Abstract
Humic acids are plant biostimulants, which can be used in horticulture as an effective and relatively inexpensive alternative to chemical means of production. The aim of the study was to assess how the fertiliser containing humic acids affected the growth, yield, and quality of strawberry fruits. In 2021, an experiment was conducted on two-year-old strawberry bushes (Fragaria× ananassa (Duchesne ex Weston) Duchesne ex Rozier) of the ‘Rumba’ cultivar growing on a horticultural farm in northern Wielkopolska, Poland. During the growing season, the soil was fertilized by the mineral fertilization and the bushes were sprayed two, three, or four times with the Humi Brown Gold fertiliser containing humic acids. In the experiment, the soil enzymatic activity, biometric parameters of strawberry leaves, fruit yield and fruit quality was assessed. It was determined that, in the experimental treatments where foliar fertilisation had been applied, the activities of proteases and dehydrogenases as well as soil respiration increased by more than double and were significantly higher than in the variants where soil fertilisers had been applied. The strawberry bushes treated with the humic acids fertiliser developed more than 60% larger surface leaves, bloomed more intensively and gave a higher yield. Fruits with significantly higher firmness were harvested from such bushes, with higher weight and extract content than those where the soil fertiliser had been applied. The difference was several tens of percent. This leads to the conclusion that the foliar application of humic acids could be an effective alternative to mineral fertilisation in strawberry plantations.
1. Introduction
Due to the health-promoting properties of fruits, especially berries, they are considered important components of a healthy human diet. The strawberry is one of the most popular soft fruit species. Its fruits contain L-ascorbic acid, polyphenolic compounds, and other easily absorbable organic compounds [1]. It also has very high antioxidative properties [2]. According to the FAO STAT data, the world’s largest strawberry producers are China (2.96 mln t) and the United States (1.29 mln t). Poland is the largest strawberry producer in Europe. In this country, the share of strawberries in the production of berries is about 35%. Strawberry plantations in Poland occupy an area of about 48,000 hectares and yield about 195,000 tonnes of fruit every year (2021).
Thanks to high yields of good-quality fruit, the production of berries, including strawberries, is profitable. Conventional production methods involving mineral fertilisation and crop-protection products are usually used. Appropriate mineral fertilisation significantly affects the vegetative growth of plants and the content of macro- and micronutrients in the substrate. However, there are also side effects of this method. Intensive, long-term mineral fertilisation, especially with nitrogen fertilisers, may increase soil acidity, disorder the balance of nutrients, lower the quality of crops [3,4], decrease the content of humic acids in the soil [5] or reduce the species biodiversity of beneficial soil microorganisms, which are of key importance to the maintenance of soil fertility.
Biostimulants can be an alternative to chemical means of production in horticulture. They are considered one of the most promising solutions improving the profitability of production, especially in organic farming [6]. Thanks to them, it is possible to reduce the difference between conventional and organic farms in the yield and quality of fruit [7]. Biostimulants may include various substances and/or microorganisms, such as humic and fulvic acids, seaweed extracts [8], mycorrhizal and Trichoderma spp. fungi [9], and rhizobacteria promoting plant growth [10]. Biostimulants may facilitate the uptake of soil nutrients, increase the yield, improve fruit quality, and increase plants’ resistance to biotic and abiotic stress factors [11,12,13]. The use of biostimulants can reduce the inputs of production and thus increase its profitability [14]. Humic acids, which are basic components of the soil humus, are also used as biostimulants. They can be delivered directly into the soil or by spraying the leaves. Humic acids may promote the proliferation of beneficial soil microorganisms [15], increase the content of soil nutrients [16,17], which can be taken up by the plant root system more easily [18,19], and increase plants’ resistance to stress factors [20,21], pests, and fungal diseases [22].
Although there have been a relatively large number of publications on the use of biostimulants in plantations, scientific information on their optimal use can still be considered incomplete. It is assumed that biostimulants can be a good source of nutrients, including nitrogen, for strawberry plants [23]. According to the assumptions of the authors of the experiment, the high yield of strawberries can be achieved through multiple foliar applications of the nutrients contained in biostimulants, without intensive mineral fertilisation. The aim of the study was to assess how the foliar application of a fertiliser containing humic acids affected the physicochemical and biological parameters of the soil as well as the growth, yield, and quality of strawberry fruits.
2. Materials and Methods
2.1. Experiment Design
The experiment was conducted in 2021 on two-year-old strawberry bushes (Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier) of the ‘Rumba’ cultivar, growing on a horticultural and nursery farm in northern Wielkopolska, Poland (52°52′48.222″ N 16°52′57.814″ E). The bushes spaced at 90 × 25 cm were planted on lessive soil (valuation class IVa). The analysis of the physical and chemical properties of soil is shown in Table 1.

Table 1.
Physicochemical and biological properties of the soil used in the experiment.
There were five treatments in the experiment. Their list and characteristics are included in Table 2. Each of the treatments occupied a total area of 240 m2. Four plots of 20 m2 within each variant were selected for close observation.

Table 2.
List of treatments used in the experiment.
The Humi Brown Gold (Generiks Sp. z o.o., Piotrków Trybunalski, Poland) preparation used in the experiment was a suspension. This organic fertiliser contained humic substances—humic and fulvic acids, obtained from leonardite—with the addition of brown coal. The Humi Brown Gold preparation was composed of nitrogen (N), 0.2% m/m, potassium (K2O), at least 0.5% m/m, humic acids, at least 65% m/m, fulvic acids, at least 15% m/m, and organic substance, 50% d.m. The fertiliser was sprayed on the leaves, using a professional tractor-mounted sprayer, on the following dates (depending on the variant): late April, mid-May, late May, and mid-June. The weather conditions on the days of the spray treatment were as follows: air temperature, 8.9–20.6 °C, humidity, 65.7–71.6%, and wind speed not higher than 1.4 m/s.
During the growing season, weeds were controlled mechanically. The plants were protected against fungal diseases, as recommended for commercial plantations. The characteristics of climatic conditions were based on the records of a weather station located about 2 km away from the experimental plots. The weather conditions during the experiment and in the previous year were shown in a climate diagram (Appendix A).
During the growing season, between mid-May and mid-July, the soil moisture in the plots was measured seven times with digital tensiometers. There was no water deficit in the soil throughout the research period. Four of the measurements showed the optimal soil moisture, i.e., 100–340 hPa (Appendix B). In early June (2 June 2021), and especially in mid-June (16 June 2021), there was too much water in the soil (31–52 hPa).
2.2. Measurements and Observations
2.2.1. Soil Analyses
In 2021, in early autumn, the content of soil macronutrients—nitrogen (N-NO3), phosphorus (P), potassium (K), magnesium (Mg), calcium (Ca), chlorine (Cl) and micronutrients: zinc (Zn), cuprum (Cu), manganese (Mn) and ferum (Fe)—was analysed. The volumetric weight of the soil, its acidity, salinity, enzyme activity and respiration were also examined. One representative mixed sample of not less than 0.5 kg was collected from each variant of the experiment. The universal method was used to analyse the content of mineral components in the soil. The following methods were applied to measure the content of soil components: N—microdistillation, P—colorimetry, K and Ca—photometry, Mg—atomic absorption spectrometry, Cl—nephelometry with AnNO3. The Lindsay solution was used to extract micronutrients from the soil and their content was measured with the AAS method. Soil acidity was measured with the potentiometric method.
