | 85.57 ± 1.56 a | 28.74 ± 1.14 c | 3.36 ± 0.56 b | 6.71 ± 0.58 a |
| CK2 | 30.64 ± 0.79 a | 85.86 ± 1.11 a | 29.69 ± 1.09 bc | 3.81 ± 0.36 a | 6.58 ± 0.63 a | |
| L | 30.93 ± 1.03 a | 85.74 ± 1.19 a | 29.99 ± 1.18 ab | 3.56 ± 0.42 ab | 6.62 ± 0.51 a | |
| M | 30.87 ± 0.66 a | 85.97 ± 1.22 a | 31.11 ± 1.67 a | 3.74 ± 0.4 ab | 6.84 ± 0.52 a | |
| H | 30.82 ± 0.66 a | 86.16 ± 0.64 a | 30.6 ± 0.96 ab | 3.56 ± 0.21 ab | 6.69 ± 0.4 a | |
| Xinluzao 67 | CK1 | 28.71 ± 0.68 a | 84.13 ± 1.05 a | 28.09 ± 1.26 a | 4.92 ± 0.23 a | 6.42 ± 0.97 a |
| CK2 | 28.25 ± 1.24 a | 83.66 ± 1.37 a | 26.94 ± 2.42 a | 4.94 ± 0.28 a | 6.19 ± 0.86 a | |
| L | 28.51 ± 0.87 a | 84.44 ± 1.6 a | 28.07 ± 1.27 a | 4.91 ± 0.3 a | 6.47 ± 0.88 a | |
| M | 28.52 ± 0.63 a | 84.01 ± 0.92 a | 27.78 ± 1.42 a | 5.08 ± 0.19 a | 6.36 ± 0.81 a | |
| H | 28.72 ± 0.9 a | 84.32 ± 1.42 a | 28.12 ± 1.54 a | 4.98 ± 0.28 a | 6.29 ± 1.02 a |
| Days after Topping (d) | 7 | 14 | 21 | 28 | 35 |
|---|---|---|---|---|---|
| CK1 ① | 71.18 ± 4.77 a | 83.53 ± 7.97 a | 87.63 ± 7.98 a | 89.00 ± 7.69 a | 89.33 ± 7.35 a |
| CK2 ① | 70.55 ± 5.12 ab | 71.90 ± 5.49 e | 71.8 ± 5.02 e | 73.25 ± 4.83 e | 73.53 ± 4.79 e |
| L ① | 70.30 ± 4.74 bc | 79.03 ± 5.89 b | 80.37 ± 6.61 b | 81.73 ± 6.15 b | 82.70 ± 6.69 b |
| M ① | 70.80 ± 4.37 bc | 76.92 ± 5.61 c | 78.38 ± 5.90 c | 78.23 ± 5.89 c | 78.40 ± 5.36 c |
| H ① | 70.17 ± 4.80 c | 75.63 ± 4.85 d | 76.45 ± 4.51 d | 76.20 ± 4.59 d | 76.78 ± 4.27 d |
| Varieties (V) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Plant topping (PT) | 0.4643 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| V × PT | 0.0002 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Days after Topping (d) | 7 | 14 | 21 | 28 | 35 |
|---|---|---|---|---|---|
| CK1 ① | 13.60 ± 1.52 a | 15.97 ± 1.27 a | 16.73 ± 1.51 a | 16.73 ± 1.62 a | 17.07 ± 1.76 a |
| CK2 ① | 13.43 ± 1.43 a | 13.33 ± 1.39 d | 13.33 ± 1.40 d | 13.67 ± 1.06 d | 13.60 ± 1.13 d |
| L ① | 13.37 ± 1.69 a | 15.00 ± 1.29 b | 15.40 ± 1.48 b | 15.70 ± 1.47 b | 15.87 ± 1.43 b |
| M ① | 13.30 ± 1.39 a | 14.77 ± 1.17 bc | 15.13 ± 1.33 bc | 15.37 ± 1.43 b | 15.23 ± 1.55 c |
| H ① | 13.57 ± 1.36 a | 14.43 ± 1.07 c | 14.77 ± 1.33 c | 14.93 ± 1.48 c | 15.20 ± 1.61 c |
