Yield, Fructans Accumulation, and Nutritional Quality of Young Chicory Plants as Related to Genotype and Nitrogen Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Non-Structural Carbohydrates (NCS) Analysis
2.3. Fructans Analysis
2.4. Inorganic Anions and Organic Acids Analysis
2.5. Statistical Analysis
3. Results
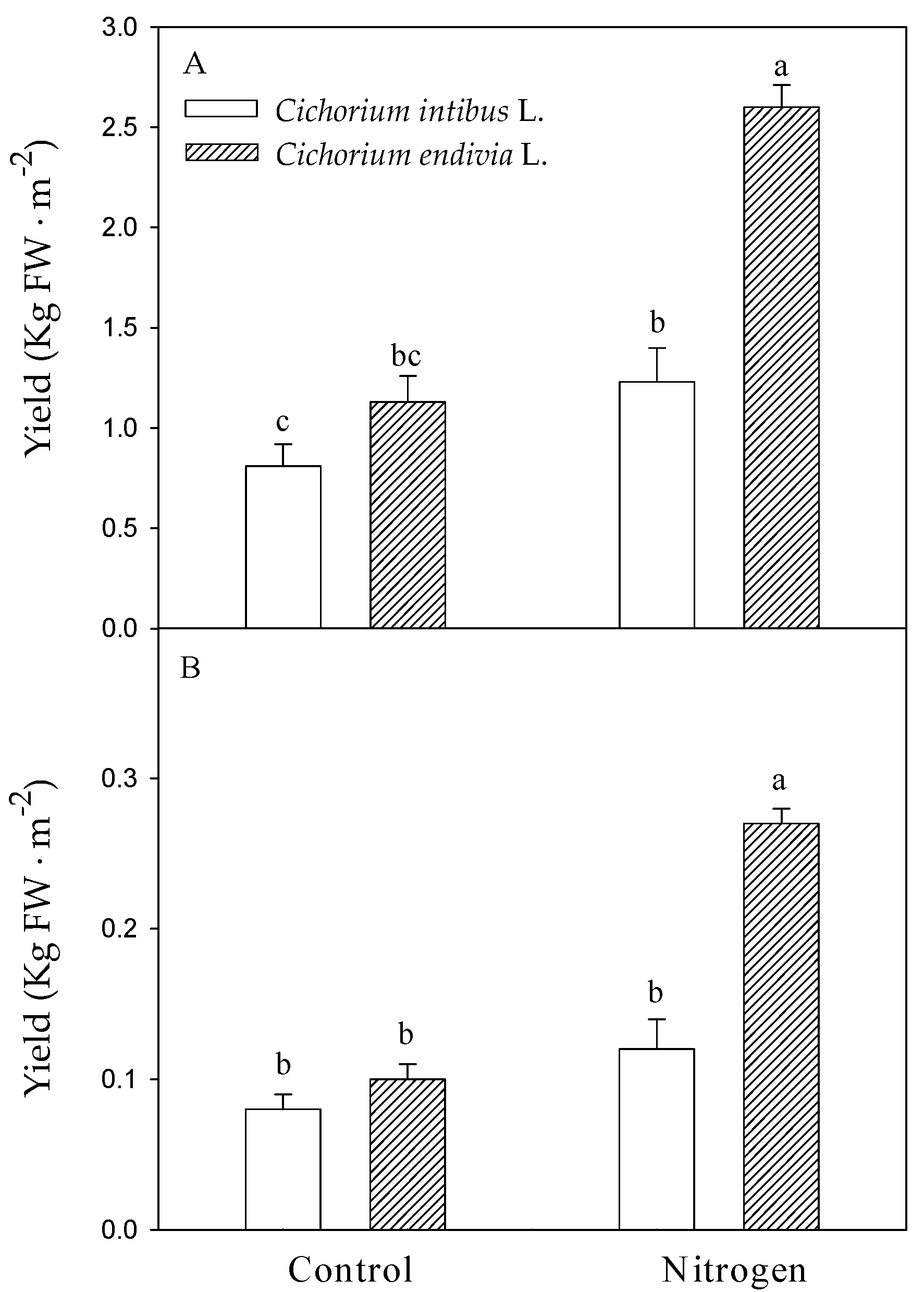
3.1. The Growth and Productivity of Chicory Plants
3.2. Non-Structural Carbohydrate and Fructans Content
3.3. Nitrogen, Protein, Anions, and Organic Acids Content
4. Discussion
4.1. The Effect of Fertilization on the Early Growth Phase and Quality of Chicory
4.2. Carbon Partitioning, NSC, and Fructans Content in the Leaves and Roots of Chicory
4.3. Nitrogen, Protein, Anions, and Organic Acids Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucchin, M.; Varotto, S.; Barcaccia, G.; Parrini, P. Chicory and Endive. In Vegetables I. Handbook of Plant Breeding; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 1, pp. 3–48. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Papa, G.; Serio, F. Nitrate and ammonium nutrition in chicory and rocket salad plants. J. Plant Nutr. 1998, 21, 1779–1789. [Google Scholar] [CrossRef]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant Metabolites and Nutritional Quality of Vegetables. J. Food Sci. 2008, 73, R48–R65. [Google Scholar] [CrossRef] [PubMed]
- Van Arkel, J.; Vergauwen, R.; Sévenier, R.; Hakkert, J.C.; van Laere, A.; Bouwmeester, H.; Koops, A.J.; van der Meer, I.M. Sink filling, inulin metabolizing enzymes and carbohydrate status in field grown chicory (Cichorium intybus L.). J. Plant Physiol. 2012, 169, 1520–1529. [Google Scholar] [CrossRef]
- Testone, G.; Sobolev, A.P.; Mele, G.; Nicolodi, C.; Gonnella, M.; Arnesi, G.; Biancari, T.; Giannino, D. Leaf nutrient content and transcriptomic analyses of endive (Cichorium endivia) stressed by downpour-induced waterlog reveal a gene network regulating kestose and inulin contents. Hortic. Res. 2021, 8, 8–92. [Google Scholar] [CrossRef]
- Malik, B.; Rehman, R.U. Chicory Inulin: A Versatile Biopolymer with Nutritional and Therapeutic Properties. Med. Aromat. Plants 2021, 16, 373–390. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 32, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Van den Ende, W. Plant fructans in stress environments: Emerging concepts and future prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin extraction from common inulin-containing plant sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Ernst, M.; Chatterton, N.; Harrison, P.A. Carbohydrate changes in chicory (Cichorium intybus L. var. foliosum) during growth and storage. Sci. Hortic. 1995, 63, 251–261. [Google Scholar] [CrossRef]
