Comparative Transcriptomics Reveal the Mechanisms Underlying the Glucosinolate Metabolic Response in Leaf Brassica juncea L. under Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cold Stress Treatment
2.2. GSLs Enzyme Immunoassay
2.3. Physiological Analyses of Cold-Treated Leaves
2.4. RNA Sequencing (RNA-Seq) and Data Analysis
2.5. Quantitative Real-Time Reverse Transcription PCR (qPCR)
2.6. Statistical Analysis
3. Results
3.1. Difference in GSLs Content in Leaf B. Juncea under Cold Stress
3.2. Sequencing and Assembly of RNA-Seq Datasets
3.3. Identification and Enrichment Analysis of DEGs
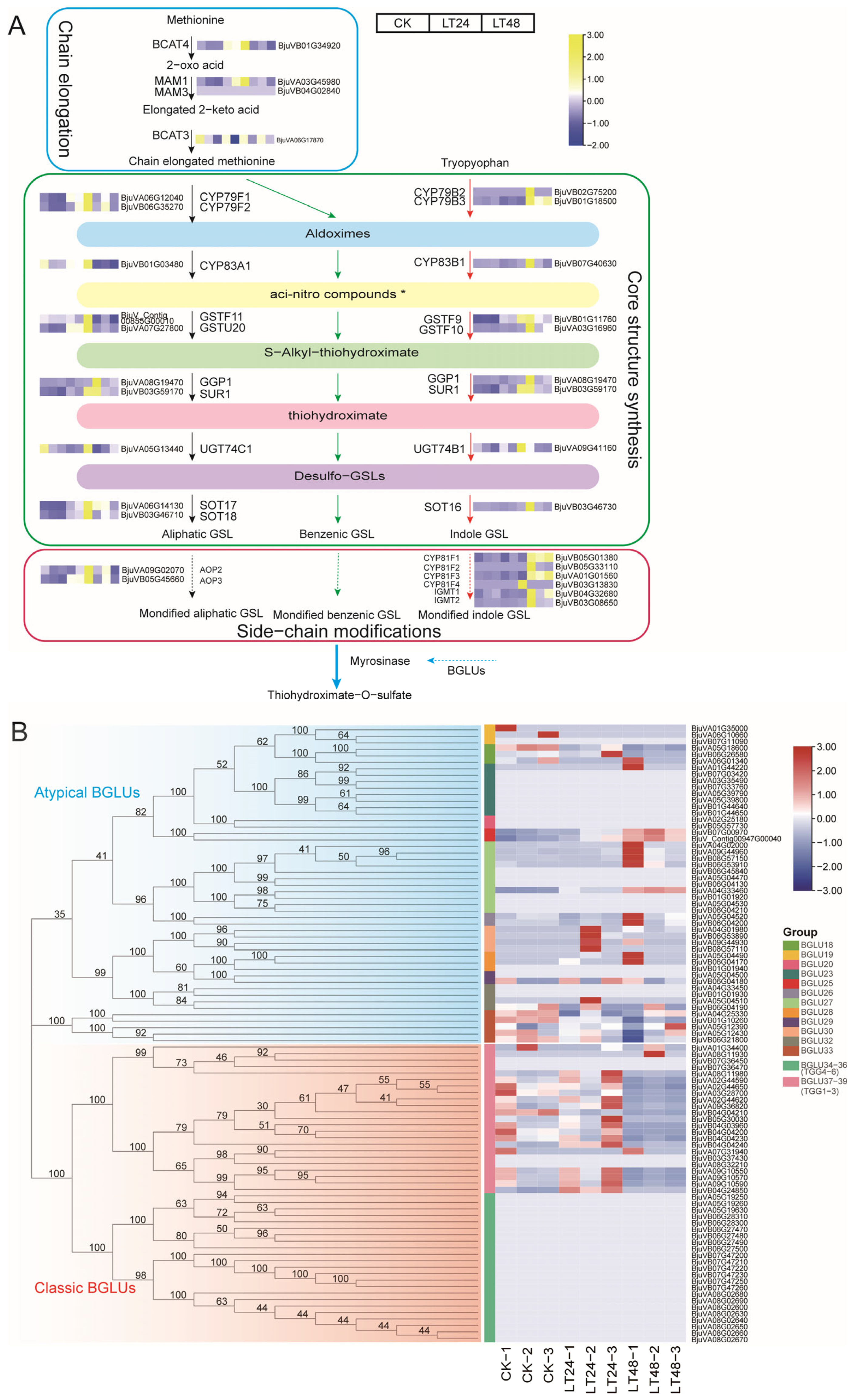
3.4. Expression Patterns of Structural DEGs Related to GSLs Biosynthesis
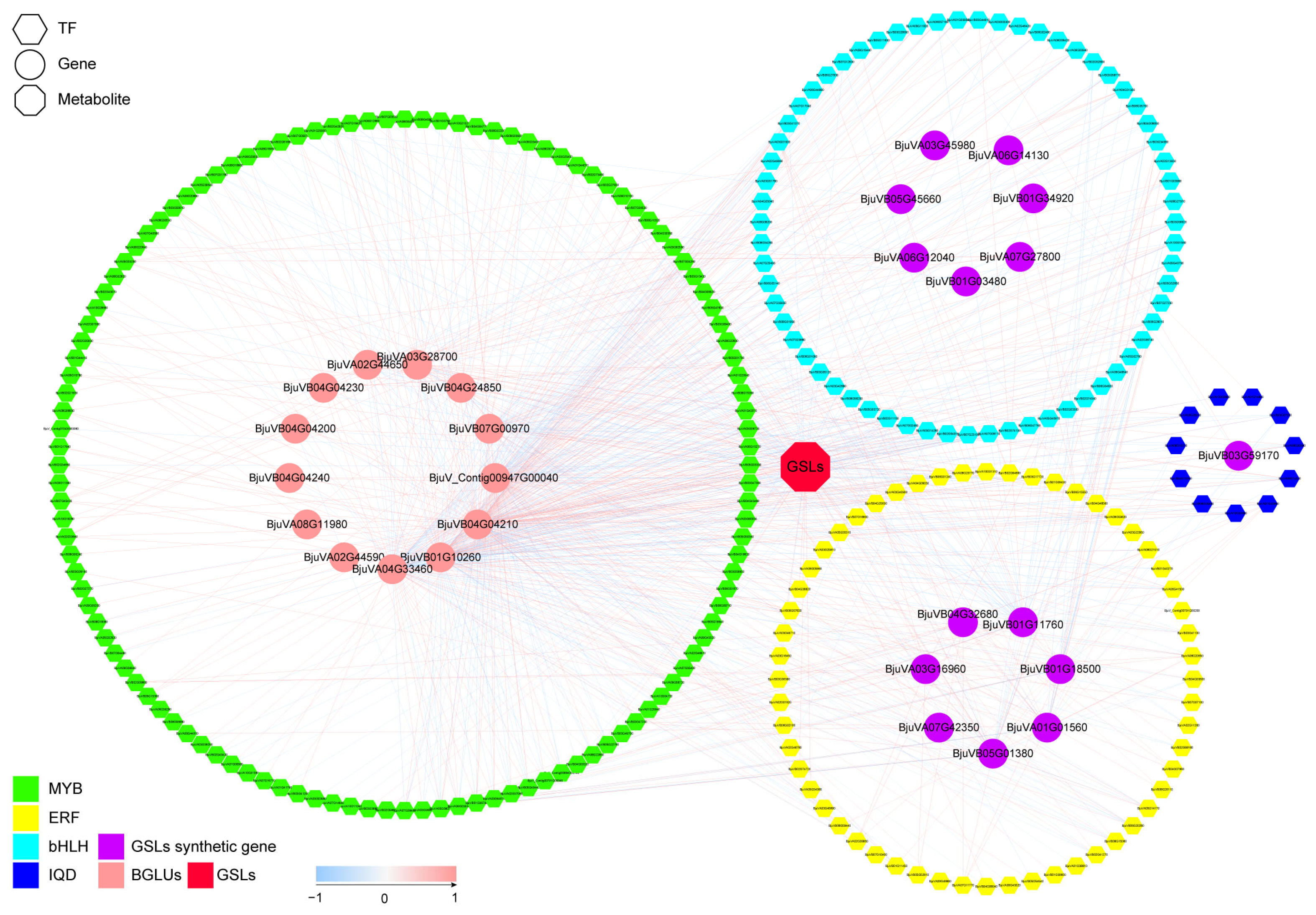
3.5. Candidate Transcription Factors Involved in GSLs Biosynthesis
3.6. Effect of Topical Application of GSLs on Cold Resistance in Leaf B. juncea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cong, L.; Chai, T.Y.; Zhang, Y.X. Characterization of the novel gene BjDREB1B encoding a dre-binding transcription factor from Brassica juncea L. Biochem. Biophy. Res. Commun. 2008, 371, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Deng, F. Phytochemistry and biological activity of mustard (Brassica juncea): A review. CyTA J. Food 2020, 18, 704–718. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Garraway, J.; Cai, Q.; Zhou, Y.; Li, X.; Hu, Z.; Zhang, M.; Yang, J. The casein kinase 2 β Subunit CK2B1 Is required for swollen stem formation via cell cycle control in vegetable Brassica juncea. Plant J. 2020, 104, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Cools, K.; Terry, L.A. The effect of processing on the glucosinolate profile in mustard seed. Food Chem. 2018, 252, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Rakariyatham, N.; Sakorn, P. Biodegradation of glucosinolates in brown mustard seed meal (Brassica juncea) by Aspergillus sp. NR-4201 in liquid and solid-state cultures. Biodegradation 2002, 13, 395–399. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, S.; Liu, S.; Chen, J.; Ma, H.; Cui, Z.; Zhang, X.; Ge, C.; Liu, R.; Li, Y. Comparative transcriptome analysis provides insights into the seed germination in cotton in response to chilling stress. Int. J. Mol. Sci. 2020, 21, 2067. [Google Scholar] [CrossRef] [Green Version]
