Chernozem Land Use Differentiation by Temperature-Dependent IR Spectra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. IR Measurements and Data Handling
3. Results
3.1. Band Assignment
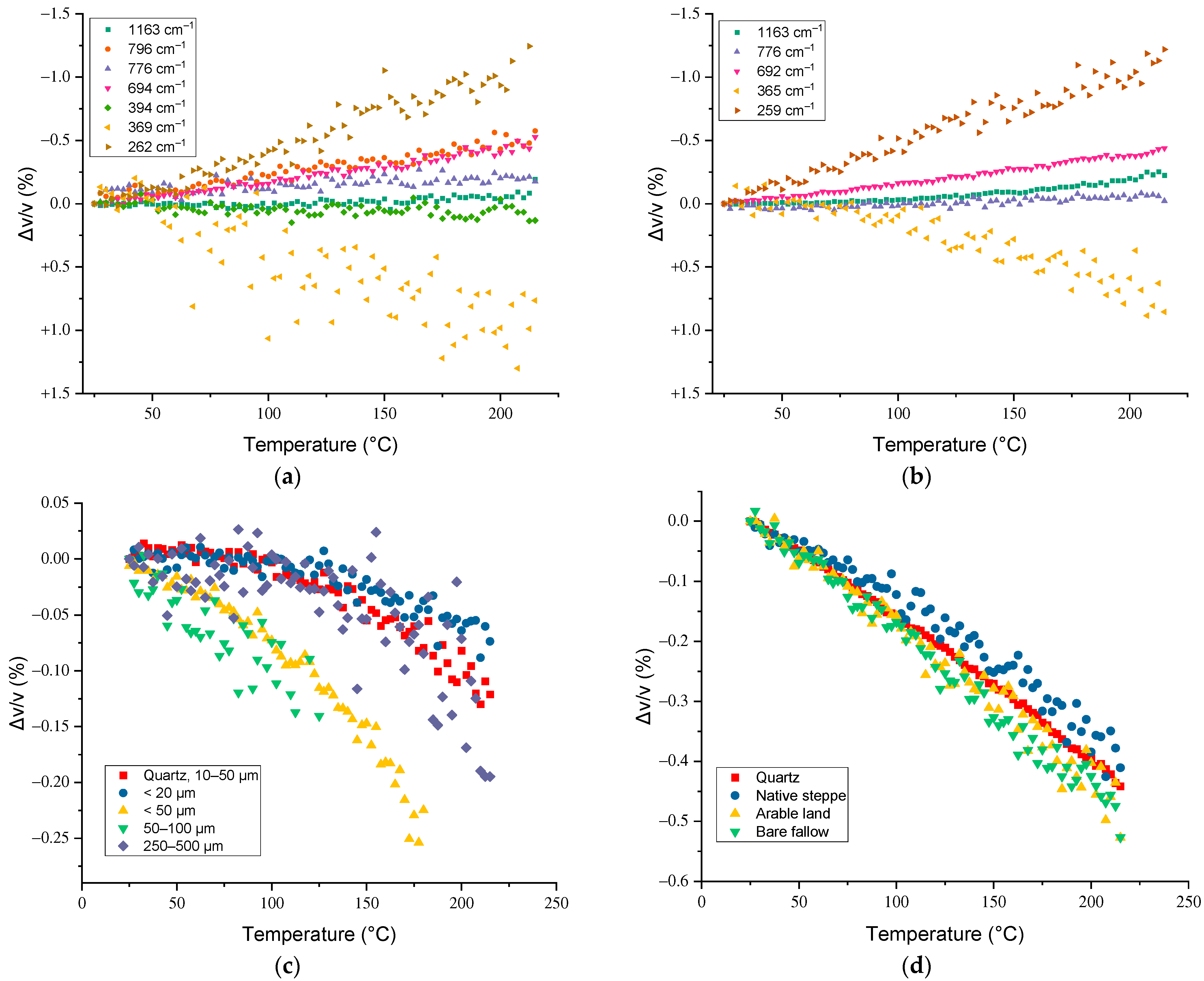
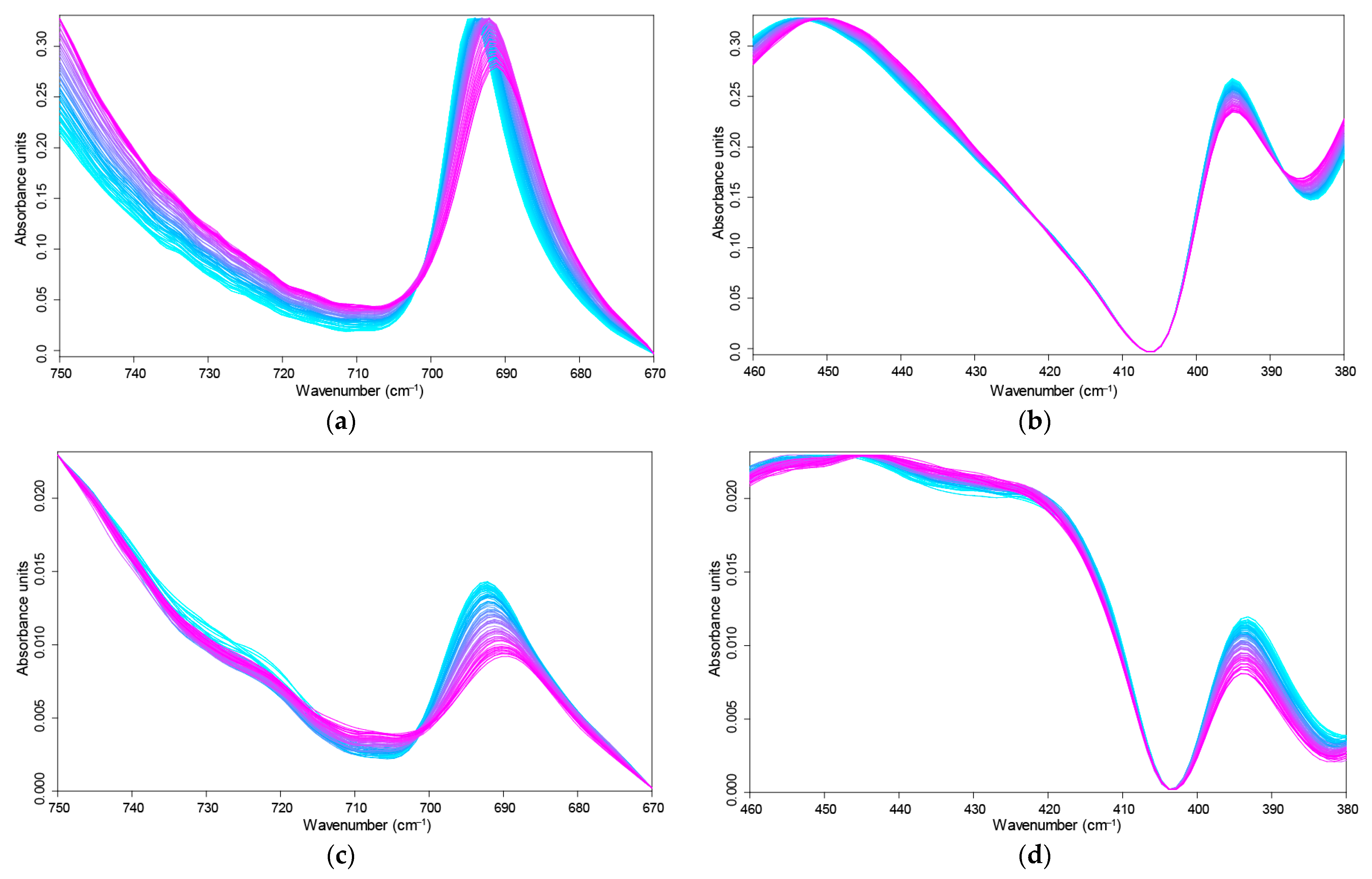
3.2. Temperature Dependences of Band Maxima
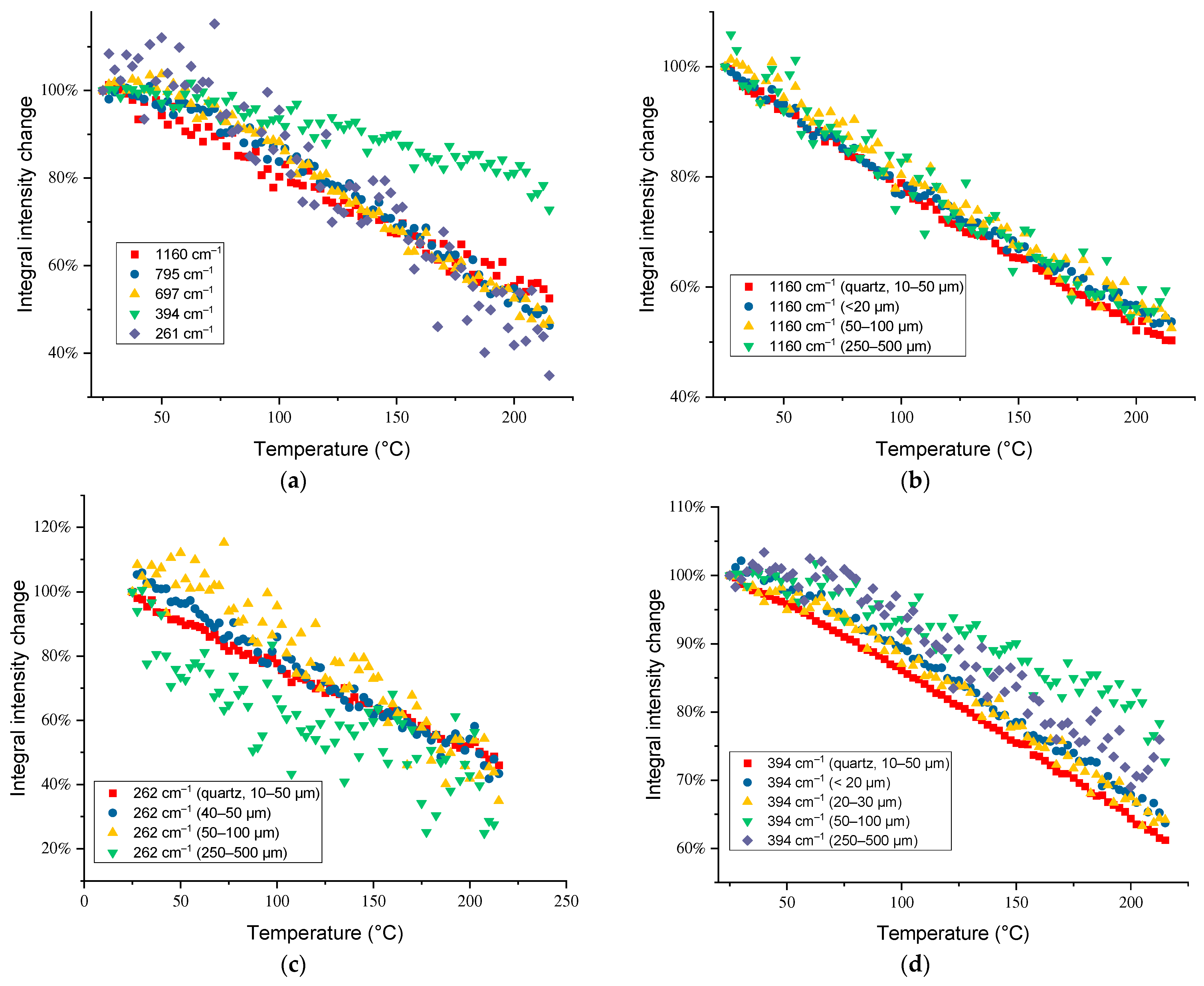
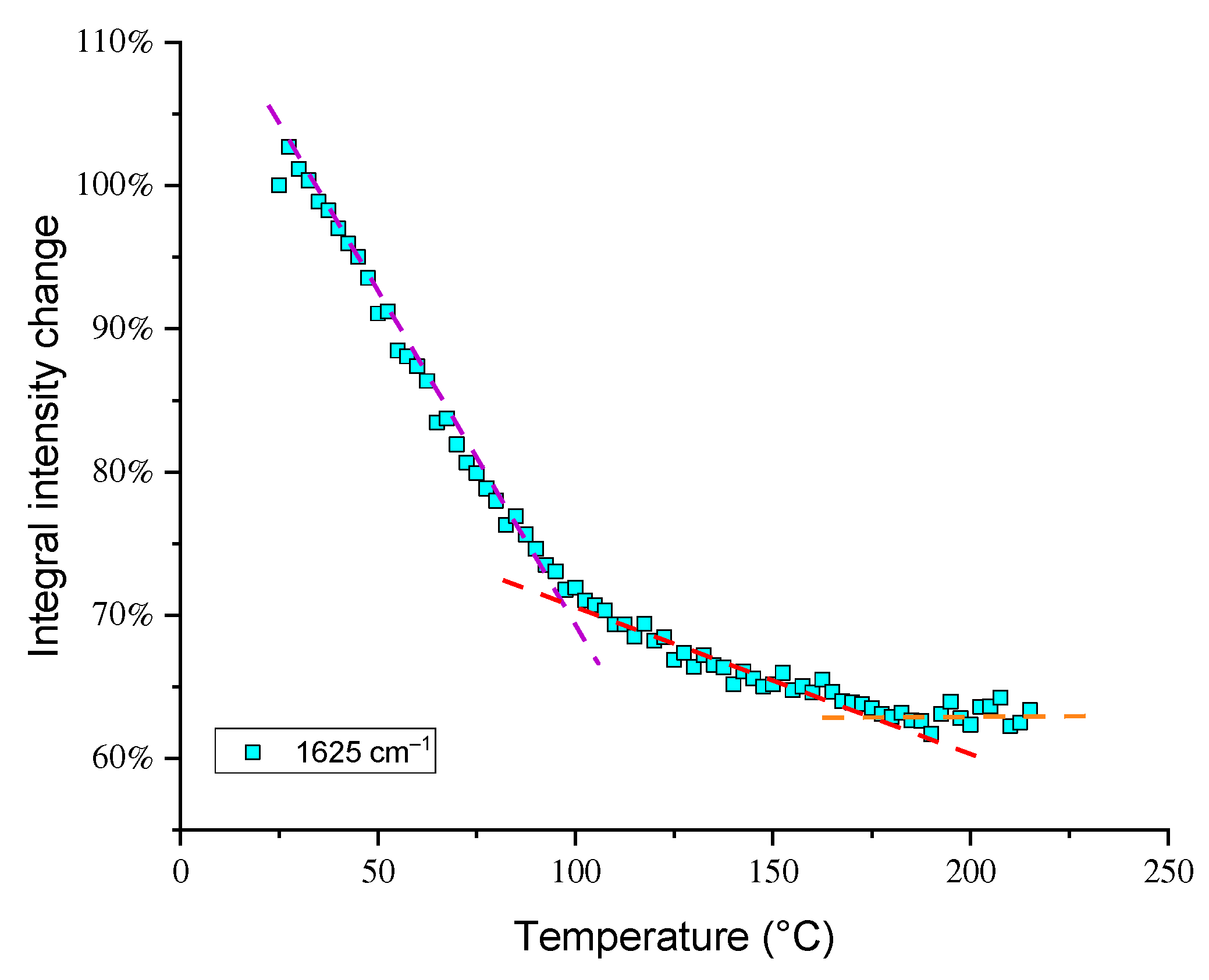
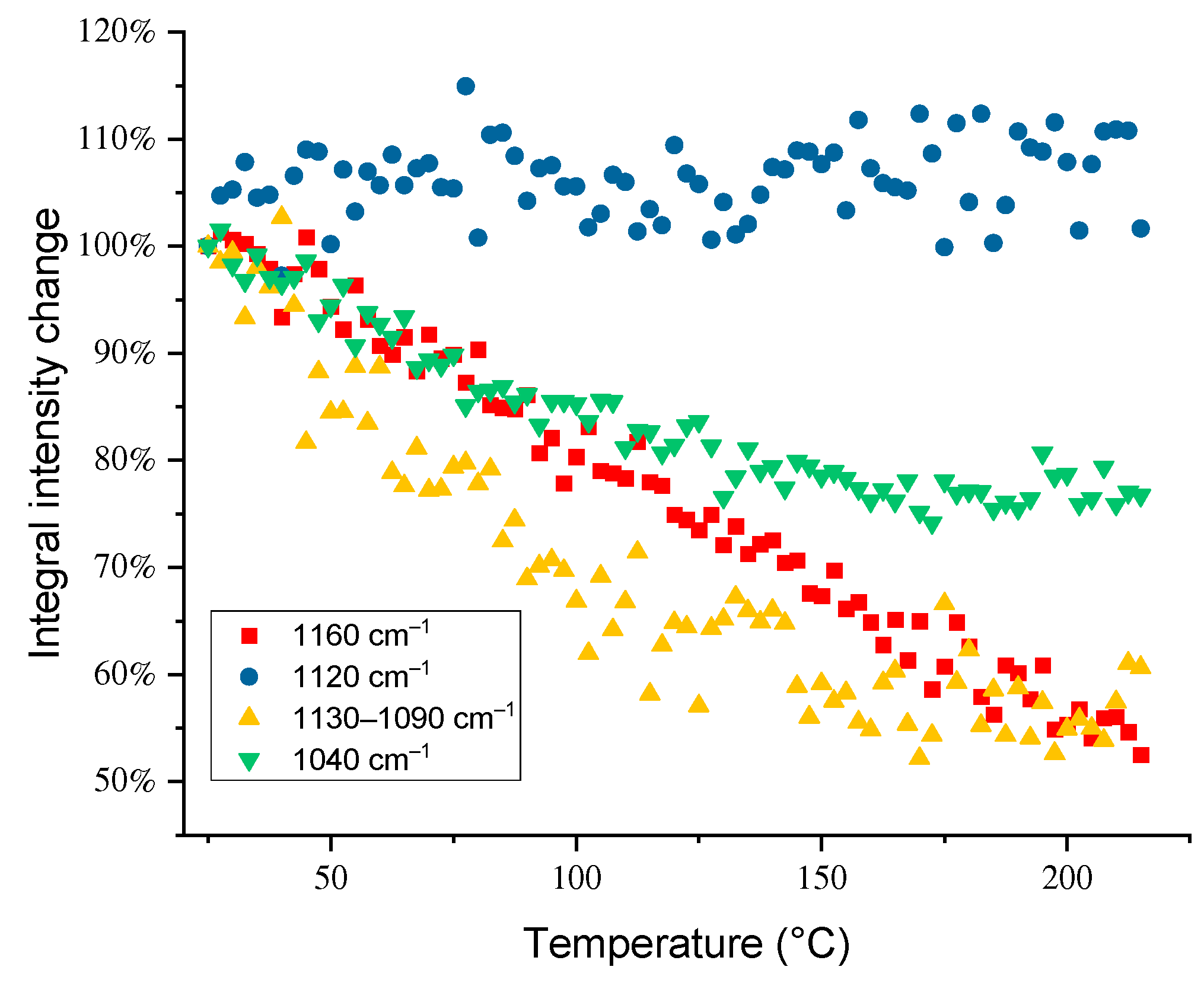
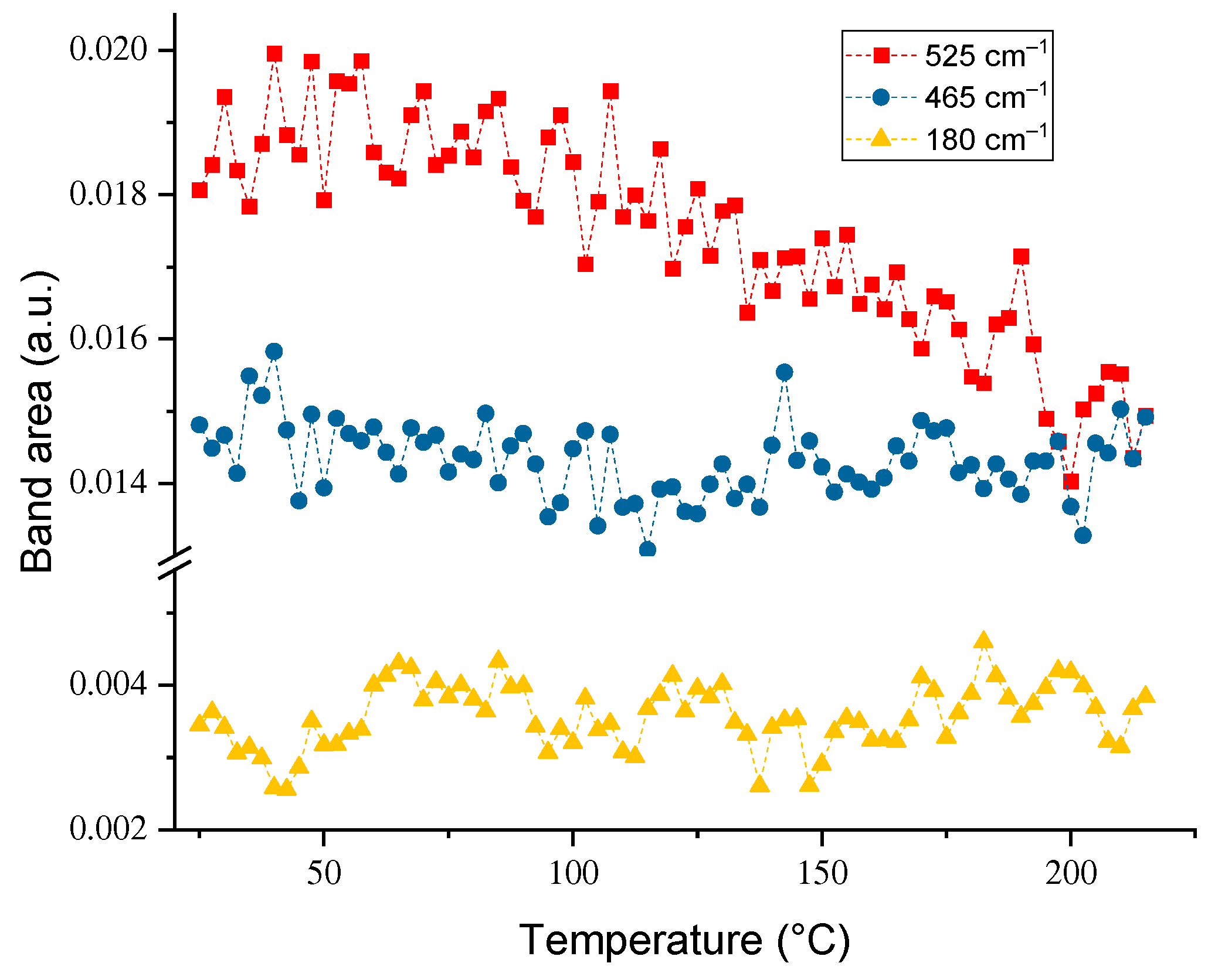
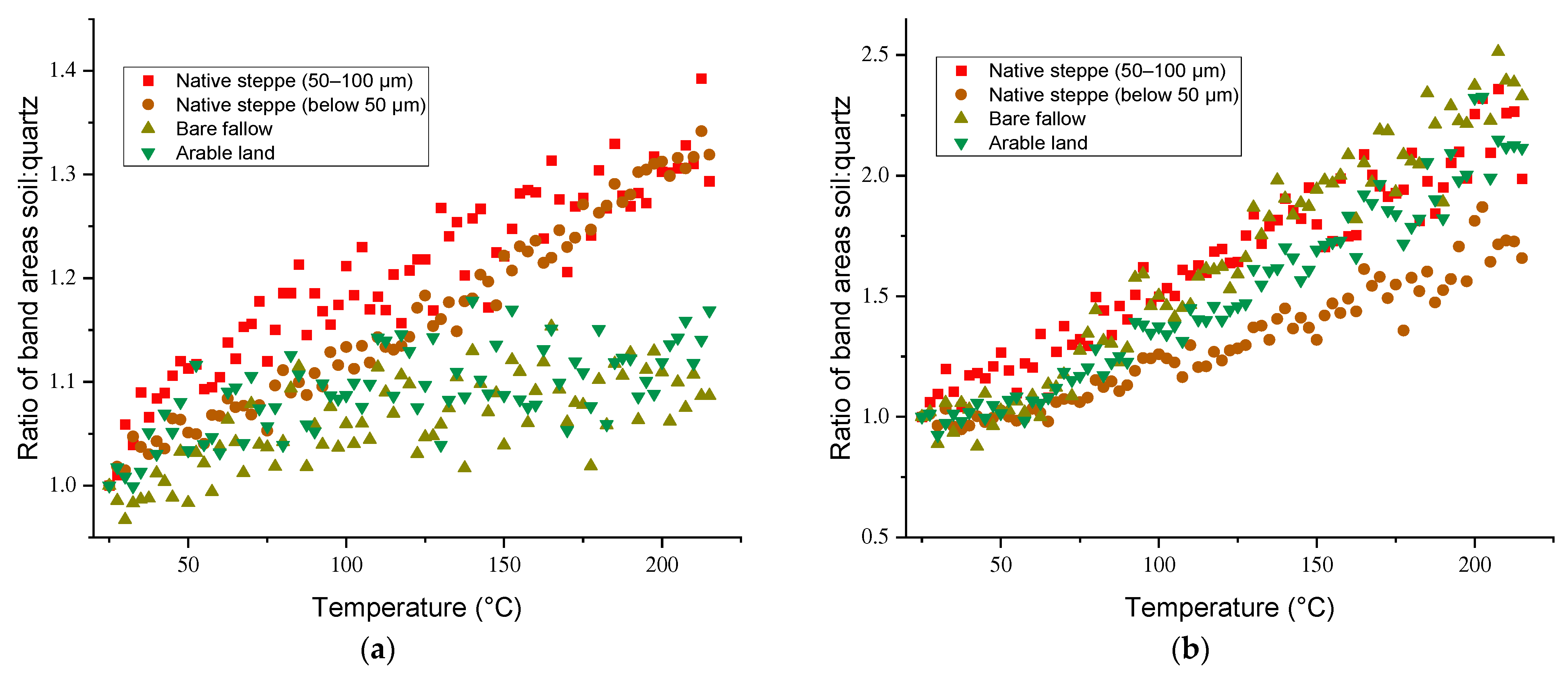
3.3. Temperature Dependences of Band Intensities
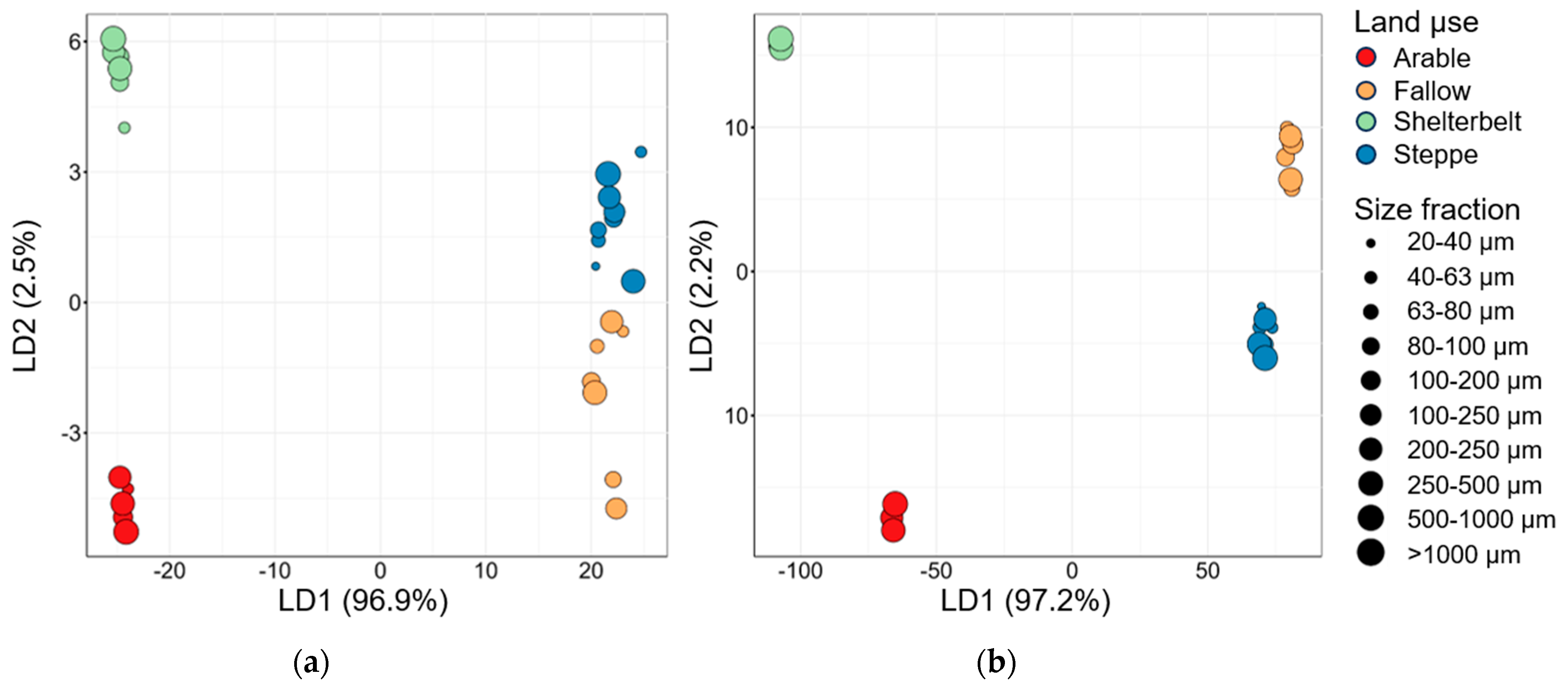
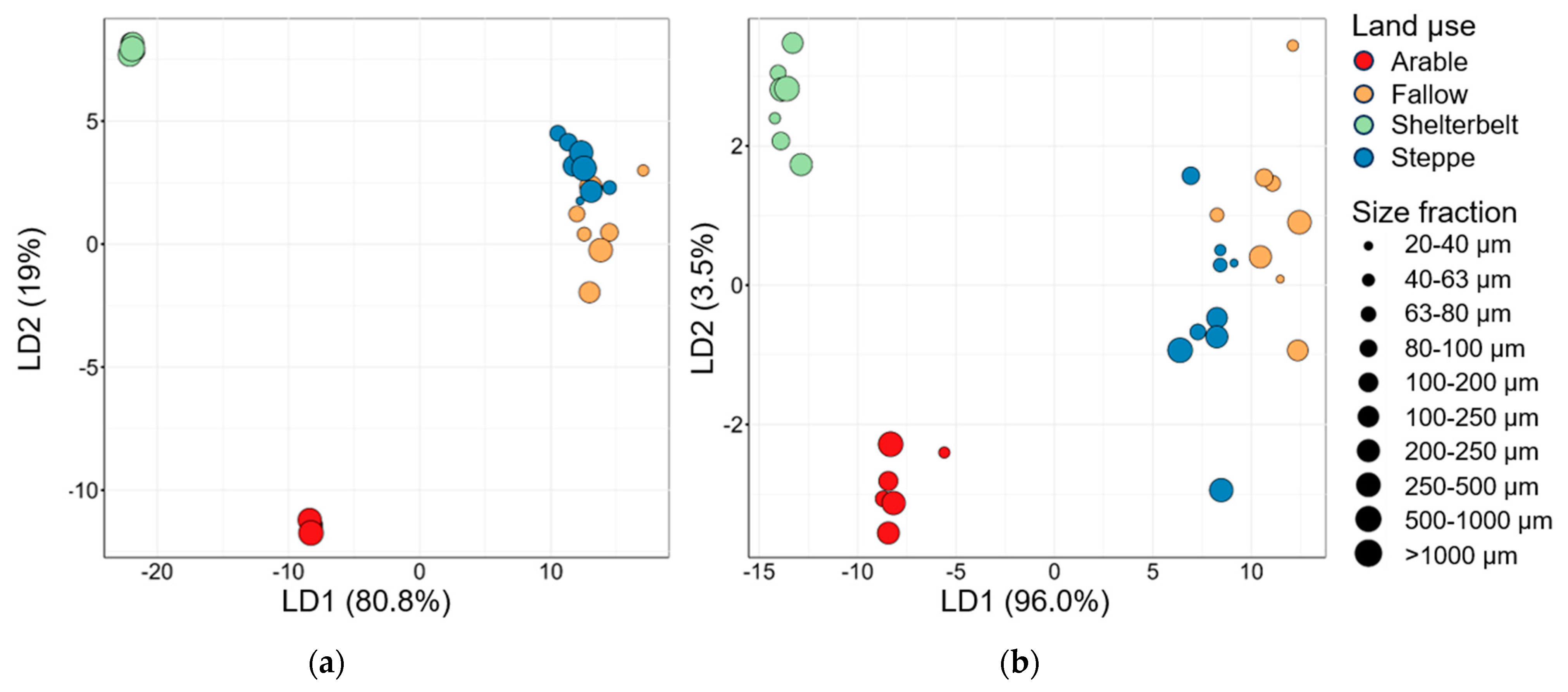
3.4. Linear Discrimination Analysis and Data Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imeson, A. Desertification, Land Degradation and Sustainability; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kononova, M.M. Soil Organic Matter, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1966. [Google Scholar]
- Huang, P.M.; Li, Y.; Sumner, M.E. (Eds.) Handbook of Soil Sciences. Properties and Processes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 1436. [Google Scholar]
- Gerke, J. The Central Role of Soil Organic Matter in Soil Fertility and Carbon Storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Jong van Lier, Q.D.; Durigon, A. Soil thermal diffusivity estimated from data of soil temperature and single soil component properties. Rev. Bras. De Ciência Do Solo 2013, 37, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Józefaciuk, G.; Sławiński, C.; Walczak, R.T.; Bieganowski, A. Review of Current Problems in Agrophysics; Institute of Agrophysics PAS: Lublin, Poland, 2005. [Google Scholar]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2017, 181, 104–136. [Google Scholar] [CrossRef] [Green Version]
- Chenu, C.; Plante, A.F. Clay-sized organo-mineral complexes in a cultivation chronosequence: Revisiting the concept of the ‘primary organo-mineral complex’. Eur. J. Soil Sci. 2006, 57, 596–607. [Google Scholar] [CrossRef]
- Chen, Y.; Tarchitzky, J. Organo-Mineral Complexes and their Effects on the Physico-Chemical Properties of Soils. In Carbon Stabilization by Clays in the Environment; Clay Minerals Society: Chantilly, VA, USA, 2009; pp. 32–49. [Google Scholar]
- Fultz, L.M.; Moore-Kucera, J.; Calderón, F.; Acosta-Martínez, V. Using Fourier-Transform Mid-Infrared Spectroscopy to Distinguish Soil Organic Matter Composition Dynamics in Aggregate Fractions of Two Agroecosystems. Soil Sci. Soc. Am. J. 2014, 78, 1940–1948. [Google Scholar] [CrossRef]
- Ge, Y.; Thomasson, J.A.; Morgan, C.L.S. Mid-infrared attenuated total reflectance spectroscopy for soil carbon and particle size determination. Geoderma 2014, 213, 57–63. [Google Scholar] [CrossRef]
- Tinti, A.; Tugnoli, V.; Bonora, S.; Francioso, O. Recent applications of vibrational mid-Infrared (IR) spectroscopy for studying soil components: A review. J. Cent. Eur. Agric. 2015, 16, 1–22. [Google Scholar] [CrossRef]
- Linker, R. Application of FTIR Spectroscopy to Agricultural Soils Analysis. In Fourier Transforms—New Analytical Approaches and FTIR Strategies; Nikolic, G., Ed.; InTech: Tokyo, Japan, 2011; pp. 385–404. [Google Scholar]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Tanykova, N.; Petrova, Y.; Kostina, J.; Kozlova, E.; Leushina, E.; Spasennykh, M. Study of Organic Matter of Unconventional Reservoirs by IR Spectroscopy and IR Microscopy. Geosciences 2021, 11, 277. [Google Scholar] [CrossRef]