- Man, S.; Liu, T.; Yao, Y.; Lu, Y.; Ma, L.; Lu, F. Friend or foe? The roles of inulin-type fructans. Carbohydr. Polym. 2021, 252, 117155. [Google Scholar] [CrossRef]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal Tolerance of Chicory Inulin Products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Seghatoleslami, M.J.; Mousavi, G.; Javadi, H. Chicory (Cichorium intybus) responses to nitrogen and plant density in Birjand, Iran. Int. J. Biosci. (IJB) 2014, 4, 56–61. [Google Scholar] [CrossRef]
- Shoorideh, H.; Peighambari, S.A.; Omidi, M.; Naghavi, M.R.; Maroufi, A. Assessing potential of Iranian Chicory geno-types for industrial application. J. Hortic. Sci. Technol. 2016, 3, 59–68. [Google Scholar]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and Nitrite in Health and Disease. Aging. Dis. 2018, 9, 938–945. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 7th ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015, 77, 343–356. [Google Scholar] [CrossRef]
- Paglialunga, G.; Proietti, S.; Cardarelli, M.; Moscatello, S.; Colla, G.; Battistelli, A. Chicory Taproot Production: Effects of Biostimulants under Partial or Full Controlled Environmental Conditions. Agronomy 2022, 12, 2816. [Google Scholar] [CrossRef]
- Scartazza, A.; Moscatello, S.; Gavrichkova, O.; Buia, M.C.; Lauteri, M.; Battistelli, A.; Lorenti, M.; Garrard, S.L.; Calfapietra, C.; Brugnoli, E. Carbon and nitrogen allocation strategy in Posidonia oceanica is altered by seawater acidification. Sci. Total. Environ. 2017, 607, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous Lighting Promotes Plant Growth, Light Conversion Efficiency, and Nutritional Quality of Eruca vesicaria (L.) Cav. in Controlled Environment With Minor Effects Due to Light Quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef]
- Verspreet, J.; Pollet, A.; Cuyvers, S.; Vergauwen, R.; Van den Ende, W.; Delcour, J.A.; Courtin, C.M. A Simple and Accurate Method for Determining Wheat Grain Fructan Content and Average Degree of Polymerization. J. Agric. Food Chem. 2012, 60, 2102–2107. [Google Scholar] [CrossRef]