The dehydrogenase activity was analysed with the colorimetric method developed by Őhlinger [24], with a 1% solution of TCC (triphenyl tetrazolium chloride) used as a substrate. Soil samples were incubated for 24 h at 30 °C. The activity of dehydrogenases was measured with a spectrophotometer. The results were expressed as cm3 H2 24 h−1 kg−1 d.m. The protease activity was analysed with the spectrophotometric method developed by Ladd and Butler [25]. A 1% sodium caseinate solution was used as the substrate. The samples were incubated for one hour at 50 °C. The protease activity was measured with a spectrophotometer at a wavelength of 578 nm. The results were expressed as mg tyrosine h−1 kg−1 d.m. The amount of CO2 emitted by the soil in the field was used to measure its respiration activity with the absorption method developed by Gołębiowska and Pędziwilk [26]. The results were expressed as mg kg−1 24 h−1.
2.2.2. Strawberry Leaf Analyses and Measurements
The strawberry leaves were analysed for their weight, surface area, content of inorganic macronutrients P, K, Ca (% d.m.) and micronutrients Zn, Cu, Mn, Fe (mg kg d.m.), and dehydration. After the fruit had been harvested, 200 leaves were collected from each variant of the experiment. The leaves were dried at 65 °C and mineralised in sulphuric acid in a mineraliser. The following methods were used to measure the content of the elements in the leaves: N—the Kjeldahl method, P—the vanadium-molybdenum method, K, Ca, Mg—atomic absorption spectrometry with a Zeiss-Jena AAS-5 apparatus (Carl Zeiss Jena GmbH, Germany). Fifty leaves were randomly selected from each variant to measure their weight (g) and area (cm2). The leaves were scanned and the DigiShape program was used to measure the area of their blades. The RWC (relative water content) and WSD (relative water deficit) indicators were used to precisely assess the leaf hydration. Ten leaves were randomly selected from each variant and 10 samples were cut out from them with a cork borer. The samples were weighed and placed in a weighing vessel. Then, they were submerged in 25 mL of distilled water and left for 24 h in a dark cool place. After 24 h, the samples were weighed again at full turgor, and then dried in the weighing vessels at 65 °C for 48 h. The weights of the samples were used to calculate the RWC and WSD coefficients according to the following formulae:
where:
W1—leaf fresh weight at full turgor minus leaf dry weight
W2—current leaf weight minus leaf dry weight
There were four replicates of all measurements in each variant.
2.2.3. Flowering, Yield, and Quality of Strawberry Fruit
The number of flowers on 30 randomly selected plants in each variant was used to assess the flowering intensity of strawberries. The number of set fruits on these plants enabled the calculation of the setting percentage. The number of fruits harvested from individual variants was converted into tonne ha to assess the yield of strawberries. Strawberries were harvested three times: in early, mid-, and late June. Due to heavy rainfall in the second half of June, fruit rotting increased and further harvesting was impossible. The quality of the fruit harvested at the three dates was evaluated on the basis of their weight (g), firmness (g mm−2), content of soluble compounds (TSS–Total Soluble Solids) (%), and juice acidity expressed as citric acid. The colour of the fruits and the degree of infestation with grey mould (Botrytis cinerea) were also assessed.
The average fruit weight was assessed in 100 strawberries randomly harvested from each variant, which were weighed with an accuracy of 0.01 g. The fruit firmness was measured with a Fruit Pressure Tester mod. 302 (Facchini FT 02, Facchini Srl, Alfonsine, Italy). The content of soluble compounds in the juice was measured with an ATAGO PR-101a digital refractometer (ATAGO Co. Ltd., Fukaya-shi, Japan). The fruit colour was evaluated with a Minolta colorimeter in the lab colour space, based on the trichromatic model of colour perception. The obtained numerical data were the absolute value and the difference based on the measurement of the difference in hue, saturation, and lightness. These values were used to calculate the colour chromaticity (Chroma = [(a ×)2 + (b ×)2]1/2). The fruit acidity, expressed as citric acid, was determined with a pH meter. A volume of 45 mL of distilled water was added to 5 mL of juice and titrated with 0.1 n NaOH until pH 8.1 was reached. The amount of sodium hydroxide used enabled the calculation of the acid content, expressed as a percentage. The percentage of the strawberry fruit’s infestation with grey mould was calculated on the basis of external symptoms of infestation. The assessment was made during the fruit harvest and on the sixth day of cold storage of the fruit at 6 °C. After the storage, the fruit firmness and extract content were also analysed.
2.3. Statistical Analysis
The results of the experiment were analysed statistically with one-way analysis of variance in the STATISTICA 12.1 program. The differences between the means were assessed with the Duncan test at a significance level of α = 0.05.
3. Results and Discussion
3.1. Soil Measurements and Analyses
The application of the fertiliser with humic acids significantly changed some physicochemical properties of the soil. In the HBG × 4 variant, the soil pH increased to 7.0, as compared with pH = 6.4 in the control variant (Table 3). Akinci et al. [27], Katkat et al. [28] and Zydlik and Zydlik [17] also observed that the application of humic acids reduced soil acidity. Soil salinity decreased from 0.30 (soil fertilisation) to 0.26 in the HBG × 3 variant and to 0.18 g NaCl dm−3 in the HBG × 4 variant. This application had little effect on the content of soil macronutrients. In comparison with the soil fertilisation variant, only the content of N-NO3 (HBG × 2) in the soil increased significantly (Table 3).

Table 3.
The content of macroelements in the soil (mg dm−3) (control—soil fertilization; water—spraying with clean water; HBG—two-, three- and four-fold spraying of Humi Brown Gold).
The content of soil macronutrients did not change after the plants had been sprayed three or four times. In comparison with soil fertilisation, the application of humic acids did not cause a change in the content of two soil micronutrients, i.e., Zn and Cu. The content of Mn and Fe decreased by about 20% (Table 4).

Table 4.
The content of microelements in the soil (mg dm−3) (control—soil fertilization; water—spraying with clean water; HBG—two-, three- and four-fold spraying of Humi Brown Gold).
The microbiological properties of soil, including the species diversity of microorganisms, are a measure of its fertility. Soil microorganisms significantly influence soil productivity through the mineralisation of organic matter. Soil respiration is a measure of the activity of microorganisms [29]. As their activity increases, so does soil respiration. In our experiment, soil respiration increased significantly. In comparison with the variants with soil fertilisation, the soil respiratory activity in variants HBG × 3 and HBG × 4 increased from 20.54 to 29.04 and 26.21 mg kg−1 24 h−1, respectively (Table 5). Thus, these observations confirmed the findings of earlier studies, which indicated the positive effect of humic acids on soil respiration [17,30].

Table 5.
The enzymatic and respiratory activity of the soil (control—soil fertilization; water—spraying with clean water; HBG—two-, three- and four-fold spraying of Humi Brown Gold).
Another measure of soil bioactivity is its enzyme activity [31,32]. The higher the soil enzyme activity is, the higher the rate of mineralisation of organic compounds is, and thus the amount of macro- and micronutrients available to plants. Dehydrogenases, belonging to the group of oxidoreductases and hydrolases, as well as proteases, are some of the most important soil enzymes. In our experiment, the application of humic acids significantly increased the activity of soil enzymes. In comparison to the variants with soil fertilisation, the content of both dehydrogenases and proteases in the HBG × 2 variant doubled from 0.24 to 0.49 cm3 H2 24 h−1 kg−1 d.m. and from 1.49 to 2.93 mg tyrosine h−1 kg−1 d.m., respectively (Table 5). The greater the number of treatments was, the higher the activity of both soil enzymes was. The quadruple application of Humi Brown Gold was the most effective. In comparison with the variant where double spraying was applied, the activity of soil dehydrogenases in the HBG × 4 variant increased by about 27%, whereas the activity of proteases increased by about 35% (Table 5). Schoebitz et al. [33] observed that the application of humic acids in a blueberry plantation increased the activity of soil enzymes.