- Artz, R.R.E.; Chapman, S.J.; Jean Robertson, A.H.; Potts, J.M.; Laggoun-Défarge, F.; Gogo, S.; Comont, L.; Disnar, J.-R.; Francez, A.-J. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem. 2008, 40, 515–527. [Google Scholar] [CrossRef]
- Unger, M.; Siesler, H.W. In situ orientation studies of a poly(3-hydroxybutyrate)/poly(epsilon-caprolactone) blend by rheo-optical fourier transform infrared spectroscopy and two-dimensional correlation spectroscopic analysis. Appl. Spectrosc. 2009, 63, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, E.; Ye, C.; Zhang, H.; Wu, P.; Noda, I.; Zhou, Q. Liquid-crystalline phase development of a mesogen-jacketed polymer-application of two-dimensional infrared correlation analysis. J. Phys. Chem. B 2005, 109, 6089–6095. [Google Scholar] [CrossRef] [PubMed]
- Arrondo, J.L.R.; Iloro, I.; Aguirre, J.; Goñi, F.M. A two-dimensional IR spectroscopic (2D-IR) simulation of protein conformational changes. J. Spectrosc. 2004, 18, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.B.; Wang, Q.; He, H.W.; Zhou, H.M. Protein thermal aggregation involves distinct regions: Sequential events in the heat-induced unfolding and aggregation of hemoglobin. Biophys. J. 2004, 86, 1682–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proskurnin, M.A.; Volkov, D.S.; Rogova, O.B. Two-Dimensional Correlation IR Spectroscopy of Humic Substances of Chernozem Size Fractions of Different Land Use. Agronomy 2023, 13, 1696. [Google Scholar] [CrossRef]
- Katon, J.E.; Phillips, D.B. Infrared Spectroscopy at Subambient Temperatures. Appl. Spectrosc. Rev. 1973, 7, 1–45. [Google Scholar] [CrossRef]
- Zallen, R.; Conwell, E.M. The effect of temperature on libron frequencies in molecular crystals: Implications for TTF-TCNQ. Solid State Commun. 1979, 31, 557–561. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Butler, I.S.; Kozinski, J.A. Supercritical Water Gasification of Lactose as a Model Compound for Valorization of Dairy Industry Effluents. Ind. Eng. Chem. Res. 2015, 54, 9296–9306. [Google Scholar] [CrossRef]
- Sirotiak, M.; Bartošová, A. Changes in Structure and Content Humic Substances in Soil During the Laboratory Simulated Fires. Trans. Všb—Tech. Univ. Ostrav. Saf. Eng. Ser. 2016, 11, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Proskurnin, M.A.; Volkov, D.S.; Rogova, O.B. Temperature Dependences of IR Spectral Bands of Humic Substances of Silicate-Based Soils. Agronomy 2023, 13, 1740. [Google Scholar] [CrossRef]
- Volkov, D.; Rogova, O.; Proskurnin, M. Temperature Dependences of IR Spectra of Humic Substances of Brown Coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Samofalova, I.A.; Rogova, O.B.; Luzyanina, O.A. Diagnostics of soils of different altitudinal vegetation belts in the Middle Urals according to group composition of iron compounds. Geogr. Nat. Resour. 2016, 37, 71–78. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N.; Rogova, O.B.; Pinskii, D.L. Application Of The Langmuir and Dubinin-Radushkevich Equations to the Description of Cu And Zn Adsorption In Rendzinas. Eurasian Soil Sci. 2000, 33, 1226–1233. [Google Scholar]
- Volkov, D.; Rogova, O.; Proskurnin, M. Organic Matter and Mineral Composition of Silicate Soils: FTIR Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection Modalities. Agronomy 2021, 11, 1879. [Google Scholar] [CrossRef]
