Enhancing Soil Remediation of Copper-Contaminated Soil through Washing with a Soluble Humic Substance and Chemical Reductant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Studied Soil
2.2. Preparation of Soluble Humic Substance
2.3. Soil Washing Experiments
2.3.1. Evaluation of Cu Removal Efficiency using Different Agents in Batch Experiments
2.3.2. Two-Step Soil Washing by Chemical Reductant and HS Solution
2.4. Chemical Faction of Cu in the Soil after Soil Washing
2.5. Plant Availability and Bioaccessibility of Cu and Soil Enzymatic Analyses
2.6. Data Analysis
3. Results and Discussion
3.1. Cu Removal Efficiency by Soil Washing
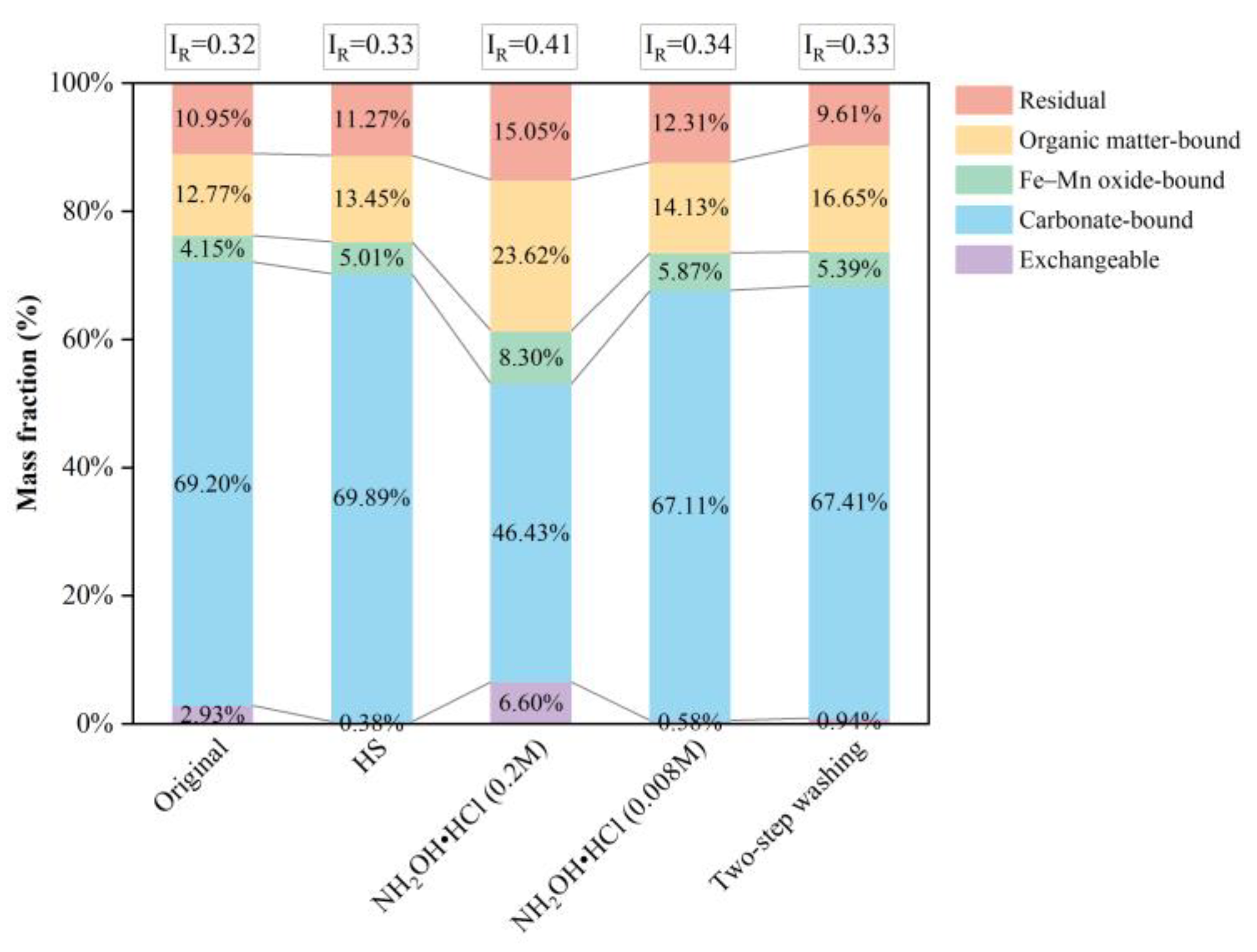
3.2. Cu Distribution and Stability in the Soil after Washing
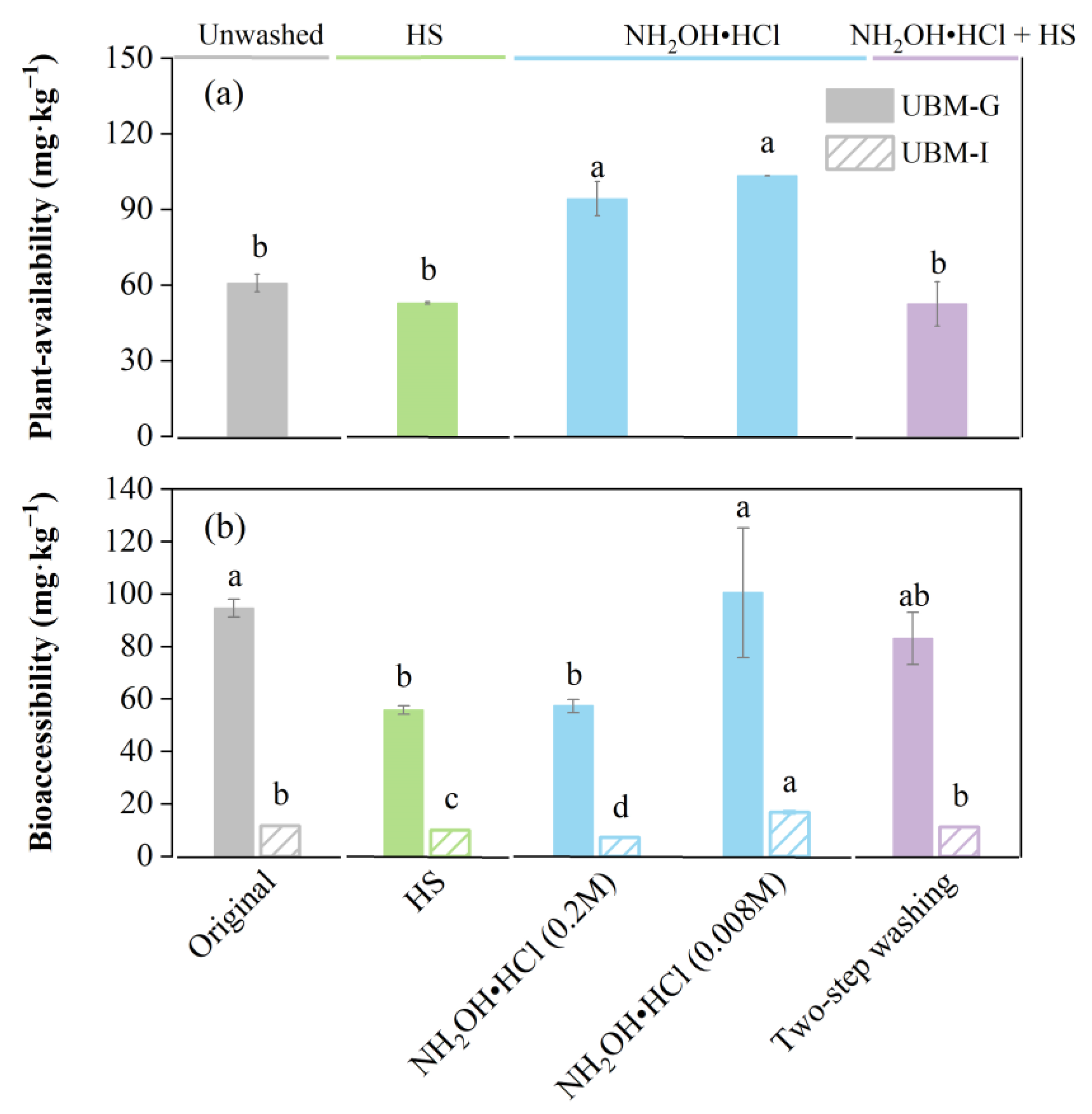
3.3. Changes in Cu Plant Availability and Bioaccessibility after Washing
3.4. Effects of Soil Washing on Soil Enzyme Activities
3.5. Environmental Significance of Using Soluble Humic Substance as a Soil-Reducing Agent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez, R.; Tapia, Y.; Antilén, M.; Casanova, M.; Vidal, C.; Silambarasan, S.; Cornejo, P. Rhizosphere Management for Phytoremediation of Copper Mine Tailings. J. Soil Sci. Plant Nutr. 2021, 21, 3091–3109. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Xu, H.; Cui, X.; Li, J.; Chen, J.; Zheng, B. Characterizations of Heavy Metal Contamination, Microbial Community, and Resistance Genes in a Tailing of the Largest Copper Mine in China. Environ. Pollut. 2021, 280, 116947. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ali, A.; Wang, P.; Li, R.; Tian, X.; Zhang, Z. Comparison of the Feasibility of Different Washing Solutions for Combined Soil Washing and Phytoremediation for the Detoxification of Cadmium (Cd) and Zinc (Zn) in Contaminated Soil. Chemosphere 2019, 230, 510–518. [Google Scholar] [CrossRef]
- Chen, X.; Yao, C.; Wang, A.; Zhang, Z.; Chen, L.; Zhang, J.; Liu, X.; Li, H. Risks of Applying Mobilising Agents for Remediation of Arsenic-Contaminated Soils: Effects of Dithionite–EDTA and Citric Acid on Arsenic Fractionation, Leachability, Oral Bioavailability/Bioaccessibility and Speciation. J. Hazard. Mater. 2023, 444, 130416. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Review on Remediation Technologies for Arsenic-Contaminated Soil. Front. Environ. Sci. Eng. 2020, 14, 24. [Google Scholar] [CrossRef]