3.2. Strawberry Leaf Analyses and Measurements
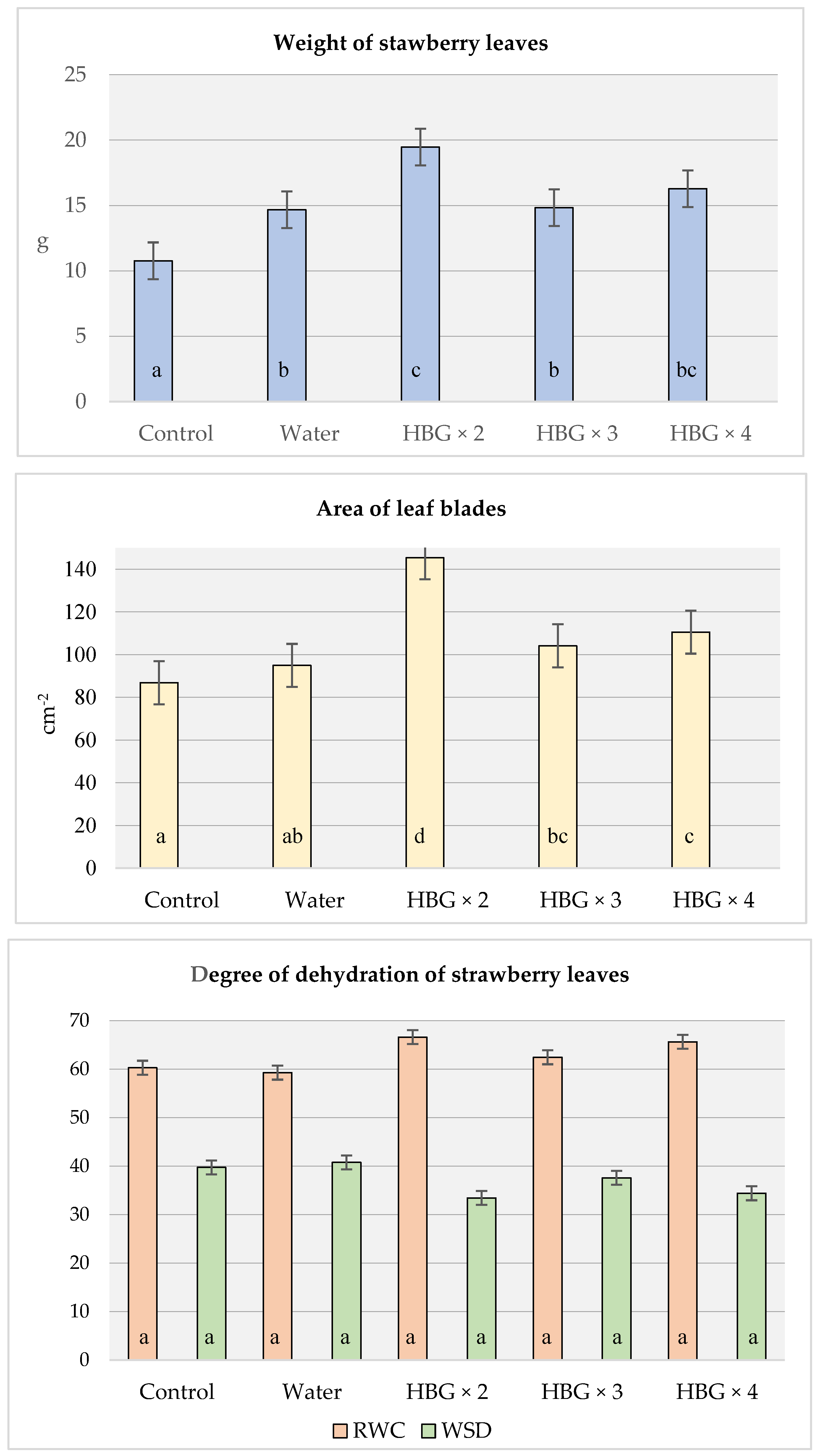
Depending on the experimental variant, the area of strawberry leaves ranged from 86.84 cm2 (soil fertilisation) to 145 cm2 (HBG × 2) (Figure 1). The treatment of strawberry plants with the preparation containing humic acids increased the area of leaf blades. In the HBG × 2 variant, it was over 60% greater than in the variant with soil fertilisation (145.34 vs. 86.84 cm2, respectively). However, when the number of treatments increased, there was no further increase in the leaf area than in the HBG × 2 variant. Rzepka-Plerens et al. [34] and Derkowska et al. [35] also observed an increase in the strawberry leaf area under the influence of biostimulants, including humic acids.
Figure 1.
Effect of humic acids fertilization on the strawberry leaves parameters (control—soil fertilization; water—spraying with clean water; HBG—two-, three and four-fold spraying of Humi Brown Gold. RWC—Relative Water Content; WSD—Water Saturation Deficit. Means marked with the same letters do not differ significantly at α = 0.05.
The analysis of the average leaf weight showed that the application of the preparation containing humic acids positively influenced the growth of strawberries. The weight of the leaves of the plants which had been treated with the fertiliser twice was almost two times greater than the weight of those treated with the soil fertiliser (19.46 vs. 10.77 g, respectively) (Figure 1). However, the average weight of strawberry leaves did not increase with the number of treatments. There were similar results of the experiment conducted by Soltaniband et al. [36], who found that the treatment of strawberries with several types of biostimulant (mycorrhizal and Trichoderma fungi, seaweed extracts, bacteria) significantly increased their biomass.
The experiment did not reveal differences in the degree of dehydration of strawberry leaves, measured with the RWC (relative water content) and WSD (relative water deficit) indicators. The variants where multiple foliar treatments with the fertiliser had been applied did not differ significantly in either of the two indicators from the variants which had received soil fertilisation (Figure 1). This may have resulted from the fact that there was sufficient soil moisture for normal growth of the plants, as evidenced by the measurements made with tensiometers (Appendix B).
The chemical composition of strawberry leaves and fruits depends on the cultivar, climatic conditions, and the degree of fruit maturity. In our experiment, the content of macronutrients in the strawberry leaves was as follows (% d.m.): N—2.11–2.18%; P—0.24–0.28%; K—1.65–1.70%; Ca—1.79–2.27%; Mg—0.31–0.35% (Table 6).

Table 6.
The content of macronutrients (in % d.m.) in the strawberry leaves (control—soil fertilization; water—spraying with clean water; HBG—two-, three- and four-fold spraying of Humi Brown Gold).
According to Wójcik [37], who published the limit content of components in strawberry leaves, the N content in our experiment was low and the P and K content was optimal, whereas the Mg content was high. These values were lower (P, K) or higher (Mg, Ca) than the content of components in strawberry leaves according to Sas-Paszt et al. [38]. In our experiment, the content of micronutrients in the strawberry leaves was as follows (% d.m.): Zn—9.77–12.0%, Cu—3.17–3.56%, Mn—47.0–70.3%, Fe—137–175%, B—35.28–36.72% (Table 7). According to the limit content of micronutrients in strawberry leaves published by Wójcik [37], the Zn and Cu content in our experiment was low, whereas the Mn, Fe, and B content was optimal.

Table 7.
The content of micronutrients (in % d.m.) in the strawberry leaves (control—soil fertilization; water—spraying with clean water; HBG—two, threeand fourfold spraying of Humi Brown Gold).