| Varieties (V) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Plant topping (PT) | 0.7503 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| V × PT | 0.7751 | 0.8636 | 0.4874 | 0.3122 | 0.4989 |
| Varieties | Plant Topping | Whole Internode Length (cm) | Upper Internode Length (cm) | Remaining Internode Length (cm) |
|---|---|---|---|---|
| Xinluzao72 | CK1 | 6.45 ± 0.54 a | 6.58 ± 0.17 b | 6.45 ± 0.89 a |
| CK2 | 6.44 ± 0.58 a | 6.77 ± 0.38 a | 6.30 ± 0.86 a | |
| L | 6.29 ± 0.48 a | 5.82 ± 0.13 c | 6.55 ± 0.98 a | |
| M | 6.16 ± 0.41 a | 5.62 ± 0.16 d | 6.39 ± 0.72 a | |
| H | 6.14 ± 0.50 a | 5.39 ± 0.10 e | 6.43 ± 0.79 a | |
| Xinluzao61 | CK1 | 5.18 ± 0.42 a | 5.42 ± 0.08 b | 5.09 ± 0.56 a |
| CK2 | 5.27 ± 0.34 a | 5.74 ± 0.22 a | 5.20 ± 0.43 a | |
| L | 5.11 ± 0.25 a | 5.10 ± 0.17 c | 5.13 ± 0.35 a | |
| M | 5.02 ± 0.33 a | 4.88 ± 0.17 d | 5.08 ± 0.44 a | |
| H | 5.04 ± 0.27 a | 4.84 ± 0.19 d | 5.17 ± 0.67 a | |
| Xinluzao67 | CK1 | 4.63 ± 0.17 b | 4.99 ± 0.52 a | 4.39 ± 0.24 a |
| CK2 | 4.88 ± 0.26 a | 5.04 ± 0.22 a | 4.56 ± 0.35 a | |
| L | 4.48 ± 0.08 c | 4.15 ± 0.24 b | 4.52 ± 0.26 a | |
| M | 4.46 ± 0.06 c | 4.17 ± 0.21 b | 4.48 ± 0.36 a | |
| H | 4.35 ± 0.10 c | 4.13 ± 0.34 b | 4.54 ± 0.31 a |
| Days after Topping (d) | 7 | 14 | 21 | 28 | 35 |
|---|---|---|---|---|---|
| CK1 ① | 8.77 ± 1.10 a | 12.22 ± 3.62 a | 13.10 ± 3.87 a | 13.60 ± 3.70 a | 13.63 ± 3.67 a |
| CK2 ① | 8.73 ± 1.01 a | 10.37 ± 3.06 d | 10.33 ± 3.24 d | 10.67 ± 3.04 d | 10.60 ± 3.06 d |
| L ① | 8.53 ± 1.28 a | 11.53 ± 5.56 b | 12.33 ± 3.52 b | 12.70 ± 3.43 b | 12.90 ± 3.35 b |
| M ① | 8.53 ± 1.14 a | 10.83 ± 3.65 c | 11.80 ± 3.62 c | 12.23 ± 3.46 c | 12.13 ± 3.54 c |
| H ① | 8.73 ± 1.01 a | 10.90 ± 3.66 c | 11.63 ± 3.42 c | 11.93 ± 3.36 c | 12.13 ± 3.44 c |
| Varieties (V) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Plant topping (PT) | 0.7718 | 0.0008 | 0.0001 | 0.0001 | 0.0001 |
| V × PT | 0.8772 | 0.2493 | 0.2535 | 0.1343 | 0.0423 |
| Varieties | Factors | Fourth Branch (cm) | Third Branch (cm) | Second Branch (cm) | First Branch (cm) |
|---|---|---|---|---|---|
| Xinluzao72 | CK1 | 10.57 ± 1.25 ab | 9.87 ± 1.55 a | 8.77 ± 0.96 b | 8.03 ± 1.22 b |
| CK2 | 11.50 + 1.10 a | 10.57 ± 0.86 a | 10.13 ± 1.11 a | 10.20 ± 1.60 a | |