- Krivoshein, P.K.; Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. FTIR photoacoustic spectroscopy for identification and assessment of soil components: Chernozems and their size fractions. Photoacoustics 2020, 18, 100162. [Google Scholar] [CrossRef]
- Kholodov, V.A.; Konstantinov, A.I.; Kudryavtsev, A.V.; Perminova, I.V. Structure of humic acids in zonal soils from 13C NMR data. Eurasian Soil Sci. 2011, 44, 976–983. [Google Scholar] [CrossRef]
- Rogova, O.B.; Kolobova, N.A.; Ivanov, A.L. Phosphorus Sorption Capacity of Gray Forest Soil as Dependent on Fertilization System. Eurasian Soil Sci. 2018, 51, 536–541. [Google Scholar] [CrossRef]
- Sukhoveeva, O.E.; Zolotukhin, A.N.; Karelin, D.V. Climate-Determined Changes of Organic Carbon Stocks in the Arable Chernozem of Kursk Region. Arid. Ecosyst. 2020, 10, 148–155. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Post, C.J. Effects of land use on soil inorganic carbon stocks in the Russian Chernozem. J. Environ. Qual. 2006, 35, 1384–1388. [Google Scholar] [CrossRef]
- Krylov, V.A.; Mamontov, V.G.; Lazarev, V.I.; Ryzhkov, O.V. The Influence of Different Land Uses on the Elemental Composition of Labile Humus Substances in Typical Chernozem Typical of Kursk Oblast. Eurasian Soil Sci. 2022, 55, 1033–1040. [Google Scholar] [CrossRef]
- Artemyeva, Z.; Danchenko, N.; Kolyagin, Y.; Kirillova, N.; Kogut, B. Chemical structure of soil organic matter and its role in aggregate formation in Haplic Chernozem under the contrasting land use variants. Catena 2021, 204, 105403. [Google Scholar] [CrossRef]
- Shein, E.V.; Lazarev, V.I.; Aidiev, A.Y.; Sakunkonchak, T.; Kuznetsov, M.Y.; Milanovskii, E.Y.; Khaidapova, D.D. Changes in the physical properties of typical chernozems of Kursk oblast under the conditions of a long-term stationary experiment. Eurasian Soil Sci. 2011, 44, 1097–1103. [Google Scholar] [CrossRef]
- Kuznetsova, I.V. Changes in the physical status of the typical and leached chernozems of Kursk oblast within 40 years. Eurasian Soil Sci. 2013, 46, 393–400. [Google Scholar] [CrossRef]
- Krylov, V.A.; Mamontov, V.G. The Impact of Different Cenoses on the Thermal Characteristics of Labile Humic Substances of Typical Chernozem in Kursk Oblast. Eurasian Soil Sci. 2022, 55, 452–459. [Google Scholar] [CrossRef]
- Ovechkin, S.V.; Bazykina, G.S. The carbonate profile and water regime of migrational-mycelial chernozems in different ecosystems of Kursk oblast. Eurasian Soil Sci. 2011, 44, 1352–1363. [Google Scholar] [CrossRef]
- Arkhangel’skaya, T.A.; Velichenko, M.V.; Tikhonravova, P.I. Thermal properties of typical chernozems in Kursk Oblast. Eurasian Soil Sci. 2016, 49, 1109–1116. [Google Scholar] [CrossRef]
- Dubovik, E.V.; Dubovik, D.V. Relationships between the Organic Carbon Content and Structural State of Typical Chernozem. Eurasian Soil Sci. 2019, 52, 150–161. [Google Scholar] [CrossRef]
- Mamontov, V.G.; Rodionova, L.P.; Artemieva, Z.S.; Krylov, V.A.; Klyshbekov, G.K. Agrogenic and postagrogenic transformation of the structure of typical chernozem Kursk region. Mezhdunarodnyi Sel’skokhozyaistvennyi Zhurnal 2019, 62, 35–39. [Google Scholar] [CrossRef]
- Kholodov, V.A.; Yaroslavtseva, N.V.; Farkhodov, Y.R.; Belobrov, V.P.; Yudin, S.A.; Aydiev, A.Y.; Lazarev, V.I.; Frid, A.S. Changes in the Ratio of Aggregate Fractions in Humus Horizons of Chernozems in Response to the Type of Their Use. Eurasian Soil Sci. 2019, 52, 162–170. [Google Scholar] [CrossRef]