The treatment of strawberry plants with the fertiliser containing humic acids did not have significant effect on the content of macro- and micronutrients in their leaves. Regardless of the number of treatments, the strawberry leaves which had received the fertiliser containing humic acids did not differ significantly in the content of each of the five macronutrients under analysis from the control variant with the soil fertiliser (Table 6). Our research finding is similar to the observations made by the authors of other studies, who found that the biostimulants they had applied did not have effect on the content of N, P, Ca, or Mg in strawberry leaves [19,36,39]. The fertilisation of strawberries with humic acids also did not significantly influence the content of micronutrients in their leaves. The content of three out of five micronutrients (Zn, Cu, B) in the strawberry leaves fertilised with the preparation containing humic acids did not differ significantly from the content of these elements in the control variant (Table 7).
3.3. Flowering and Yield of Strawberry Fruit
The application of the fertiliser with humic acids (HBG × 3 and HBG × 4) positively influenced the flowering intensity of strawberries and the setting of fruit. For example, the number of flowers per plant in the HBG × 3 variant was significantly greater (by about 50%) than in the variant with soil fertilisation (Table 8). Also, the average number of fruits on the plants in the HBG × 3 variant (21 pcs.) was more than two times greater than in the control variant (10 pcs.). This effect was not observed in the HBG × 2 variant. Nevertheless, the percentage of strawberry fruits set in this variant (about 70%) was much higher than in the control variant (50%) (Table 8). In the HBG × 4 variant, the percentage of fruits set amounted to 79%.

Table 8.
The flowering intensity of strawberries and the setting of fruit (control—soil fertilization; water—spraying with clean water; HBG—two, three and fourfold spraying of Humi Brown Gold).
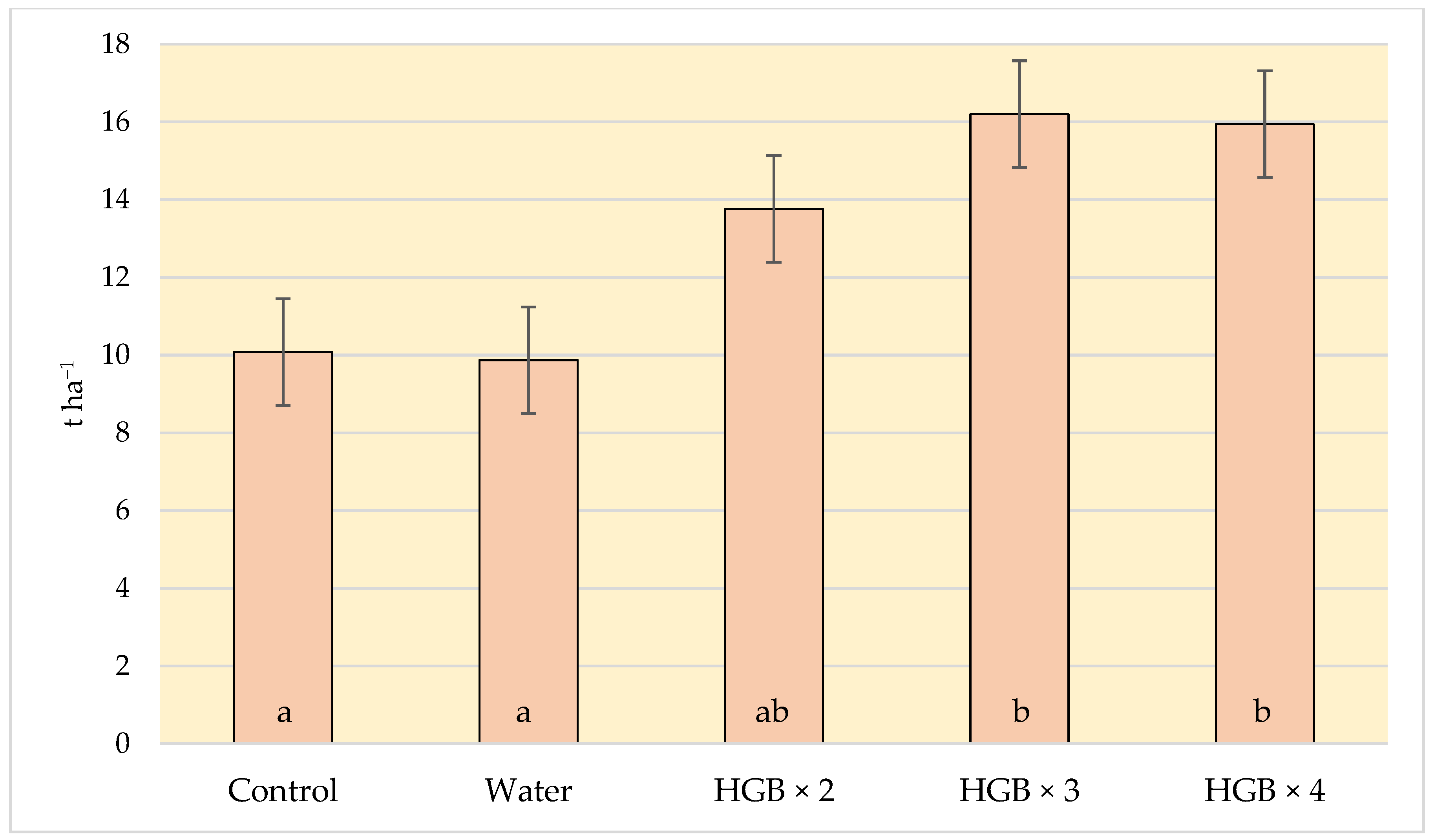
The profitability of cultivation depends on the yield of crops. The application of the fertiliser with humic acids had a positive effect on the yield of strawberries. The yield from the plants in variants HBG × 3 and HBG × 4 was about 60% higher than from the plants where the soil fertiliser had been applied (Figure 2). The yield of strawberries in the HBG × 2 variant (13.76 t ha−1) was slightly higher than in the variant with soil fertilization (10.08 t ha−1). However, the differences were not statistically significant.
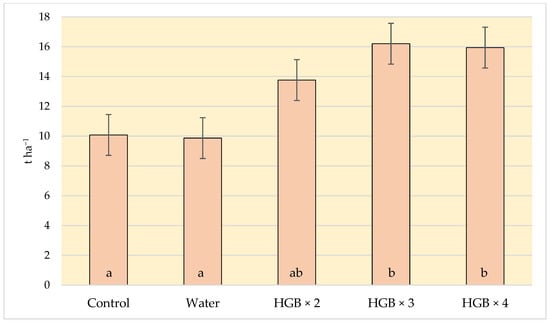
Figure 2.
Effect of humic acid fertilization on the yield of strawberries (t ha−1) (control—soil fertilization; water—spraying with clean water; HBG—two, three and fourfold spraying of Humi Brown Gold). Means marked with the same letters do not differ significantly at α = 0.05.
The result of our experiment is in line with the findings of other studies. For example, Shehat et al. [39] observed that the strawberries treated with humic acids gave a higher yield than those treated with a mineral fertiliser. Bogunovic et al. [40] also observed that the yield of strawberries treated with biostimulants increased by several dozen per cent.
The increase in the yield of the strawberries treated with humic acids may have been caused by their easier uptake of macro- and micronutrients from the soil [18,41]. This uptake could have been facilitated by a well-developed root system of the plants, which grew better under the influence of humic acids [42]. This conclusion was drawn from the observations of agricultural crops, such as, sunflowers [27], vegetables and berries, e.g., blueberries [35,42]. However, our experiment did not confirm the hypothesis of the easier uptake of nutrients. The content of macro- and micronutrients in the strawberry leaves treated with the fertiliser containing humic acids did not differ significantly from the content of these elements in the leaves of the plants which had received the soil fertiliser (Table 6 and Table 7). The high yield of the strawberries in the variants treated with humic acids may also have been caused by the fact that the roots of these plants retained more water [43]. However, this effect is the most noticeable when there is water shortage in the substrate [44]. In our experiment, during the growing season, the moisture content in the soil was optimal or elevated (Appendix B).