| L | 9.97 ± 1.59 b | 8.93 ± 1.08 b | 7.83 ± 0.99 c | 4.93 ± 0.92 c | |
| M | 9.7 ± 1.52 b | 7.67 ± 0.88 c | 6.43 ± 1.15 d | 4.10 ± 1.43 cd | |
| H | 9.77 ± 1.22 b | 7.30 ± 1.24 c | 5.33 ± 1.26 e | 3.53 ± 1.01 d | |
| Xinluzao61 | CK1 | 9.43 ± 1.37 a | 8.97 ± 0.90 a | 7.67 ± 1.21 b | 5.43 ± 1.18 b |
| CK2 | 9.47 ± 1.38 a | 9.67 ± 1.37 a | 9.80 ± 1.33 a | 8.87 ± 0.93 a | |
| L | 8.27 ± 1.61 b | 6.53 ± 1.19 b | 5.53 ± 0.83 c | 3.47 ± 1.17 c | |
| M | 8.20 ± 1.53 b | 5.97 ± 1.32 bc | 4.87 ± 1.26 cd | 3.03 ± 1.14 c | |
| H | 7.80 ± 1.49 b | 5.63 ± 1.03 c | 4.13 ± 2.00 d | 2.93 ± 1.02 c | |
| Xinluzao67 | CK1 | 17.40 ± 2.51 a | 16.40 ± 1.41 a | 12.30 ± 1.55 a | 11.40 ± 2.50 a |
| CK2 | 16.50 ± 2.13 a | 15.90 ± 1.68 a | 12.00 ± 2.87 a | 11.00 ± 2.06 a | |
| L | 16.70 ± 1.09 a | 14.05 ± 1.62 b | 9.65 ± 1.81 b | 5.80 ± 1.40 b | |
| M | 16.85 ± 2.80 a | 13.65 ± 1.86 b | 9.35 ± 1.55 b | 5.15 ± 1.42 b | |
| H | 16.30 ± 1.74 a | 13.40 ± 1.88 b | 9.00 ± 1.65 b | 4.75 ± 2.20 b | |
| Source of variance | Varieties (V) | <0.0001 | <0.0001 | <0.0001 | 0.0027 |
| Plant topping (PT) | 0.0266 | <0.0001 | 0.0004 | 0.0001 | |
| V × PT | ns | ns | 0.0243 | 0.0002 |
| Correlation Degree between Cotton Yield and Plant Architecture | Correlation between Cotton Plant Height and Other Plant Architecture Parameters | ||||
|---|---|---|---|---|---|
| Trait | Correlation degree γ | Rank | Trait | Correlation degree γ | Rank |
| Plant height | 0.826 | 1 | Upper internode length | 0.8675 | 1 |
| Whole internode length | 0.8058 | 2 | Whole internode length | 0.8539 | 2 |
| Remaining internode length | 0.7988 | 3 | Remaining internode length | 0.8414 | 3 |
| Number of fruit branches | 0.7961 | 4 | Number of fruit branches | 0.8314 | 4 |
| Number of main stem internodes | 0.7929 | 5 | Number of main stem internodes | 0.8029 | 5 |
| Upper internode length | 0.7894 | 6 | Second branch length | 0.6174 | 6 |
| Fourth branch length | 0.6642 | 7 | Fourth branch length | 0.6144 | 7 |
| Second branch length | 0.66 | 8 | Third branch length | 0.6019 | 8 |
| Third branch length | 0.6567 | 9 | First branch length | 0.5853 | 9 |
| First branch length | 0.5715 | 10 | |||
| Days after Topping (d) | 7 | 14 | 21 | 28 | 35 |