- Cassan, L.; Corbineau, F.; Limami, A.M. Genetic variability of nitrogen accumulation during vegetative development and remobilization during the forcing process in witloof chicory tuberized root (Cichorium intybus L.). J. Plant Physiol. 2008, 165, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; Ciampitti, I. Crop Mass and N Status as Prerequisite Covariables for Unraveling Nitrogen Use Efficiency across Genotype-by-Environment-by-Management Scenarios: A Review. Plants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2020, 158, 76–82. [Google Scholar] [CrossRef]
- Ameziane, R.; Cassan, L.; Dufossé, C.; Rufty, T.W., Jr.; Limami, A.M. Phosphate availability in combination with nitrate availability affects root yield and chicon yield and quality of Belgian endive (Cichorium intybus). Plant Soil 1997, 191, 269–277. [Google Scholar] [CrossRef]
- Neuweiler, R.; Krauss, J.; Konrad, P.; Imhof, T. Optimized N-fertilization in the production of chicory. Agrarforschung 2007, 14, 530–535, ISSN 1022-663X. [Google Scholar]
- Adamczewska-Sowińska, K.; Uklańska, C.M. Effect of Nitrogen Fertilization on Yield and Quality of Endive. J. Fruit Ornam. Plant Res. 2009, 70, 193–201. [Google Scholar] [CrossRef]
- Biesiada, A.; Kołota, E. The effect of nitrogen fertilization on yielding and chemical composition of radicchio chicory for au-tumn–harvest cultivation. Acta Sci. Pol. Hortorum. Cultus. 2010, 9, 85–91. [Google Scholar]
- Custic, M.; Poljak, M.; Ćosić, T. Nitrate content in leafy vegetables as related to nitrogen fertilization in Croatia. Acta Hortic. 1994, 371, 407–412. [Google Scholar] [CrossRef]
- Gromaz, A.; Torres, J.F.; Bautista, A.S.; Pascual, B.; López-Galarza, S.; Maroto, J.V. Effect of different levels of nitrogen in nutrient solution and crop system on nitrate accumulation in endive. J. Plant Nutr. 2017, 40, 2045–5053. [Google Scholar] [CrossRef]
- Corriveau, J.; Gaudreau, L.; Caron, J.; Jenni, S.; Gosselin, A. Testing irrigation, day/night foliar spraying, foliar calcium and growth inhibitor as possible cultural practices to reduce tipburn in lettuce. Can. J. Plant Sci. 2012, 92, 889–899. [Google Scholar] [CrossRef]
- Pressman, E.; Shaked, R.; Arcan, L. The Effect of Flower-Inducing Factors on Leaf Tipburn Formation in Chinese Cabbage. J. Plant Physiol. 1993, 141, 210–214. [Google Scholar] [CrossRef]
- Samarakoon, U.; Palmer, J.; Ling, P.; Altland, J. Effects of Electrical Conductivity, pH, and Foliar Application of Calcium Chloride on Yield and Tipburn of Lactuca sativa Grown Using the Nutrient–Film Technique. Hortscience 2020, 55, 1265–1271. [Google Scholar] [CrossRef]