- Kholodov, V.A.; Yaroslavtseva, N.V.; Yashin, M.A.; Frid, A.S.; Lazarev, V.I.; Tyugai, Z.N.; Milanovskiy, E.Y. Contact angles of wetting and water stability of soil structure. Eurasian Soil Sci. 2015, 48, 600–607. [Google Scholar] [CrossRef]
- Khaidapova, D.D.; Chestnova, V.V.; Shein, E.V.; Milanovskii, E.Y. Rheological properties of typical chernozems (Kursk oblast) under different land uses. Eurasian Soil Sci. 2016, 49, 890–897. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Bryant, R.B.; Vassenev, I.I.; Schwager, S.J.; Post, C.J. Cultivation Effects on Soil Carbon and Nitrogen Contents at Depth in the Russian Chernozem. Soil Sci. Soc. Am. J. 2000, 64, 738–745. [Google Scholar] [CrossRef]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A.; Farkhodov, Y.R.; Markeeva, L.B. Thermal stability of organic matter of typical chernozems under different land uses. Soil Tillage Res. 2020, 197, 104500. [Google Scholar] [CrossRef]
- Krivoshein, P.K.; Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. FTIR Photoacoustic and ATR Spectroscopies of Soils with Aggregate Size Fractionation by Dry Sieving. ACS Omega 2022, 7, 2177–2197. [Google Scholar] [CrossRef]
- Figueroa-Cisterna, J.; Bagur-Gonzalez, M.G.; Morales-Ruano, S.; Carrillo-Rosua, J.; Martin-Peinado, F. The use of a combined portable X ray fluorescence and multivariate statistical methods to assess a validated macroscopic rock samples classification in an ore exploration survey. Talanta 2011, 85, 2307–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Weihs, C.; Ligges, U.; Luebke, K.; Raabe, N. klaR Analyzing German Business Cycles. In Data Analysis and Decision Support; Baier, D., Decker, R., Schmidt-Thieme, L., Eds.; Studies in Classification, Data Analysis, and Knowledge Organization; Springer: Berlin/Heidelberg, Germany, 2005; pp. 335–343. [Google Scholar]
- Wickham, H. ggplot2; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Garczarek, U. Classification Rules in Standardized Partition Spaces; Universität Dortmund: Dortmund, Germany, 2002. [Google Scholar]
- Kronenberg, A.K. Chapter 4. Hydrogen Speciation and Chemical Weakening of Quartz. In Silica; De Gruyter: Berlin, Germany, 1994; pp. 123–176. [Google Scholar]
- Russell, J.D.; Fraser, A.R. Infrared methods. In Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Wilson, M.J., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 11–67. [Google Scholar]
- Madejová, J. Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Raichlin, Y.; Millo, A.; Katzir, A. Investigations of the structure of water using mid-IR fiberoptic evanescent wave spectroscopy. Phys. Rev. Lett. 2004, 93, 185703. [Google Scholar] [CrossRef]
- Calderón, F.J.; Mikha, M.M.; Vigil, M.F.; Nielsen, D.C.; Benjamin, J.G.; Reeves, J.B. Diffuse-Reflectance Mid-infrared Spectral Properties of Soils under Alternative Crop Rotations in a Semi-arid Climate. Commun. Soil Sci. Plant Anal. 2011, 42, 2143–2159. [Google Scholar] [CrossRef]
- Calderón, F.J.; Reeves, J.B.; Collins, H.P.; Paul, E.A. Chemical Differences in Soil Organic Matter Fractions Determined by Diffuse-Reflectance Mid-Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2011, 75, 568–579. [Google Scholar] [CrossRef] [Green Version]
- Changwen, D.; Jing, D.; Jianmin, Z.; Huoyan, W.; Xiaoqin, C. Characterization of Greenhouse Soil Properties Using Mid-infrared Photoacoustic Spectroscopy. Spectrosc. Lett. 2011, 44, 359–368. [Google Scholar] [CrossRef]