- Sehrawat, A.; Deswal, R. S-nitrosylation analysis in Brassica juncea apoplast highlights the importance of nitric oxide in cold-stress signaling. J. Proteome. Res. 2014, 13, 2599–2619. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trend. Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trend Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Malka, S.K.; Cheng, Y. Possible interactions between the biosynthetic pathways of indole glucosinolate and auxin. Front. Plant Sci. 2017, 8, e2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa-Ichinose, T.; Miura, D.; Zhang, L.; Kim, S.-J.; Maruyama-Nakashita, A. Involvement of BGLU30 in glucosinolate catabolism in the Arabidopsis leaf under dark conditions. Plant Cell Physiol. 2020, 61, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Böttcher, C.; Baatout, D.; Gigolashvili, T. Glucosinolates are produced in trichomes of Arabidopsis thaliana. Front Plant Sci. 2012, 3, e242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L. The Biosynthesis of Glucosinolates: Insights, inconsistencies, and unknowns. In Annual Plant Reviews Online; Roberts, J.A., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 969–1000. ISBN 978-1-119-31299-4. [Google Scholar]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, e112100. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Schuster, J.; Knill, T.; Reichelt, M.; Gershenzon, J.; Binder, S. Branched-chain aminotransferase 4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell 2006, 18, 2664–2679. [Google Scholar] [CrossRef] [Green Version]
- Textor, S.; de Kraker, J.-W.; Hause, B.; Gershenzon, J.; Tokuhisa, J.G. MAM3 Catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiol. 2007, 144, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Yoo, M.-J.; Koh, J.; Liu, L.; Chen, Y.; Acikgoz, D.; Wang, Q.; Chen, S. Molecular reprogramming of Arabidopsis in response to perturbation of jasmonate signaling. J. Proteome Res. 2014, 13, 5751–5766. [Google Scholar] [CrossRef]
- Hirai, M.Y.; Sugiyama, K.; Sawada, Y.; Tohge, T.; Obayashi, T.; Suzuki, A.; Araki, R.; Sakurai, N.; Suzuki, H.; Aoki, K. Omics-based identification of Arabidopsis MYB transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 6478–6483. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Hansen, B.G.; Bjarnholt, N.; Ticconi, C.; Halkier, B.A.; Kliebenstein, D.J. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE 2007, 2, e1322. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Yatusevich, R.; Berger, B.; Müller, C.; Flügge, U.-I. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana: HAG1 and glucosinolate biosynthesis. Plant J. 2007, 51, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Fröschel, C.; Iven, T.; Walper, E.; Bachmann, V.; Weiste, C.; Dröge-Laser, W. A Gain-of-function screen reveals redundant ERF transcription factors providing opportunities for resistance breeding toward the vascular fungal pathogen Verticillium longisporum. Mol. Plant-Microbe Interact. 