3.4. Strawberry Fruit Quality
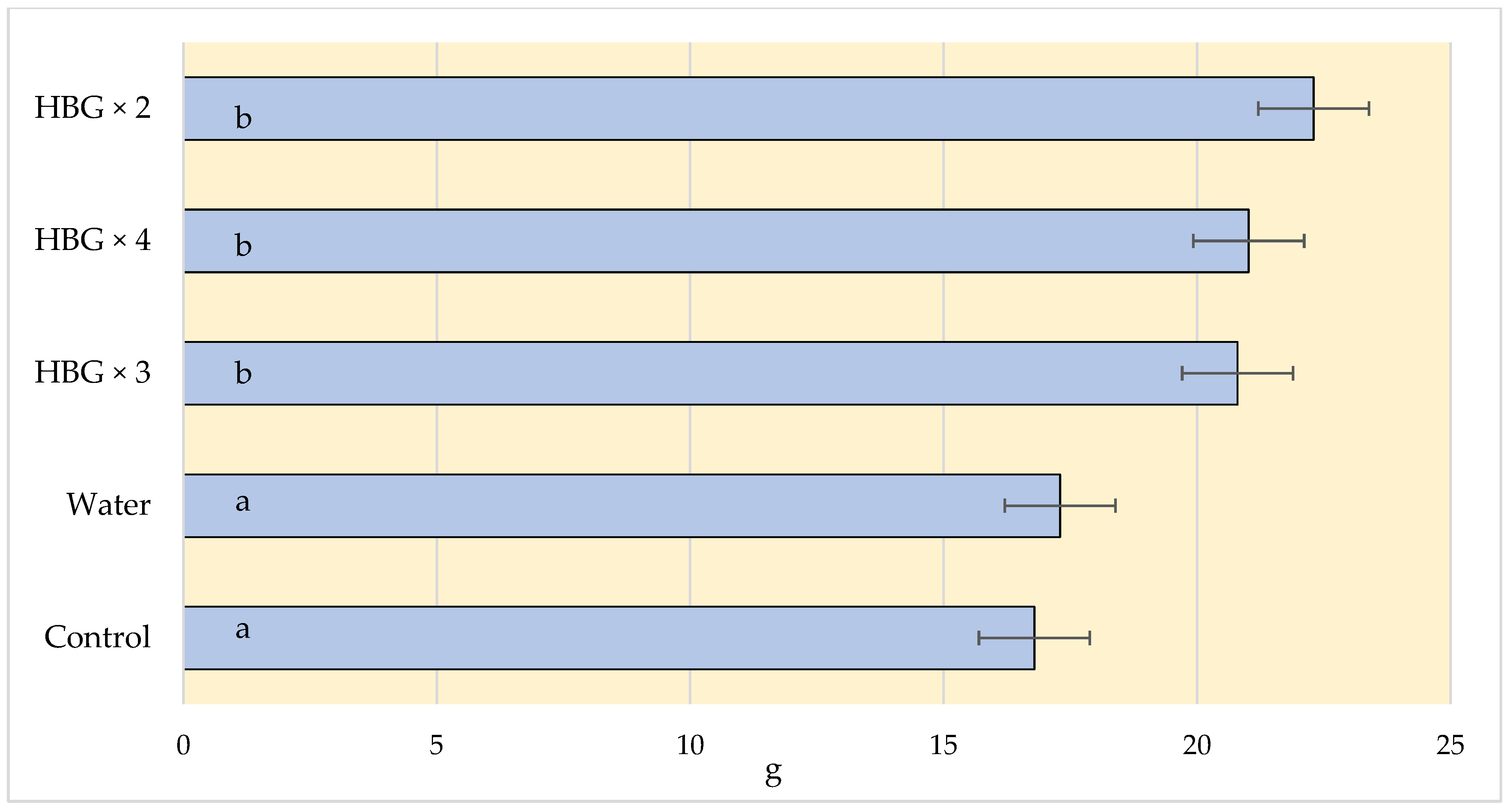
Due to the increasing market requirements, producers are forced to pay more attention to the quality of fruit. It is usually determined by such parameters as weight, firmness, extract content, and the percentage of acids. It is easier to sell large fruits, which is crucial for the profitability of production. In our experiment, the strawberries harvested from the plants treated with the soil fertiliser had the lowest weight. The application of the Humi Brown Gold fertiliser had a positive influence on the weight of the strawberries. The weight of the fruits in the HBG × 2 variant was over 30% higher than in the variant where the soil fertiliser had been applied (22.3 g vs. 16.39 g, respectively) (Figure 3).
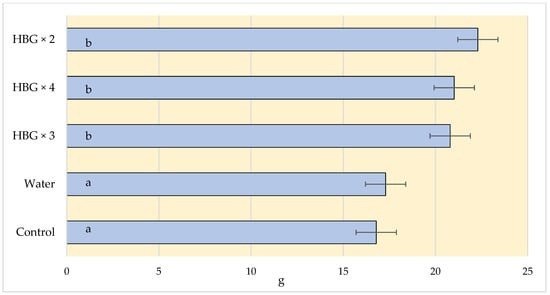
Figure 3.
Effect of humic acid fertilization on the weight (g) of strawberries (control—soil fertilization; water—spraying with clean water; HBG—two, three and fourfold spraying of Humi Brown Gold). Means marked with the same letters do not differ significantly at α = 0.05.
The number of fertilisation treatments did not have significant effect on the average weight of strawberries. The authors of other experiments on the use of biostimulants, including humic acids, observed their positive effect on the weight of not only strawberries [45] but also apples [46,47]. However, the experiments conducted by Soldaniband et al. [36] did not reveal any significant effect of the biostimulants on the weight of strawberries. The effects of treatment of plants with humic acids may depend on various factors, such as the climatic and soil conditions, the way they are brought in or the species cultivated. For example, the treatment of blue huckleberry plants with humic acids did not improve the growth of shoots, yield, or fruit quality [15,20].
The suitability of soft fruits for transport is largely determined by their firmness. Firm fruits are more attractive to consumers [48] and less susceptible to damage caused by Botrytis cinerea. In our experiment, the strawberries from the variant with soil fertilization were characterised by the lowest firmness (158.8 g mm−2). The firmness of the fruit harvested from the bushes in the HBG × 2 variant (198.0 g mm−2) was significantly higher than that of the fruit harvested from the plants treated with the soil fertiliser (Table 9). The fruit firmness increased along with the number of spray treatments, i.e., up to 206.7 g mm−2 (HBG × 2) and 212.5 g mm−2 (HBG × 3). Farahi et al. [49] also observed an increase in the firmness of strawberries harvested from plants treated with humic acids.

Table 9.
Effect of humic acid fertilization on strawberry fruit quality (control—soil fertilization; water—spraying with clean water; HBG—two-, three and fourfold spraying of Humi Brown Gold).
The high content of extract (TSS) in fruit increases its dessert value. The strawberries harvested from the plants treated with the soil fertiliser had the lowest TSS content (8.54%). The highest TSS content in the strawberries was 10.2% (Table 9). It was lower than the values noted in the experiments conducted by Wysocki et al. [50] (13.23%) and Mikiciuk et al. [45] (12.37%). The application of humic acids had a positive effect on the content of the extract in strawberries. The double treatment of the plants with the fertiliser containing humic acids caused a significant increase in the TSS content, i.e., up to 9.28%. The TSS content in the strawberries increased along with the number of spray treatments, i.e., by 14% in the HBG × 3 variant and by 17% in the HBG × 4 variant (Table 9).