- Wei, J.; Deng, S.; Lu, J. A Single Soil Washing with Humic Substance Can Achieve the Risk-Based Remedial Target for Nickel Contaminated Soil. Bull. Environ. Contam. Toxicol. 2022, 109, 623–629. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and Artificial Humic Substances to Manage Minerals, Ions, Water, and Soil Microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic Acids: Structural Properties and Multiple Functionalities for Novel Technological Developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Klik, B. Feasibility of Using Humic Substances from Compost to Remove Heavy Metals (Cd, Cu, Ni, Pb, Zn) from Contaminated Soil Aged for Different Periods of Time. J. Hazard. Mater. 2015, 300, 882–891. [Google Scholar] [CrossRef]
- Yang, T.; Hodson, M.E. Investigating the Use of Synthetic Humic-like Acid as a Soil Washing Treatment for Metal Contaminated Soil. Sci. Total Environ. 2019, 647, 290–300. [Google Scholar] [CrossRef]
- Hartley, N.R.; Tsang, D.C.W.; Olds, W.E.; Weber, P.A. Soil Washing Enhanced by Humic Substances and Biodegradable Chelating Agents. Soil Sediment Contam. Int. J. 2014, 23, 599–613. [Google Scholar] [CrossRef]
- Piccolo, A.; De Martino, A.; Scognamiglio, F.; Ricci, R.; Spaccini, R. Efficient Simultaneous Removal of Heavy Metals and Polychlorobiphenyls from a Polluted Industrial Site by Washing the Soil with Natural Humic Surfactants. Environ. Sci. Pollut. Res. 2021, 28, 25748–25757. [Google Scholar] [CrossRef]
- Damian, G.E.; Micle, V.; Sur, I.M. Mobilization of Cu and Pb from Multi-Metal Contaminated Soils by Dissolved Humic Substances Extracted from Leonardite and Factors Affecting the Process. J. Soils Sediments 2019, 19, 2869–2881. [Google Scholar] [CrossRef]
- Rui, D.; Wu, Z.; Ji, M.; Liu, J.; Wang, S.; Ito, Y. Remediation of Cd- and Pb- Contaminated Clay Soils through Combined Freeze-Thaw and Soil Washing. J. Hazard. Mater. 2019, 369, 87–95. [Google Scholar] [CrossRef]