- Olle, M.; Bender, I. Causes and control of calcium deficiency disorders in vegetables: A review. J. Hortic. Sci. Biotechnol. 2009, 84, 577–584. [Google Scholar] [CrossRef]
- Bizzarri, M.; Delledonne, M.; Ferrarini, A.; Tononi, P.; Zago, E.; Vittori, D.; Damiani, F.; Paolocci, F. Whole-Transcriptome Analysis Unveils the Synchronized Activities of Genes for Fructans in Developing Tubers of the Jerusalem Artichoke. Front. Plant Sci. 2020, 11, 101. [Google Scholar] [CrossRef]
- Puhlmann, M.-L.; de Vos, W.M. Back to the Roots: Revisiting the Use of the Fiber-Rich Cichorium intybus L. Taproots. Adv. Nutr. Int. Rev. J. 2020, 11, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Teferra, T.F. Possible actions of inulin as prebiotic polysaccharide: A review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA J. 2015, 13, 3951. [Google Scholar]
- Ćustić, M.; Horvatić, M.; Butorac, A. Effects of nitrogen fertilization upon the content of essential amino acids in head chicory (Cichorium intybus L. var. foliosum). Sci. Hortic. 2002, 92, 205–215. [Google Scholar] [CrossRef]
- Habermeyer, M.; Roth, A.; Guth, S.; Diel, P.; Engel, K.-H.; Epe, B.; Fürst, P.; Heinz, V.; Humpf, H.-U.; Joost, H.-G.; et al. Nitrate and nitrite in the diet: How to assess their benefit and risk for human health. Mol. Nutr. Food Res. 2014, 59, 106–128. [Google Scholar] [CrossRef]
- EFSA. Nitrate in Vegetables—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 689, 1e79. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Staccini, B.; Ranucci, D.; Miraglia, D.; Altissimi, M.S.; Mercuri, M.L.; Haouet, N.M. Contribution of vegetables and cured meat to dietary nitrate and nitrite intake in Italian population: Safe level for cured meat and controversial role of vegetables. Ital. J. Food Saf. 2018, 7, 7692. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Burns, I.G.; Zhang, K.; Turner, M.K.; Meacham, M.; Al-Redhiman, K.; Lynn, J.; Broadley, M.R.; Hand, P.; Pink, D. Screening for genotype and environment effects on nitrate accumulation in 24 species of young lettuce. J. Sci. Food Agric. 2011, 91, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, S.; Jia, L.; Chen, W.; Shen, Q. The Mechanism of Nitrate Accumulation in Pakchoi [Brassica campestris L. ssp. Chinensis (L.)]. Plant Soil 2006, 282, 291–300. [Google Scholar] [CrossRef]
- Razgallah, N.; Rouhou, H.C.; Abid, G.; M’hamdi, M. Identification of Differentially Expressed Putative Nitrate Transporter Genes in Lettuce. Int. J. Veg. Sci. 2017, 23, 390–399. [Google Scholar] [CrossRef]