- Changwen, D.; Jianmin, Z.; Goyne, K.W. Organic and inorganic carbon in paddy soil as evaluated by mid-infrared photoacoustic spectroscopy. PLoS ONE 2012, 7, e43368. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Janik, L.J.; Raupach, M. Diffuse reflectance infrared fourier transform (DRIFT) spectroscopy in soil studies. Soil. Res. 1991, 29, 49–67. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Bowey, J.E. Quantitative Infrared Spectra of Hydrosilicates and Related Minerals. Mon. Not. R. Astron. Soc. 2006, 367, 577–591. [Google Scholar] [CrossRef]
- Bock, J.A.N.; Su, G.-J. Interpretation of the Infrared Spectra of Fused Silica. J. Am. Ceram. Soc. 1970, 53, 69–73. [Google Scholar] [CrossRef]
- Spitzer, W.G.; Kleinman, D.A. Infrared Lattice Bands of Quartz. Phys. Rev. 1961, 121, 1324–1335. [Google Scholar] [CrossRef]
- Fung, M.F.K.; Senterman, M.K.; Mikhael, N.Z.; Lacelle, S.; Wong, P.T.T. Pressure-tuning fourier transform infrared spectroscopic study of carcinogenesis in human endometrium. Biospectroscopy 1998, 2, 155–165. [Google Scholar] [CrossRef]
- Wang, H.P.; Wang, H.C.; Huang, Y.J. Microscopic FTIR studies of lung cancer cells in pleural fluid. Sci. Total Environ. 1997, 204, 283–287. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Koike, C.; Noguchi, R.; Chihara, H.; Suto, H.; Ohtaka, O.; Imai, Y.; Matsumoto, T.; Tsuchiyama, A. Infrared Spectra of Silica Polymorphs and the Conditions of Their Formation. Astrophys. J. 2013, 778, 60. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Watanabe, T. Infrared Spectra of Interstratified Illite/Smectite from Hydrothermally Altered Tuffs (Shinzan, Japan) and Diagenetic Bentonites (Kinnekulle, Sweden). Clay Sci. 1989, 7, 263–275. [Google Scholar] [CrossRef]
- Ahmed, S.; Pasti, A.; Fernandez-Teran, R.J.; Ciardi, G.; Shalit, A.; Hamm, P. Aqueous solvation from the water perspective. J. Chem. Phys. 2018, 148, 234505. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-G.; Nyman, G. The Infrared and UV-Visible Spectra of Polycyclic Aromatic Hydrocarbons Containing (5, 7)-Member Ring Defects: A Theoretical Study. Astrophys. J. 2012, 751, 3. [Google Scholar] [CrossRef]
- Workman, J. The Handbook of Organic Compounds, Three-Volume Set: NIR, IR, R, and UV-Vis Spectra Featuring Polymers and Surfactants; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- San Andrés, E.; del Prado, A.; Mártil, I.; González-Díaz, G.; Bravo, D.; López, F.J.; Fernández, M.; Bohne, W.; Röhrich, J.; Selle, B.; et al. Bonding configuration and density of defects of SiOxHy thin films deposited by the electron cyclotron resonance plasma method. J. Appl. Phys. 2003, 94, 7462–7469. [Google Scholar] [CrossRef]
- Tong, Y.; Kampfrath, T.; Campen, R.K. Experimentally probing the libration of interfacial water: The rotational potential of water is stiffer at the air/water interface than in bulk liquid. Phys. Chem. Chem. Phys. 2016, 18, 18424–18430. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.P.; Song, R.; Mou, B.; Li, K.P.; Chiang, P.; Wang, D.; Tse, W.S.; Ho, L.T. Fourier transform Raman spectroscopy of carcinogenic polycyclic aromatic hydrocarbons in biological systems: Binding to heme proteins. J. Raman Spectrosc. 1999, 30, 551–555. [Google Scholar] [CrossRef]