|---|---|---|---|---|---|
| CK1 ① | 3.90 ± 0.76 ab | 5.38 ± 0.66 a | 5.63 ± 0.77 a | 5.50 ± 0.88 a | 4.51 ± 0.68 b |
| CK2 ① | 4.15 ± 1.18 a | 5.30 ± 0.42 a | 5.27 ± 0.44 b | 5.02 ± 0.34 b | 4.43 ± 0.66 b |
| L ① | 4.00 ± 0.69 a | 4.88 ± 0.81 b | 5.39 ± 1.08 b | 5.00 ± 1.17 b | 4.77 ± 0.65 a |
| M ① | 3.70 ± 0.43 b | 4.42 ± 0.84 c | 4.90 ± 0.91 c | 5.13 ± 1.22 b | 4.90 ± 0.82 a |
| H ① | 3.25 ± 0.58 c | 4.20 ± 0.97 d | 4.75 ± 0.95 c | 4.95 ± 1.35 b | 4.49 ± 1.09 b |
| Varieties (V) | 0.0219 | 0.0001 | 0.0001 | 0.0031 | 0.0621 |
| Plant topping (PT) | 0.4492 | 0.0031 | 0.0045 | 0.8182 | 0.8882 |
| V × PT | 0.0001 | 0.0001 | 0.3202 | 0.0001 | 0.0001 |
| Days after Topping (d) | 10 | 20 | 27 | 34 | 41 |
|---|---|---|---|---|---|
| CK1 ① | 25.47 ± 2.60 c | 23.21 ± 2.95 c | 21.21 ± 2.18 c | 13.17 ± 1.03 d | 9.01 ± 1.08 d |
| CK2 ① | 25.71 ± 3.15 c | 25.88 ± 3.09 b | 21.45 ± 2.27 c | 19.63 ± 1.95 c | 18.38 ± 2.23 a |
| L ① | 25.35 ± 3.25 c | 23.55 ± 4.24 c | 22.51 ± 3.18 b | 22.19 ± 1.89 b | 12.74 ± 3.43 c |
| M ① | 29.140 ± 2.84 a | 27.87 ± 1.36 a | 23.64 ± 2.23 a | 22.73 ± 2.29 ab | 15.21 ± 3.46 b |
| H ① | 27.65 ± 3.16 b | 27.48 ± 1.78 a | 24.37 ± 2.70 a | 23.43 ± 2.45 a | 15.07 ± 3.88 b |
| Varieties (V) | 0.0001 | 0.0008 | 0.0066 | 0.2273 | 0.2265 |
| Plant topping (PT) | 0.0028 | 0.0091 | 0.1764 | 0.0010 | 0.0546 |
| V × PT | 0.0407 | 0.0057 | 0.0001 | 0.0001 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Tang, J.; Tian, J.; Du, M.; Gou, L.; Zhang, Y.; Zhang, W. Different Concentrations of Chemical Topping Agents Affect Cotton Yield and Quality by Regulating Plant Architecture. Agronomy 2023, 13, 1741. https://doi.org/10.3390/agronomy13071741
Wu Y, Tang J, Tian J, Du M, Gou L, Zhang Y, Zhang W. Different Concentrations of Chemical Topping Agents Affect Cotton Yield and Quality by Regulating Plant Architecture. Agronomy. 2023; 13(7):1741. https://doi.org/10.3390/agronomy13071741
Chicago/Turabian StyleWu, Yanqin, Jiyuan Tang, Jingshan Tian, Mingwei Du, Ling Gou, Yali Zhang, and Wangfeng Zhang. 2023. "Different Concentrations of Chemical Topping Agents Affect Cotton Yield and Quality by Regulating Plant Architecture" Agronomy 13, no. 7: 1741. https://doi.org/10.3390/agronomy13071741