2019, 32, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Berger, B.; Gigolashvili, T. bHLH05 is an interaction partner of MYB51 and a novel regulator of glucosinolate biosynthesis in Arabidopsis. Plant Physiol. 2014, 166, 349–369. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Wang, Q.; Kaspi, R.; Parrella, M.P.; Abel, S. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense: IQD1, a positive regulator of glucosinolate accumulation. Plant J. 2005, 43, 79–96. [Google Scholar] [CrossRef]
- del Carmen Martínez-Ballesta, M.; Moreno, D.; Carvajal, M. The Physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [Green Version]
- Aarabi, F.; Kusajima, M.; Tohge, T.; Konishi, T.; Gigolashvili, T.; Takamune, M.; Sasazaki, Y.; Watanabe, M.; Nakashita, H.; Fernie, A.R. Sulfur deficiency–induced repressor proteins optimize glucosinolate biosynthesis in plants. Sci. Adv. 2016, 2, e1601087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, H.; Cai, C.; Wei, J.; Huang, J.; Chang, J.; Qian, H.; Zhang, X.; Zhao, Y.; Sun, B.; Wang, B. Glucose enhances indolic glucosinolate biosynthesis without reducing primary sulfur assimilation. Sci. Rep. 2016, 6, 31854. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Analy. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Vieira, S.M.; Silva, T.M.; Glória, M.B.A. Influence of processing on the levels of amines and proline and on the physico-chemical characteristics of concentrated orange juice. Food Chem. 2010, 119, 7–11. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Li, Z.; Li, X.; He, Z.; Zhang, L.; Sha, T.; Lyu, X.; Chen, S.; Gu, Y. Genomic signatures of vegetable and oilseed allopolyploid Brassica juncea and genetic loci controlling the accumulation of glucosinolates. Plant Biotech. J. 2021, 19, 2619–2628. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative Toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Trick, M.; Higgins, J.; Fraser, F.; Catchpole, L.; Wells, R.; Hattori, C.; Werner, C.; Bancroft, I. Associative transcriptomics of traits in the polyploid crop species brassica napus. Nat. Biotech. 2012, 30, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.L.; He, Z.; Langer, S.; Havlickova, L.; Wang, L.; Fellgett, A.; Gupta, V.; Kumar Pradhan, A.; Bancroft, I. Validation of an associative transcriptomics platform in the polyploid crop species Brassica juncea by dissection of the genetic architecture of agronomic and quality traits. Plant J. 2020, 103, 1885–1893. [Google Scholar] [CrossRef]