- Schenzel, K.; Almlöf, H.; Germgård, U. Quantitative analysis of the transformation process of cellulose I → cellulose II using NIR FT Raman spectroscopy and chemometric methods. Cellulose 2009, 16, 407–415. [Google Scholar] [CrossRef]
- Zelsmann, H.R. Temperature dependence of the optical constants for liquid H2O and D2O in the far IR region. J. Mol. Struct. 1995, 350, 95–114. [Google Scholar] [CrossRef]
- Max, J.J.; Chapados, C. Isotope effects in liquid water by infrared spectroscopy. III. H2O and D2O spectra from 6000 to 0 cm–1. J. Chem. Phys. 2009, 131, 184505. [Google Scholar] [CrossRef]
- Baes, A.U.; Bloom, P.R. Diffuse Reflectance and Transmission Fourier Transform Infrared (DRIFT) Spectroscopy of Humic and Fulvic Acids. Soil Sci. Soc. Am. J. 1989, 53, 695–700. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy; Elsevier Science: San Diego, CA, USA, 1990. [Google Scholar]
- Kholodov, V.A.; Yaroslavtseva, N.V.; Konstantinov, A.I.; Perminova, I.V. Preparative yield and properties of humic acids obtained by sequential alkaline extractions. Eurasian Soil Sci. 2015, 48, 1101–1109. [Google Scholar] [CrossRef]
- Day, K.L. Temperature Dependence of Mid-Infrared Silicate Absorption. Astrophys. J. 1976, 203, L99. [Google Scholar] [CrossRef]
- Chan, T.F.; Su, J.H.; Chung, Y.F.; Chang, H.L.; Yuan, S.S. Decreased serum leptin levels in women with uterine leiomyomas. Acta Obstet. Gynecol. Scand. 2003, 82, 173–176. [Google Scholar] [CrossRef]
- Boguta, P.; Sokolowska, Z.; Skic, K. Use of thermal analysis coupled with differential scanning calorimetry, quadrupole mass spectrometry and infrared spectroscopy (TG-DSC-QMS-FTIR) to monitor chemical properties and thermal stability of fulvic and humic acids. PLoS ONE 2017, 12, e0189653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasimova, М.I.; Bronnikova, М.A.; Khitrov, N.B.; Shorkunov, I.G. Hierarchical morphogenetic analysis of Kursk chernozem. Dokuchaev Soil Bull. 2016, 86, 64–76. [Google Scholar] [CrossRef]
- Bazykina, G.S.; Ovechkin, S.V. Migration-mycelial chernozems in biospheric cycles within the Kursk region. Dokuchaev Soil Bull. 2012, 70, 3–18. [Google Scholar] [CrossRef]
- Chendev, Y.G.; Sauer, T.J.; Ramirez, G.H.; Burras, C.L. History of East European Chernozem Soil Degradation; Protection and Restoration by Tree Windbreaks in the Russian Steppe. Sustainability 2015, 7, 705–724. [Google Scholar] [CrossRef] [Green Version]
- Lützow, M.v.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Role of Hydrophobic Components of Soil Organic Matter in Soil Aggregate Stability. Soil Sci. Soc. Am. J. 1999, 63, 1801–1810. [Google Scholar] [CrossRef]
- Shein, E.V.; Milanovskiy, E. The Role of Organic Matter in the Formation and Stability of Soil Aggregates. Eurasian Soil Sci. 2003, 36, 51–58. [Google Scholar]
- Andriopoulou, N.C.; Christidis, G.E. Multi-analytical characterisation of wheat biominerals: Impact of methods of extraction on the mineralogy and chemistry of phytoliths. Archaeol. Anthropol. Sci. 2020, 12, 186. [Google Scholar] [CrossRef]
- Silantyeva, M.; Solomonova, M.; Speranskaja, N.; Blinnikov, M.S. Phytoliths of temperate forest-steppe: A case study from the Altay, Russia. Rev. Palaeobot. Palynol. 2018, 250, 1–15. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; Van Damme, S.; Meire, P.; Conley, D.J. The Global Biogeochemical Silicon Cycle. Silicon 2010, 1, 207–213. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Wang, H.; Wang, C. The effects of chemical composition and distribution on the preservation of phytolith morphology. Appl. Phys. A 2013, 114, 503–507. [Google Scholar] [CrossRef]