The application of humic acids had positive influence on the quality of strawberries both after harvesting and after storage. The stored fruits from the variants with HBG fertilisation were firmer than those harvested from the plants treated with the soil fertiliser (Table 9). There were significant differences observed in the fruits from the HBG × 2 variant. The firmness of stored strawberries increased along with the number of treatments up to 192.13 g mm−2. Also, the content of the extract in the stored strawberries harvested from the plants in all variants with humic acids was significantly higher than in the fruits harvested from the plants treated with the soil fertiliser (Table 9).
The taste of fruits is also affected by the amount of sugars and organic acids dissolved in them [51]. Citric and malic acids are the most important for the taste of strawberries. The fertilisation of strawberry plants with humic acids had a relatively small effect on the hydrolytic acidity of the fruit juice, expressed as citric acid. The variants with soil fertilisation (0.55%) and the HBG × 2 and HBG × 3 variants (0.54%) did not differ significantly in the hydrolytic acidity. The acidity of strawberries decreased to 0.42% only in the HBG × 4 variant. This result is similar to the findings of the study conducted by Mikiciuk et al. [45], who found no changes in the acidity of strawberries after the treatment with biostimulants.
The colour of fruits affects their commercial value. In our experiment, the colour intensity (parameter L) of the strawberries harvested from the plants which had received the HBG fertiliser was significantly lower (paler fruits) than the colour intensity of the fruits from the plants treated with the soil fertiliser (darker fruits). The number of treatments did not have significant effect on the colour of strawberries. Similarly to the colour intensity, the values of the a-coordinate (the colour range from green to red) and the b-coordinate (the colour range from blue to yellow) and the chromaticity of fruits treated with the fertiliser containing humic acids were significantly lower than in the fruits from the variant with soil fertilization (Table 10).

Table 10.
Effect of humic acid fertilization on the colour of strawberries (control—soil fertilization; water—spraying with clean water; HBG—two, three and fourfold spraying of Humi Brown Gold).
The strawberries harvested from the plants treated with the fertiliser containing humic acids were more resistant to fungal diseases. In these variants, during the harvest the percentage of fruits infested by grey mould (Botrytis cinerea) ranged from 0.25% (HBG × 3) to 0.42% (HBG × 2), whereas in the soil fertilisation variant it was almost nine times greater (2.25%). The analysis of the condition of strawberries after storage led to a similar conclusion. The amount of stored fruits affected by grey mould increased significantly. There were particularly big differences in the soil fertilisation variant—2.25% (freshly harvested fruits) vs. 17.60% (stored fruits). By comparison, in the variants where humic acids had been applied, the percentage of strawberries infested by grey mould after storage did not exceed 5%.
Both the physical and biochemical characteristics of fruits largely depend on the course of weather conditions [52]. They change depending on the growing season. The harvest date was another parameter affecting all the qualitative parameters of strawberries. On the first harvest date, i.e., in early June, the fruits were characterised by the highest weight and the lowest hydrolytic acidity (Table 11).

Table 11.
Effect of harvest date on the qualitative parameters of strawberries.
However, the large fruits harvested at that time had the lowest extract content—8.16%. The TSS content in the fruits harvested in the last period was 10.87%, which may have resulted from the high air temperature. Temperature is an important factor for both the yield and the content of various substances in strawberries [53,54]. In late June (the third harvest date), the air temperature was higher than at the beginning of the month (Appendix A). The fruits from the second harvest date, i.e., mid-June, were characterised by the greatest firmness and the highest hydrolytic acidity (Table 11).
4. Conclusions
Crops provided with the optimal amount of nutrients maintain their physiological processes at the right level, which determines the yield volume and quality. The experiment showed the positive influence of the fertiliser containing humic acids on the physicochemical and biological properties of the soil, the growth rate, and the quality of strawberries. The application of the Humi Brown Gold fertiliser significantly increased the soil pH and reduced the salinity. The effectiveness of fertilisation increased along with the number of spray treatments. The biological properties of the soil also changed. The Humi Brown Gold fertiliser significantly increased the activity of soil enzymes—proteases and dehydrogenases—as well as soil respiration. The improvement in the physicochemical and biological properties of the soil positively influenced the vegetative growth of the plants. The strawberry leaf area in the variants fertilised with the preparation containing humic acids was two times larger than in the variants where soil fertilisation had been applied. The number of treatments did not have significant effect on this parameter. Much more fruits were set in the variants where humic acid fertilisation had been applied, which resulted in high yields. Apart from the yield, the profitability of production was considerably influenced by the quality of fruit. The strawberry bushes treated with the fertiliser containing humic acids yielded significantly heavier and firmer fruits with a higher extract content than the plants where soil fertilisation had been applied. The quality characteristics of strawberries improved along with the increasing number of foliar spray treatments with the fertiliser. The methods of fertilisation did not cause any differences in the hydrolytic acidity of strawberry cell juice. The results of our experiment allow us to conclude that fertilisers containing humic acids can be a valuable alternative to standard mineral fertilisation in strawberry plantations. Such preparations can be successfully used in organic berry plantations.
Author Contributions
Conceptualization, Z.Z. and P.Z.; methodology, Z.Z.; resources, P.Z.; writing—original draft preparation, P.Z. and Z.Z.; crop management and samplings, Z.Z. and P.Z.; laboratory analyses, Z.Z.; writing—review and editing, P.Z.; project administration, Z.Z.; funding acquisition, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
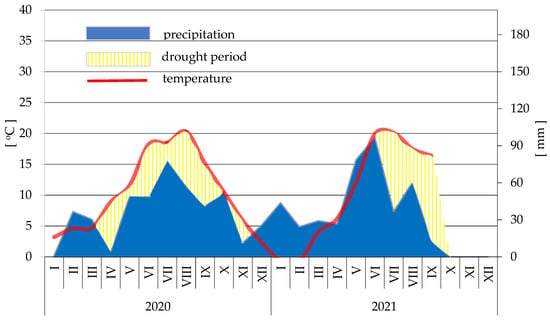
Figure A1.
A climatogram of the years 2020 and 2021.
Figure A1.
A climatogram of the years 2020 and 2021.