- Xu, Z.; Bevan, D.R.; Winkel, B.S.J.; Mohamed, A.; Cheng, C.-L.; Poulton, J.E.; Esen, A. Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family. Plant Mol. Biol. 2004, 55, 343–367. [Google Scholar] [CrossRef]
- Nakano, R.T.; Piślewska-Bednarek, M.; Yamada, K.; Edger, P.P.; Miyahara, M.; Kondo, M.; Böttcher, C.; Mori, M.; Nishimura, M.; Schulze-Lefert, P. PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana. Plant J. 2017, 89, 204–220. [Google Scholar] [CrossRef]
- Celenza, J.L.; Quiel, J.A.; Smolen, G.A.; Merrikh, H.; Silvestro, A.R.; Normanly, J.; Bender, J. The Arabidopsis ATR1 MYB transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 2005, 137, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, F.; Fernández-Calvo, P.; Zander, M.; Diez-Diaz, M.; Fonseca, S.; Glauser, G.; Lewsey, M.G.; Ecker, J.R.; Solano, R.; Reymond, P. Arabidopsis basic Helix-Loop-Helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 2013, 25, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Meng, J.; Meng, X.; Zhao, Y.; Liu, J.; Sun, T.; Liu, Y.; Wang, Q.; Zhang, S. Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell 2016, 28, 1144–1162. [Google Scholar] [CrossRef] [Green Version]
- Prof, D.; Gupta, C. Glucosinolates: The phytochemicals of nutraceutical importance. J. Complement. Integr. Med. 2012, 9, e13. [Google Scholar] [CrossRef]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Steindal, A.L.H.; Rødven, R.; Hansen, E.; Mølmann, J. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in Kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef]
- Johansen, T.J.; Hagen, S.F.; Bengtsson, G.B.; Mølmann, J.A.B. Growth temperature affects sensory quality and contents of glucosinolates, vitamin C and sugars in swede roots (Brassica napus L. ssp. Rapifera Metzg.). Food Chem. 2016, 196, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, X.; Han, X.; Li, W.; Liu, T.; Chen, B.; Chen, X.; Wang-Pruski, G. Comparative transcriptome analyses revealed different heat stress responses in high- and low-GS Brassica alboglabra sprouts. BMC Genom. 2019, 20, e269. [Google Scholar] [CrossRef]
- Jasper, J.; Wagstaff, C.; Bell, L. Growth Temperature influences postharvest glucosinolate concentrations and hydrolysis product formation in first and second cuts of rocket salad. Postharvest Biol. Technol. 2020, 163, e111157. [Google Scholar] [CrossRef]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, e4021. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Abass Ahanger, M.; Nasser Alyemeni, M.; Alam, P. Photosynthesis, Productivity and Environmental Stress, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; ISBN 978-1-119-50177-0. [Google Scholar]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, pp. 305–350. ISBN 978-0-12-816567-6. [Google Scholar]