- Begum, Z.A.; Rahman, I.M.M.; Tate, Y.; Sawai, H.; Maki, T.; Hasegawa, H. Remediation of Toxic Metal Contaminated Soil by Washing with Biodegradable Aminopolycarboxylate Chelants. Chemosphere 2012, 87, 1161–1170. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Xia, F.; Yang, L.; Chen, Q.; Chen, Y.; Deng, S.; Yuan, G.; Wang, H.; Jeyakumar, P. Enhanced Removal of Arsenic and Cadmium from Contaminated Soils Using a Soluble Humic Substance Coupled with Chemical Reductant. Environ. Res. 2023, 220, 115120. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods for Soil and Agricultural Chemistry; Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and Bacteria Inoculated Biochar Enhanced Cd and Cu Immobilization and Enzymatic Activity in a Polluted Soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Klik, B.; Gusiatin, Z.M.; Kulikowska, D. A Holistic Approach to Remediation of Soil Contaminated with Cu, Pb and Zn with Sewage Sludge-Derived Washing Agents and Synthetic Chelator. J. Clean. Prod. 2021, 311, 127664. [Google Scholar] [CrossRef]
- Klik, B.K.; Gusiatin, Z.M.; Kulikowska, D. Suitability of Environmental Indices in Assessment of Soil Remediation with Conventional and next Generation Washing Agents. Sci. Rep. 2020, 10, 20586. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.; Basta, N.; Brandon, E.; Casteel, S.; Denys, S.; Gron, C.; Oomen, A.; Reimer, K.; Tack, K. An Inter-Laboratory Trial of the Unified BARGE Bioaccessibility Method for Arsenic, Cadmium and Lead in Soil. Sci. Total Environ. 2011, S0048969711005213. [Google Scholar] [CrossRef]
- Jorge-Mardomingo, I.; Soler-Rovira, P.; Casermeiro, M.Á.; de la Cruz, M.T.; Polo, A. Seasonal Changes in Microbial Activity in a Semiarid Soil after Application of a High Dose of Different Organic Amendments. Geoderma 2013, 206, 40–48. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of Carbon and Microbial Activity in Salt-Induced Soil by Application of Peanut Shell Biochar during Short-Term Incubation Study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Makhinova, A.F.; Makhinov, A.N. Role of Humus Substances in Chemical Soil Pollution during Deposit Exploitation in Priokhotye and Priamurye. Environ. Res. 2020, 188, 109766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, Y.; Peng, H.; Zhang, L.; Zhou, Y.; Zhang, J.; Du, C.; Liu, J.; Lin, X.; Wang, N. Silicon Fertilizers, Humic Acid and Their Impact on Physicochemical Properties, Availability and Distribution of Heavy Metals in Soil and Soil Aggregates. Sci. Total Environ. 2022, 822, 153483. [Google Scholar] [CrossRef] [PubMed]