- Chen, B.-M.; Wang, Z.-H.; Li, S.-X.; Wang, G.-X.; Song, H.-X.; Wang, X.-N. Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Sci. 2004, 167, 635–643. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A survey of nitrate and oxalate content in fresh vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. EFSA J. 2017, 15, e04787. [Google Scholar] [CrossRef]
- Smith, F.W.; Jackson, W.A. Nitrogen Enhancement of Phosphate Transport in Roots of Zea mays L. I. Effects of Ammonium and Nitrate Pretreatment. Plant Physiol. 1987, 84, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Di Mola, I.; Cozzolino, E.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Nutrient and Nutraceutical Quality of Rocket as a Function of Greenhouse Cover Film, Nitrogen Dose and Biostimulant Application. Agronomy 2023, 13, 638. [Google Scholar] [CrossRef]
- Deane-Drummond, C.E. The regulation of sulphate uptake following growth of Pisum sativum L. seedlings in S nutrient limiting conditions. Interaction between nitrate and sulphate transport. Plant Sci. 1987, 50, 27–35. [Google Scholar] [CrossRef]
- Haynes, R.J.; Goh, K.M. Ammonium and nitrate nutrition of plants. Biol. Rev. 1978, 53, 465–510. [Google Scholar] [CrossRef]
| Leaf FW (g/Plant) | Leaf DW (g/Plant) | Leaf DM% | SLDW (mg cm−2) | Tap Root FW (g/Plant) | Tap Root DW (g/Plant) | Tap Root DM% | Shoot/Root | |
|---|---|---|---|---|---|---|---|---|
| Species | ||||||||
| C. intybus | 13.19 ± 1.91 | 1.33 ± 0.20 | 10.04 ± 0.21 | 2.31 ± 0.08 | 1.02 ± 0.13 | 0.19 ± 0.03 | 18.02 ± 0.98 | 8.36 ± 1.53 |
| C. endivia | 22.22 ± 4.22 | 2.25 ± 0.46 | 9.71 ± 0.37 | 2.35 ± 0.22 | 1.61 ± 0.30 | 0.32 ± 0.07 | 18.36 ± 1.06 | 7.29 ± 0.54 |
| TR | ||||||||
| C | 9.21± 0.53 | 0.87 ± 0.05 | 9.52 ± 0.33 | 2.09 ± 0.10 | 0.93 ± 0.08 | 0.17 ± 0.02 | 17.73 ± 1.00 | 5.68 ± 0.57 |
| N | 26.19 ± 3.12 | 2.70 ± 0.34 | 10.23 ± 0.22 | 2.57 ± 0.17 | 1.70 ± 0.30 | 0.34 ± 0.07 | 18.65 ± 1.01 | 9.96 ± 1.17 |
| Species × TR | ||||||||
| C. intybus-C | 8.63 ± 0.57 c | 0.87 ± 0.04 c | 10.10 ± 0.31 a | 2.35 ± 0.06 b | 1.04 ± 0.13 b | 0.21 ± 0.03 b | 19.75 ± 1.11 a | 4.48 ± 0.55 c |
| C. intybus-N | 17.74 ± 2.40 b | 1.79 ± 0.28 b | 9.98 ± 0.33 ab | 2.27 ± 0.15 bc | 1.01 ± 0.25 b | 0.17 ± 0.05 b | 16.29 ± 1.25 b | 12.23 ± 1.66 a |
| C. endivia-C | 9.79 ± 0.88 c | 0.88 ± 0.10 c | 8.94 ± 0.48 b | 1.83 ± 0.08 c | 0.83 ± 0.08 b | 0.13 ± 0.02 b | 15.72 ± 1.13 b | 6.88 ± 0.65 bc |