- Haynes, R.J. The nature of biogenic Si and its potential role in Si supply in agricultural soils. Agric. Ecosyst. Environ. 2017, 245, 100–111. [Google Scholar] [CrossRef]
- Shillito, L.M.; Almond, M.J.; Nicholson, J.; Pantos, M.; Matthews, W. Rapid characterisation of archaeological midden components using FT-IR spectroscopy, SEM-EDX and micro-XRD. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 133–139. [Google Scholar] [CrossRef]
- Zancajo, V.M.R.; Diehn, S.; Filiba, N.; Goobes, G.; Kneipp, J.; Elbaum, R. Spectroscopic Discrimination of Sorghum Silica Phytoliths. Front. Plant Sci. 2019, 10, 1571. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, M.; Gnappi, G.; Montenero, A.; Bersani, D.; Lottici, P.P.; Kaciulis, S.; Mattogno, G.; Fini, M. Hydroxy- and fluorapatite films on Ti alloy substrates: Sol-gel preparation and characterization. J. Mater. Sci. 2001, 36, 3253–3260. [Google Scholar] [CrossRef]
- Noda, L.; Sensato, F.; Gonçalves, N. Titanyl Sulphate, an Inorganic Polymer: Structural Studies and Vibrational Assignment. Química Nova 2019, 42, 1112–1115. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Schiavon, M.; Francioso, O.; Dalla Vecchia, F.; Ertani, A.; Nardi, S. Bioactivity of Size-Fractionated and Unfractionated Humic Substances from Two Forest Soils and Comparative Effects on N and S Metabolism, Nutrition, and Root Anatomy of Allium sativum L. Front. Plant Sci. 2020, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Hamkalo, Z.; Bedernichek, T. Labile pool of soil organic matter as an indicator of its ecological quality under different land use conditions. Ekosyistemy Ikh Optim. I Okhrana (Optim. Prot. Ecosyst.) 2014, 10, 193–200. [Google Scholar]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Photoacoustic and photothermal methods in spectroscopy and characterization of soils and soil organic matter. Photoacoustics 2020, 17, 100151. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jin, S.; Noda, I.; Jung, Y.M. Continuing progress in the field of two-dimensional correlation spectroscopy (2D-COS), part I. Yesterday and today. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 281, 121573. [Google Scholar] [CrossRef]
- Park, Y.; Noda, I.; Jung, Y.M. Novel developments and applications of two-dimensional correlation spectroscopy. J. Mol. Struct. 2016, 1124, 11–28. [Google Scholar] [CrossRef]


| Band Maximum *, cm−1 | Band Intensities and Changes of Integral Intensity with Temperature * | SiO2/Mineral and Water Constituents | Organic Matter |
|---|---|---|---|
| 3690, 3620 | m ↑ | SiO–H…OH2 stretch (amorph.), kaolin [59,60,61] | n/a |
| 3520–3370 | s ↓ | water, v3 | O–H: phenolic, alcohol, carboxylic |
| 3350 | s ↓ | SiO–H… OH2 SiO–H stretch (α-quartz) [59]; water, v1, v3, and v2 overtone [62] | n/a |
| 3270 | s ↑ | water, v1 | O–H, phenolic, alcohol, carboxylic C=O overtone |
| B 2920, B 2860–2850 | w ↑ | n/a | C–H, CH2 stretch [63,64,65,66] |
| 1845 Q, 1790–1775 Q | w = | SiO2 combination | C=O stretching |
| R 1740 | w = | n/a | C=O stretching |
| R 1628 | s ↓ | SiOH…HOH; HO–H stretching (amorph.) [59]; water, v2 | N–H bending, C=O stretching |
| 1580 Q | w = | SiO2 overtone | C–C stretching, aromatic rings |
| 1450–1400 | m = | SiO2 amorph. combination; carbonates [67] | O–H, C–H scissoring |
| R 1360 | w = | SiO2 combination [68] | n/a |