- Beiyuan, J.; Lau, A.Y.T.; Tsang, D.C.W.; Zhang, W.; Kao, C.; Baek, K.; Ok, Y.S.; Li, X. Chelant-Enhanced Washing of CCA-Contaminated Soil: Coupled with Selective Dissolution or Soil Stabilization. Sci. Total Environ. 2018, 612, 1463–1472. [Google Scholar] [CrossRef]
- Chien, S.W.C.; Wang, H.; Chen, Y.; Wang, M.; Liu, C. Removal of Heavy Metals from Contaminated Paddy Soils Using Chemical Reductants Coupled with Dissolved Organic Carbon Solutions. J. Hazard. Mater. 2021, 403, 123549. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Front. Earth Sci. 2022, 10, 901570. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil Washing for Metal Removal: A Review of Physical/Chemical Technologies and Field Applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of Soil Washing on Heavy Metal Removal and Soil Quality: A Two-Sided Coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef]
- Roussel, H.; Waterlot, C.; Pelfrêne, A.; Pruvot, C.; Mazzuca, M.; Douay, F. Cd, Pb and Zn Oral Bioaccessibility of Urban Soils Contaminated in the Past by Atmospheric Emissions from Two Lead and Zinc Smelters. Arch. Environ. Contam. Toxicol. 2010, 58, 945–954. [Google Scholar] [CrossRef]
- Bi, D.; Yuan, G.; Wei, J.; Xiao, L.; Feng, L.; Meng, F.; Wang, J. A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils. Int. J. Environ. Res. Public. Health 2019, 16, 4999. [Google Scholar] [CrossRef]
- Qu, C.; Chen, W.; Hu, X.; Cai, P.; Chen, C.; Yu, X.; Huang, Q. Heavy Metal Behaviour at Mineral-Organo Interfaces: Mechanisms, Modelling and Influence Factors. Environ. Int. 2019, 131, 104995. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Zhao, Y.; Gueret Yadiberet Menzembere, E.R. Remediation of Vanadium-Contaminated Soils by the Combination of Natural Clay Mineral and Humic Acid. J. Clean. Prod. 2021, 279, 123874. [Google Scholar] [CrossRef]
- Huang, W.; Lin, T.; Huang, C.; Chen, T.; Yeh, Y. Copper Distribution and Binding Affinity of Size-Fractioned Humic Substances Taken from Paddy Soil and Correlation with Optical Characteristics. Agronomy 2022, 12, 1689. [Google Scholar] [CrossRef]
- Feng, L.; Gao, Z.; Ma, H.; He, S.; Liu, Y.; Jiang, J.; Zhao, Q.; Wei, L. Carbonate-Bound Pb Percentage Distribution in Agricultural Soil and Its Toxicity: Impact on Plant Growth, Nutrient Cycling, Soil Enzymes, and Functional Genes. J. Hazard. Mater. 2023, 451, 131205. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Egli, T. Environmental Fate and Microbial Degradation of Aminopolycarboxylic Acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef]