| C. endivia-N | 34.65 ± 1.47 a | 3.62 ± 0.11 a | 10.48 ± 0.29 a | 2.87 ± 0.26 a | 2.39 ± 0.31 a | 0.50 ± 0.07 a | 21.01 ± 0.50 a | 7.70 ± 0.90 b |
| Chicory Leaves | |||
|---|---|---|---|
| Total Soluble | Total NSC | Starch | |
| Species | |||
| C. intybus | 9.13 ± 1.05 b | 10.10 ± 1.08 b | 0.97 ± 0.13 b |
| C. endivia | 11.51 ± 0.75 a | 13.89 ± 0.64 a | 2.39 ± 0.19 a |
| TR | |||
| C | 8.51 ± 0.52 b | 10.54 ± 0.73b | 2.03 ± 0.29 a |
| N | 12.13 ± 0.99 a | 13.46 ± 1.17a | 1.33 ± 0.23 b |
| Species × TR | |||
| C. intybus-C | 7.71 ± 0.95 | 8.96 ± 1.03 | 1.25 ± 0.12 |
| C. intybus-N | 10.55 ± 1.75 | 11.24 ± 1.88 | 0.69 ± 0.15 |
| C. endivia-C | 9.31 ± 0.09 | 12.11 ± 0.30 | 2.80 ± 0.23 |
| C. endivia-N | 13.70 ± 0.31 | 15.68 ± 0.41 | 1.97 ± 0.14 |
| Chicory Roots | ||||
|---|---|---|---|---|
| Total Soluble | Total NSC | Fructans | Fructans (g m−2) | |
| Species | ||||
| C. intybus | 4.57 ± 0.48 b | 43.52 ± 2.39 b | 38.94 ± 2.30 | 4.02 ± 0.44 |
| C. endivia | 5.94± 0.25 a | 50.16 ± 2.94 a | 44.20 ± 2.92 | 7.77 ± 2.04 |
| TR | ||||
| C | 4.66± 0.47 b | 43.16 ± 1.69 b | 38.49 ± 1.80 | 3.26 ± 0.40 |
| N | 5.86± 0.31 a | 50.52 ± 3.30 a | 44.65 ± 3.16 | 8.53 ± 1.86 |
| Species × TR | ||||
| C. intybus-C | 3.52 ± 0.50 | 43.58 ± 2.57 | 40.05 ± 2.37 b | 4.03 ± 0.41 b |
| C. intybus-N | 5.62 ± 0.50 | 43.46 ± 4.36 | 37.83 ± 4.18 b | 4.01 ± 0.84 b |
| C. endivia-C | 5.79 ± 0.32 | 42.74 ± 2.49 | 36.92 ± 2.77 b | 2.49 ± 0.48 c |
| C. endivia-N | 6.09 ± 0.40 | 57.58 ± 2.26 | 51.47 ± 2.08 a | 13.06 ± 2.17 a |
| Chicory Leaves | Chicory Roots | |||
|---|---|---|---|---|
| N (% DM) | Protein (% DM) | N (% DM) | Protein (% DM) | |
| Species | ||||
| C. intybus | 3.04 ± 0.35 | 11.15 ± 0.91 | 1.18 ± 0.20 | 5.64 ± 0.85 |
| C. endivia | 2.68 ± 0.32 | 9.95 ± 0.91 | 1.02 ± 0.16 | 5.62 ± 0.74 |
| TR | ||||
| C | 2.01 ± 0.12 b | 9.66 ± 0.87 | 0.78 ± 0.12 b | 4.68 ± 0.75 |
| N | 3.71 ± 0.13 a | 11.44 ± 0.88 | 1.42 ± 0.16 a | 6.57 ± 0.67 |
| Species × TR | ||||
| C. intybus-C | 2.16 ± 0.13 | 10.54 ± 1.10 | 0.76 ± 0.23 | 4.52 ± 0.31 |
| C. intybus-N | 3.92 ± 0.21 | 11.77 ± 1.60 | 1.60 ± 0.16 | 6.75 ± 0.70 |
| C. endivia-C | 1.87 ± 0.20 | 8.70 ± 1.40 | 0.73 ± 0.12 | 4.41 ± 0.70 |
| C. endivia-N | 3.49 ± 0.09 | 11.03 ± 1.00 | 1.51 ± 0.05 | 7.63 ± 0.30 |
| Chicory Leaves | Chicory Roots | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrate (ppm) | Sulphate | Phosphate | Malic Acid | Citric Acid | Nitrate (ppm) | Sulphate | Phosphate | Malic Acid | Citric Acid | |