| R 1265 | m ↑ | SiO2 combination | Amide III, C–O stretching, aromatic rings and carboxylic acids [65], CH2 rocking; C–N stretching |
| R 1175 Q | w ↓ | SiO2 combination [68], amorph. [69] | CH2 wagging |
| B 1153–1163 Q | s ↑ | SiO2 lattice [70] | C–O of carbohydrates; H-bonded stretching of C–OH; CH deformation [71,72,73] |
| 1120 | m = | amorph. silica [74] | n/a |
| B 1100–1080 (sh) Q | s = | O–Si–O lattice stretch [70] | in plane C–H bending (aromatic); C=C bending |
| 1070 Q | s ↓ | SiO2 (kaolinite, illite) O–Si–O lattice, stretching [70] | n/a |
| R 1037 (sh) Q | s ↓ | silicate (kaolinite, illite) Si–O as stretching; Al–O stretching [67] | in plane C–H bending (non-aromatic) and (?) carbohydrates |
| 1000 | s ↓ | SiO2 Si–O stretching, lattice | in plane C–H bending (non-aromatic) and (?) carbohydrates; carboxyl out-of-plane C–O–H bending |
| 930–910 (sh) | s ↓ | silicate, aluminosilicates, overtone [69] | n/a |
| B 830 (sh) | w ↓ | Al–OH (clay minerals), smectite, illite [75] | Cellulose |
| R 796 Q | s ↓ | SiO2 silicate, lattice s Si–O–Si stretching [69,70] | out-of-plane C–H bend (non-aromatic) |
| R 776–775 Q | s ↓ | α-quartz [70,74]; water librations, wagging (L3) [76] | (?) polyaromatic; –C–H bending (substituted) |
| R 750–740 (sh) | w = | Mg–OH, Al–OH (clay minerals) | polyaromatic [77]; out of plane –C–H bending (aromatic); in-phase rocking of alkanes C4+ [78] |
| R 722 (sh) | w = | (?) water, librations | out-of-plane –C–H bend (aromatic); in-phase rocking of alkanes C4+ [78] |
| R 697 Q | m = | SiO2 Si–O–Si bend [70] | aromatic, polyaromatic |
| R 650 | m = | Kaolin, clinochlore, Mg-Al-silicates | n/a |
| R 625 Q | w = | Structural vibrations, quartz [79]; water, librations, rocking (L2) [76,80] | non-carboxylic, out-of-plane C–O–H bend; –C–H out-of-plane bending, in biosystems [81] |
| 530–520 Q | m ↓ | α-quartz (?) [70] kaolinite silicate O–Si–O bend [70], | n/a |
| 460 Q | s = | SiO2 O–Si–O bending [69]; O–Al–O [67] | n/a |
| R 445 Q | s = | SiO2 O–Si–O bend lattice [69,70] | C–C–C bending [82] |
| 430–420 Q | s ↑ | Mg–OH, Al–OH (clay minerals) water, librations twisting (L1) [76] | C–C–C bending [82] |
| 394 Q | s = | SiO2 O–Si–O, bend lattice [70]; water, librations, twisting mode (L1) [83] | n/a |
| B 368–364 Q | s ↓ | R(SiO4) [68]; amorph. silica [69]; SiO2 lattice | n/a |
| B 330 Q | w = | α-quartz, kaolin (?) Mg–O stretch [68] | n/a |
| 303 | w = | montmorillonite | n/a |
| 279 | w = | (?) amorphous silica | n/a |
| R 263–258 Q | s ↓ | α-quartz [74] | n/a |
| 228 | w = | α-quartz [74] | n/a |
| 190–180 | w = | α-quartz [74]; hydrogen bond stretching | n/a |
| 140 | w = | α-quartz [74] | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proskurnin, M.A.; Volkov, D.S.; Timofeev, Y.V.; Fomin, D.S.; Rogova, O.B. Chernozem Land Use Differentiation by Temperature-Dependent IR Spectra. Agronomy 2023, 13, 1967. https://doi.org/10.3390/agronomy13081967
Proskurnin MA, Volkov DS, Timofeev YV, Fomin DS, Rogova OB. Chernozem Land Use Differentiation by Temperature-Dependent IR Spectra. Agronomy. 2023; 13(8):1967. https://doi.org/10.3390/agronomy13081967
Chicago/Turabian StyleProskurnin, Mikhail A., Dmitry S. Volkov, Yaroslav V. Timofeev, Dmitry S. Fomin, and Olga B. Rogova. 2023. "Chernozem Land Use Differentiation by Temperature-Dependent IR Spectra" Agronomy 13, no. 8: 1967. https://doi.org/10.3390/agronomy13081967