- Zhou, L.X.; Wong, J.W.C. Effect of Dissolved Organic Matter from Sludge and Sludge Compost on Soil Copper Sorption. J. Environ. Qual. 2001, 30, 878–883. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Olds, W.E.; Weber, P.A.; Yip, A.C.K. Soil Stabilisation Using AMD Sludge, Compost and Lignite: TCLP Leachability and Continuous Acid Leaching. Chemosphere 2013, 93, 2839–2847. [Google Scholar] [CrossRef]
- Beiyuan, J.; Tsang, D.C.W.; Ok, Y.S.; Zhang, W.; Yang, X.; Baek, K.; Li, X. Integrating EDDS-Enhanced Washing with Low-Cost Stabilization of Metal-Contaminated Soil from an e-Waste Recycling Site. Chemosphere 2016, 159, 426–432. [Google Scholar] [CrossRef]
- Subirés-Muñoz, J.D.; García-Rubio, A.; Vereda-Alonso, C.; Gómez-Lahoz, C.; Rodríguez-Maroto, J.M.; García-Herruzo, F.; Paz-García, J.M. Feasibility Study of the Use of Different Extractant Agents in the Remediation of a Mercury Contaminated Soil from Almaden. Sep. Purif. Technol. 2011, 79, 151–156. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Xu, X.; Zhong, Q.; Zhang, C.; Jia, Y.; Li, T.; Deng, O.; Li, Y. Heavy Metal Removal by GLDA Washing: Optimization, Redistribution, Recycling, and Changes in Soil Fertility. Sci. Total Environ. 2016, 569–570, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D.; Klik, B.K.; Gusiatin, Z.M.; Jabłoński, R. Sewage Sludge Can Provide a Washing Agent for Remediation of Soil from a Metallurgical Area. CATENA 2019, 173, 22–28. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Jensen, J.K.; Soleimani, M.; Strobel, B.W. Cleaning Heavy Metal Contaminated Soil with Soluble Humic Substances Instead of Synthetic Polycarboxylic Acids. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2011, 61, 577–581. [Google Scholar] [CrossRef]
- Yuan, G.; Bi, D.; Wei, J.; Xiao, L. Calcined Oyster Shell-Humic Complex as Soil Amendment to Remediate Cd- and As-Contaminated Soil. Agronomy 2022, 12, 1413. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Weber, J.; Naidu, R.; Gancarz, D.; Rofe, A.; Todor, D.; Smith, E. Determination of Cadmium Relative Bioavailability in Contaminated Soils and Its Prediction Using in vitro Methodologies. Environ. Sci. Technol. 2010, 44, 5240–5247. [Google Scholar] [CrossRef]