| Species | ||||||||||
| C. intybus | 5332 ± 1121 | 0.28 ± 0.08 | 0.31 ± 0.06 | 1.86 ± 0.25 a | 2.73 ± 0.39 | 1805 ± 525 a | 0.13 ± 0.12 | 0.23 ± 0.12 | 1.40 ± 0.12 a | 3.10 ± 0.12 a |
| C. endivia | 4542 ± 996 | 0.21 ± 0.06 | 0.27 ± 0.05 | 0.85 ± 0.12 b | 2.23 ± 0.16 | 1471 ± 441 b | 0.18 ± 0.12 | 0.18 ± 0.12 | 0.66 ± 0.12 b | 0.56 ± 0.12 b |
| TR | ||||||||||
| C | 2564 ± 641 b | 0.38 ± 0.11 a | 0.42 ± 0.04 a | 1.39 ± 0.31 | 2.59 ± 0.40 | 215.3 ± 21.7 b | 0.24 ± 0.12 a | 0.31 ± 0.12 a | 0.86 ± 0.12 b | 1.80 ± 0.12 |
| N | 7310 ± 788 a | 0.11 ± 0.03 b | 0.17 ± 0.03b | 1.32 ± 0.19 | 2.37 ± 0.17 | 3062 ± 162.7 a | 0.07 ± 0.12 b | 0.10 ± 0.12 b | 1.20 ± 0.12 a | 1.86 ± 0.12 |
| Species × TR | ||||||||||
| C. intybus-C | 3343 ± 1240 | 0.41 ± 0.12 | 0.42 ± 0.08 | 2.02 ± 0.46 | 3.13 ± 0.73 | 266 ± 23.0 | 0.21 ± 0.02 | 0.34 ± 0.03 | 1.11 ± 0.10 | 2.97 ± 0.18 |
| C. intybus-N | 7321 ± 1461 | 0.15 ± 0.05 | 0.21 ± 0.06 | 1.70 ± 0.23 | 2.34 ± 0.27 | 3346 ± 2367 | 0.05 ± 0.01 | 0.12 ± 0.03 | 1.69 ± 0.13 | 3.23 ± 0.43 |
| C. endivia-C | 1785 ± 590 | 0.35 ± 0.09 | 0.42 ± 0.04 | 0.76 ± 0.17 | 2.06 ± 0.22 | 165 ± 17.9 | 0.26 ± 0.04 | 0.28 ± 0.05 | 0.61 ± 0.22 | 0.63 ± 0.10 |
| C. endivia-N | 7299 ± 812 | 0.07 ± 0.01 | 0.13 ± 0.01 | 0.94 ± 0.19 | 2.40 ± 0.23 | 2778 ± 151 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.72 ± 0.04 | 0.49 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscatello, S.; Battistelli, A.; Mattioni, M.; Proietti, S. Yield, Fructans Accumulation, and Nutritional Quality of Young Chicory Plants as Related to Genotype and Nitrogen Fertilization. Agronomy 2023, 13, 1752. https://doi.org/10.3390/agronomy13071752
Moscatello S, Battistelli A, Mattioni M, Proietti S. Yield, Fructans Accumulation, and Nutritional Quality of Young Chicory Plants as Related to Genotype and Nitrogen Fertilization. Agronomy. 2023; 13(7):1752. https://doi.org/10.3390/agronomy13071752
Chicago/Turabian StyleMoscatello, Stefano, Alberto Battistelli, Michele Mattioni, and Simona Proietti. 2023. "Yield, Fructans Accumulation, and Nutritional Quality of Young Chicory Plants as Related to Genotype and Nitrogen Fertilization" Agronomy 13, no. 7: 1752. https://doi.org/10.3390/agronomy13071752
APA StyleMoscatello, S., Battistelli, A., Mattioni, M., & Proietti, S. (2023). Yield, Fructans Accumulation, and Nutritional Quality of Young Chicory Plants as Related to Genotype and Nitrogen Fertilization. Agronomy, 13(7), 1752. https://doi.org/10.3390/agronomy13071752