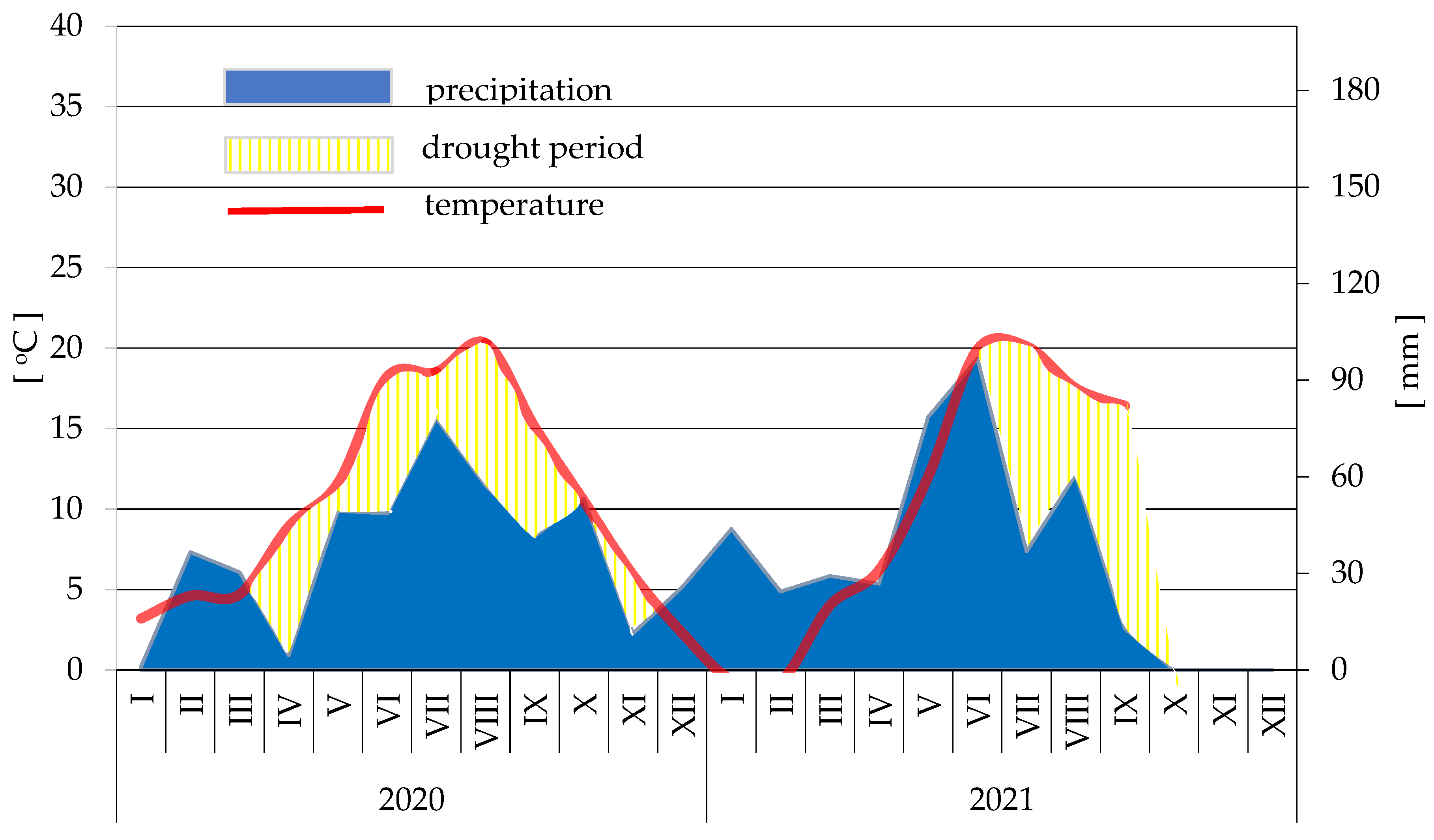
Appendix B

Table A1.
hPa is too high: from 80 to 100 hPa—high; from 100 to 350 hPa—optimal; from 350 to 500 hPa—low; from 500 to 600 hPa—too low.
Table A1.
hPa is too high: from 80 to 100 hPa—high; from 100 to 350 hPa—optimal; from 350 to 500 hPa—low; from 500 to 600 hPa—too low.
| Treatments | Measurement Dates | ||||||
|---|---|---|---|---|---|---|---|
| 19 May 2021 | 25 May 2021 | 2 June 2021 | 10 June 2021 | 16 June 2021 | 29 June 2021 | 8 July 2021 | |
| Control | 108 | 68 | 91 | 234 | 86 | 327 | 200 |
| Water | 91 | 77 | 37 | 175 | 43 | 238 | 188 |
| HBG × 2 | 136 | 70 | 39 | 173 | 52 | 220 | 340 |
| HBG × 3 | 142 | 73 | 100 | 169 | 39 | 211 | 334 |
| HBG × 4 | 105 | 89 | 107 | 148 | 38 | 194 | 127 |
References
- Muthukumaran, S.; Tranchant, C.; Shi, J.; Ye, X.; Xue, S.J. Ellagic acid in strawberry (Fragaria spp.): Biological, technological, stability, and human health aspects. Food Qual. Saf. 2017, 1, 227–252. [Google Scholar] [CrossRef]
- Fijoł-Adach, E.; Feldyn-Szewczyk, B.; Kazimierczak, R.; Stalenga, J. Effect of an agricultu ral production system on the presence of bioactive substances in strawberry fruits. Postęp Tech. Przetwórstwa Spożywczego 2016, 1, 78–86. (In Polish) [Google Scholar]
- Khan, H.Z.; Malik, M.A.; Saleem, M.F. Effect of rate and source of organic material on the production potential of spring maize (Zea mays L.). Pak. J. Agric. Sci. 2008, 45, 40–43. [Google Scholar]
- Kobi, H.B.; Martins, M.C.; Silva, P.I.; Souza, J.L.; Carneiro, J.C.S.; Heleno, F.; Queiroz, M.E.L.R.; Costa, N.M. Organic and conventional strawberries: Nutritional quality, antioxidant characteristics and pesticide residues. Fruits 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Zavyalova, N.E.; Vasbieva, M.T.; Yamaltdinova, V.R.; Shlyapina, Y.V. Characteristics of humic acids in sod-podzolic soil under long-term exposure to different fertilization systems. Dokuchaev Soil Bull. 2022, 111, 97–115. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hort. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Švecová, E.B.; Ruzzi, M. Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Front. Plant Sci. 2019, 10, 60. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Schoebitz, M.; L’opez, M.D.; Serri, H.; Martínez, O.; Zagal, E. Combined application of microbial consortium and humic substances to improve the growth performance of blueberry seedlings. J. Plant. Nutr. Soil Sci. 2016, 16, 1010–1023. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Zamboni, A.; Sega, D.; Varanini, Z.; Pinton, R. Waterextractable humic substances speed up transcriptional response of maize roots to nitrate. Environ. Exp. Bot. 2018, 147, 167–178. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P. Effect of a preparation containing humic acids on selected physico-chemical and biological properties of replanted soil. J. Elem. 2020, 25, 993–1004. [Google Scholar] [CrossRef]
- Nazli, R.I.; Kuşvuran, A.; Inal, I.; Demirbaş, A.; Tansi, V. Effects of different organic materials on forage yield and quality of silage maize (Zea mays L.). Turk. J. Agric. Forest. 2014, 38, 23–31. [Google Scholar] [CrossRef]
- Ennab, H. Effect of humic acid on growth and productivity of Egyptian Lime trees (Citrus aurantifolia Swingle) under salt stress conditions. J. Agric. Res. 2016, 42, 494–505. [Google Scholar]
- Nunes, R.O.; Domiciano, G.A.; Alves, W.S.; Melo, A.C.A.; Nogueira, F.C.S.; Canellas, L.P.; Olivares, F.L.; Zingali, R.B.; Soares, M.R. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 2019, 9, 12019. [Google Scholar] [CrossRef]
- Saidimoradi, D.; Ghaderi, N.; Javadi, T. Salinity stress mitigation by humic acid application in strawberry (Fragaria ananassa Duch). Sci. Hort. 2019, 256, 108594. [Google Scholar] [CrossRef]
- Olivares, F.L.; Aguiar, N.O.; Rosa, R.C.C.; Calellas, L.P. Substrate Biofortification in Combination with Foliar Sprays of Plant Growth Promoting Bacteria and Humic Substances Boosts Production of Organic Tomatoes. Sci. Hortic. 2015, 183, 100–108. [Google Scholar] [CrossRef]
- Filipczak, J.; Żurawicz, E.; Sas-Paszt, L. Influence of selected biostimulants on the growth and yielding of ‘Elkat’ strawberry plants. Zesz. Nauk. Inst. Ogrod. 2016, 24, 43–58. [Google Scholar]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Ladd, N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Gołębiowska, J.; Pędziwilk, Z. CO2 release as on index of biological activity of cultivated soils. Acta Microbiol. Pol. 1984, 33, 249–256. [Google Scholar] [PubMed]
- Akinci, S.; Büyükkeskin, T.; Eroğlu, A.; Erdoğan, B.E. The effect of humic acid on nutrient composition in broad bean (Vicia faba L.) Roots. Not. Sci. Biol. 2009, 1, 81–87. [Google Scholar] [CrossRef]
- Katkat, A.V.; Çelik, H.; Turan, M.A.; Aşık, B.B. Effects of soil and foliar applications of humic substances on dry weight and mineral nutrients uptake of wheat under calcareous soil conditions. Aust. J. Basic Appl. Sci. 2009, 3, 1266–1273. [Google Scholar]
- Gajda, A.M.; Przewłoka, B.; Gawryjołek, K. Changes in soil quality associated with tillage system applied. Int. Agrophys. 2013, 27, 133–141. [Google Scholar] [CrossRef]