- Jelusic, M.; Lestan, D. Effect of EDTA Washing of Metal Polluted Garden Soils. Part I: Toxicity Hazards and Impact on Soil Properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil Washing with Biodegradable Chelating Agents and EDTA: Effect on Soil Properties and Plant Growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef]
- Basak, B.; Biswas, D.; Pal, S. Soil Biochemical Properties and Quality as Affected by Organic Manures and Mineral Fertilizers in Soil under Maize-Wheat Rotation. Agrochimica 2013, 57, 49–66. [Google Scholar]
- Klik, B.K.; Kulikowska, D.; Gusiatin, Z.M. Flushing of Soils Highly Contaminated with Cd Using Various Washing Agents Derived from Sewage Sludge. Energies 2022, 15, 349. [Google Scholar] [CrossRef]
- Yuan, B.; Yue, D. Soil Microbial and Enzymatic Activities Across a Chronosequence of Chinese Pine Plantation Development on the Loess Plateau of China. Pedosphere 2012, 22, 1–12. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Jensen, J.K.; Borggaard, O.K. A Laboratory Test of NOM-Assisted Remediation of Arsenic and Copper Contaminated Soils. J. Environ. Chem. Eng. 2015, 3, 3020–3023. [Google Scholar] [CrossRef]


| Properties | pH | SOM | CEC | Total Cu |
|---|---|---|---|---|
| Unit | - | g·kg−1 | cmol(+)·kg−1 | mg·kg−1 |
| Value | 7.36 | 6.66 | 10.52 | 1205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wei, J.; Yang, L.; Chen, Y.; Wang, M.; Xiao, L.; Yuan, G. Enhancing Soil Remediation of Copper-Contaminated Soil through Washing with a Soluble Humic Substance and Chemical Reductant. Agronomy 2023, 13, 1754. https://doi.org/10.3390/agronomy13071754
Wang L, Wei J, Yang L, Chen Y, Wang M, Xiao L, Yuan G. Enhancing Soil Remediation of Copper-Contaminated Soil through Washing with a Soluble Humic Substance and Chemical Reductant. Agronomy. 2023; 13(7):1754. https://doi.org/10.3390/agronomy13071754
Chicago/Turabian StyleWang, Lina, Jing Wei, Lu Yang, Yun Chen, Mengjie Wang, Liang Xiao, and Guodong Yuan. 2023. "Enhancing Soil Remediation of Copper-Contaminated Soil through Washing with a Soluble Humic Substance and Chemical Reductant" Agronomy 13, no. 7: 1754. https://doi.org/10.3390/agronomy13071754