- Yan, X.; Chen, S. Regulation of Plant Glucosinolate Metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Guan, R.; Li, S.; Xu, X.; Zhang, S.; Xu, J. Co-regulation of indole glucosinolates and camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6 signaling pathways. J. Integr. Plant Biol. 2020, 62, 1780–1796. [Google Scholar] [CrossRef]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant. 2013, 147, 28–35. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Berger, B.; Mock, H.-P.; Müller, C.; Weisshaar, B.; Flügge, U.-I. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007, 50, 886–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premachandra, G.S.; Saneoka, H.; Fujita, K.; Ogata, S. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in Sorghum. J. Exp. Bot. 1992, 43, 1569–1576. [Google Scholar] [CrossRef]
- Huang, X.; Shi, H.; Hu, Z.; Liu, A.; Amombo, E.; Chen, L.; Fu, J. ABA is involved in regulation of cold stress response in bermudagrass. Front. Plant Sci. 2017, 8, e01613. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.; Zhang, B.-H.; Mo, C.-Y.; Fu, W.-Y.; Yang, W.; Wang, Q.-Q.; Ao, N.; Qu, F.; Tan, G.-F.; Tao, L.; et al. Comparative Transcriptomics Reveal the Mechanisms Underlying the Glucosinolate Metabolic Response in Leaf Brassica juncea L. under Cold Stress. Agronomy 2023, 13, 1922. https://doi.org/10.3390/agronomy13071922
Tang B, Zhang B-H, Mo C-Y, Fu W-Y, Yang W, Wang Q-Q, Ao N, Qu F, Tan G-F, Tao L, et al. Comparative Transcriptomics Reveal the Mechanisms Underlying the Glucosinolate Metabolic Response in Leaf Brassica juncea L. under Cold Stress. Agronomy. 2023; 13(7):1922. https://doi.org/10.3390/agronomy13071922
Chicago/Turabian StyleTang, Bing, Bao-Hui Zhang, Chuan-Yuan Mo, Wen-Yuan Fu, Wei Yang, Qing-Qing Wang, Ning Ao, Fei Qu, Guo-Fei Tan, Lian Tao, and et al. 2023. "Comparative Transcriptomics Reveal the Mechanisms Underlying the Glucosinolate Metabolic Response in Leaf Brassica juncea L. under Cold Stress" Agronomy 13, no. 7: 1922. https://doi.org/10.3390/agronomy13071922
APA StyleTang, B., Zhang, B.-H., Mo, C.-Y., Fu, W.-Y., Yang, W., Wang, Q.-Q., Ao, N., Qu, F., Tan, G.-F., Tao, L., & Deng, Y. (2023). Comparative Transcriptomics Reveal the Mechanisms Underlying the Glucosinolate Metabolic Response in Leaf Brassica juncea L. under Cold Stress. Agronomy, 13(7), 1922. https://doi.org/10.3390/agronomy13071922