- Nunez, G.H.; Buzzi, G.; Heller, C.R. Southern highbush blueberry responses to humic acid application in soilless substrates. Sci. Hort. 2023, 308, 111541. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India . Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- Schoebitz, M.; L’opez, M.D.; Serri, H.; Aravena, V.; Zagal, E.; Rold’an, A. Characterization of bioactive compounds in blueberry and their impact on soil properties in response to plant biostimulants. Commun. Soil Sci. Plant Anal. 2019, 50, 2482–2494. [Google Scholar] [CrossRef]
- Rzepka-Plevnes, D.; Kulpa, D.; Gołębiowska, D.; Porwolik, D. Effects of auxins and humic acids on in vitro rooting of strawberry (Fragaria × ananassa Duch.). J. Food Agric. Environ. 2011, 9, 592–595. [Google Scholar]
- Derkowska, E.; Sas Paszt, L.; Trzciński, P.; Przybył, M.; Weszczak, K. Influence of biofertilizers on plant growth and rhizosphere microbiology of greenhouse-grown strawberry cultivars. Acta Sci. Pol. Hortorum Cultus 2015, 14, 83–96. [Google Scholar]
- Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants Promote Plant Development, Crop Productivity, and Fruit Quality of Protected Strawberries. Agronomy 2022, 12, 1684. [Google Scholar] [CrossRef]
- Wójcik, P. Analiza Mineralna Liści—Uaktulnione Kryterium Diagnostyczne w Nawożeniu Roślin Sadowniczyh; Instytut Ogrodnictwa—Państwowy Instytut Badawczy: Skierniewice, Poland, 2021. (In Polish) [Google Scholar]
- Sas-Paszt, L.; Sumorok, B.; Derkowska, E.; Trzciński, P.; Lisek, A.; Grzyb, Z.; Sitarek, M.; Przybył, M.; Frąc, M. Effect of microbiologically enriched fertilizers on the vegetative growth of strawberry plants under field conditions in the first year of plantation. J. Res. Appl. Agric. Eng. 2019, 64, 29–37. [Google Scholar]
- Shehata, S.A.; Gharib, A.A.; Mohamed, M.; El-Mogy, K.F.; Abdel Gawad, E.A. Influence of compost, amino and humic acids on the growth, yield and chemical parameters of strawberries. J. Med. Plants Res. 2011, 5, 2304–2308. [Google Scholar]
- Bogunovic, I.; Duralija, B.; Gadze, J.; Kisic, I. Biostimulant usage for preserving strawberries to climate damages. Hort. Sci. 2015, 42, 132–140. [Google Scholar] [CrossRef]
- Baqir, H.A.; Zeboon, N.H. Response of some wheat growth traits for foliar spraying with humic acid glutamic acid. Iraqi J. Agric. Sci. 2019, 50, 1455–1464. Available online: https://jcoagri.uobaghdad.edu.iq/index.php/intro/article/view/833/632 (accessed on 19 December 2019).
- Bryla, D.R.; Strik, B.C. Nutrient requirements, leaf tissue standards, and new options for fertigation of northern highbush blueberry. Horttechnology 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. Available online: https://www.agriculturejournals.cz/publicFiles/4_2010-SWR.pdf (accessed on 1 March 2011). [CrossRef]
- Alfatlawi, Z.H.C.; Alrubaiee, S. Effect of spraying different concentrations of humic acid on the growth and yield of wheat crop (ipa 99 cultivar) in different stages. Plant Arch. 2020, 20, 1517–1521. Available online: http://www.plantarchives.org/SPL%20ISSUE%2020-2/249__1517-1521_.pdf (accessed on 10 July 2020).
- Mikiciuk, L.; Sas-Paszt, L.; Mikiciuk, M.; Derkowska, E.; Trzciński, P.; Głuszek, A.; Rudnicka, J. Mycorrhizal frequency, physiological parameters, and yield of strawberry plants inoculated with endomycorrhizal fungi and rhizosphere bacteria. Mycorrhiza 2019, 29, 489–501. [Google Scholar] [CrossRef]
- Boray, M.S.S.; Mostafa, M.F.M.; Abd El-Galel, M.M.; Somaa, I.A.I. Effect of humic and fulvic acids with some nutrients at different time of application on yield and fruits quality of Anna apple trees. J. Plant Product. 2015, 6, 307–321. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P.; Kayzer, D. The influence of a soil activator containing humic acids on the yield and quality of apples in conditions of replantation. J. Elem. 2021, 26, 333–347. [Google Scholar] [CrossRef]
- Lobos, G.A.; Bravo, C.; Valdés, M.; Graell, J.; Lara Ayala, I.; Beaudry, R.M.; Moggia, C. Within-plant variability in blueberry (Vaccinium Corymbosum, L.): Maturity at harvest and position within the canopy influence fruit firmness at harvest and postharvest. Postharvest Biol. Technol. 2018, 146, 26–35. [Google Scholar] [CrossRef]
- Farahi, M.H.; Aboutaleb, A.; Eshghi, S.; Dastyaran, M.; Yosefi, F. Foliar application of humic acid on quantitative and qualitative characteristics of‘ Aromas’ strawberry in soilless culture. Agric. Commun. 2013, 1, 13–16. [Google Scholar]
- Wysocki, K.; Kopytowski, J.; Bieniek, A.; Bojarska, J. The effect of substrates on yield and quality of strawberry fruits cultivated in a heated foil tunnel. Zemdirb. Agric. 2017, 104, 283–286. [Google Scholar] [CrossRef]
- Milukic-Petkovsek, M.; Ivancic, A.; Schimitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Gonçalves, B.; Aires, A.; Silva, A.; Ferreira, L.; Carvalho, R.; Fernandes, H.; Freitas, C.; Carnide, V.; Silva, A.P. Effect of Harvest Year and Altitude on Nutritional and Biometric Characteristics of Blueberry Cultivars. J. Chem. 2016, 2016, 8648609. [Google Scholar] [CrossRef]
- MacKenzie, S.J.; Chandler, C.K.; Hasing, T.; Whitaker, V.M. The role of temperature in the late-season decline in soluble solids content of strawberry fruit on a subtropical production system. HortScience 2011, 46, 1562–1566. [Google Scholar] [CrossRef]
- Palencia, P.; Martinez, F.; Medina, J.J.; Medina, J.L. Strawberry yield efficiency and its cor relation with temperature and solar radiation. Hortic. Bras. 2013, 31, 93–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).