Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Gas Sampling
2.1.1. Soil Sample Collection and Soil Physicochemical Properties Analysis
2.1.2. Measurement of Soil Respiration
2.2. High-Throughput DNA Sequencing
2.3. Data Processing
3. Results
3.1. Physicochemical Properties of Paddy Soils in Karst and Non-Karst Areas
3.2. In Situ Soil Respiration and Cumulative CO2 Emissions of Paddy Fields during Rice Growth in KA and NKA
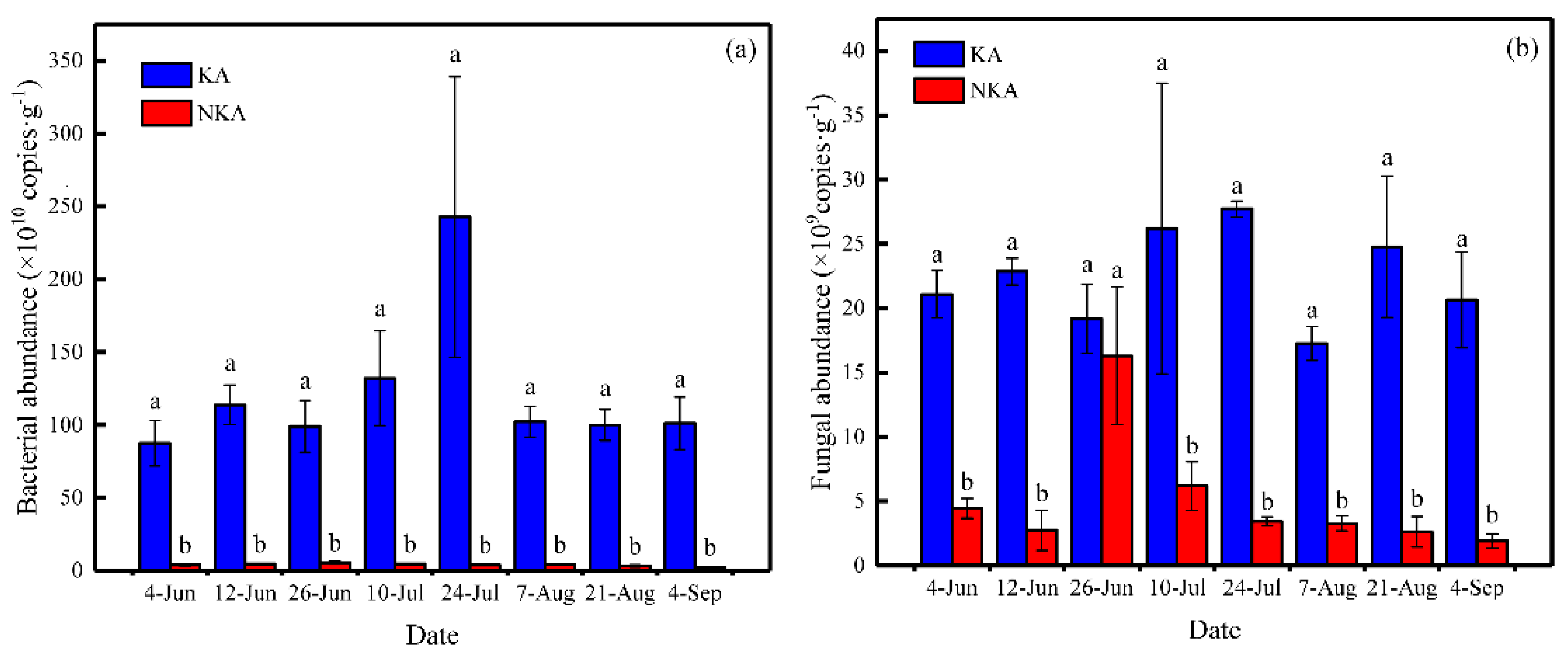
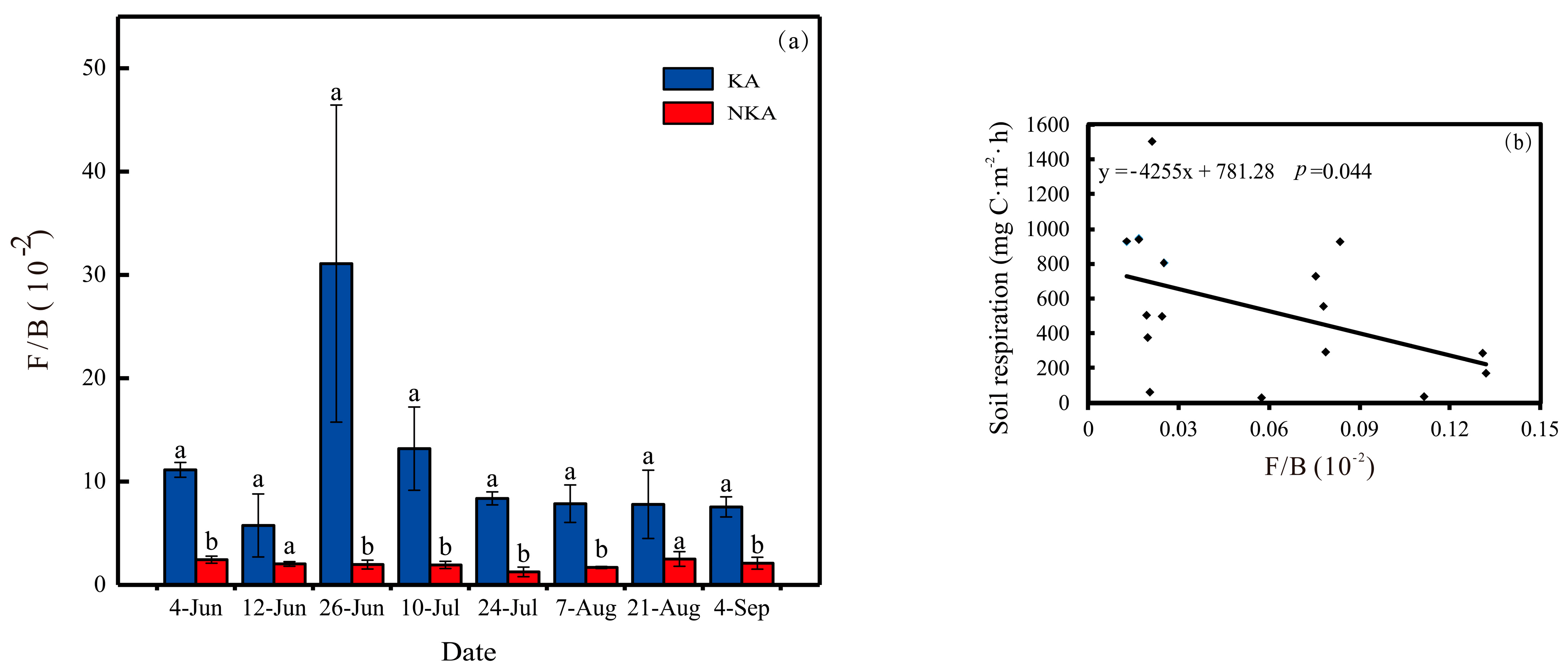
3.3. The Abundance of Bacteria and Fungi and the Ratio of Fungi to Bacteria (F/B) in Paddy Field Soils of KA and NKA
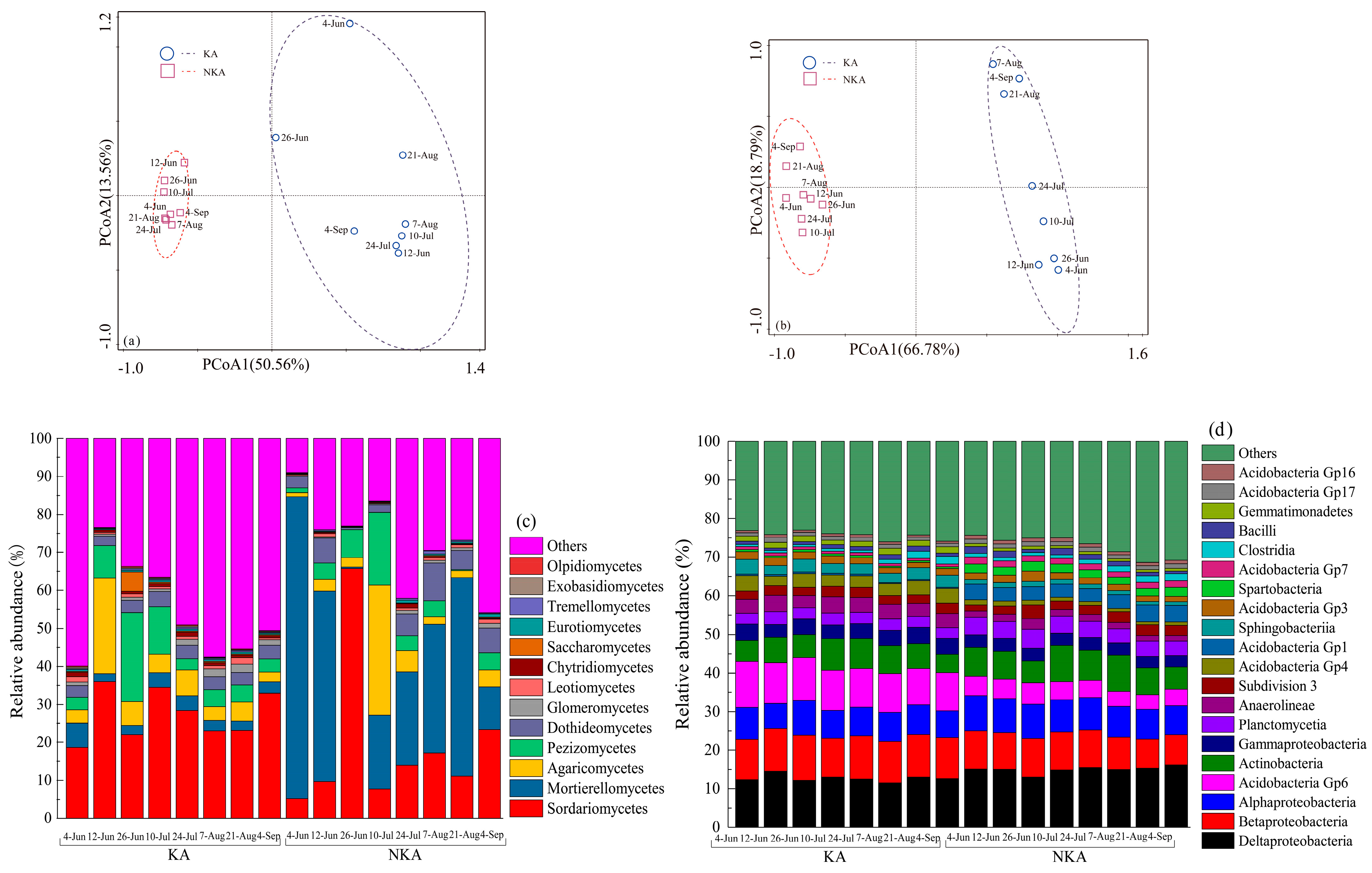
3.4. Variation in Fungal and Bacterial Communities in Paddy Fields of KA and NKA
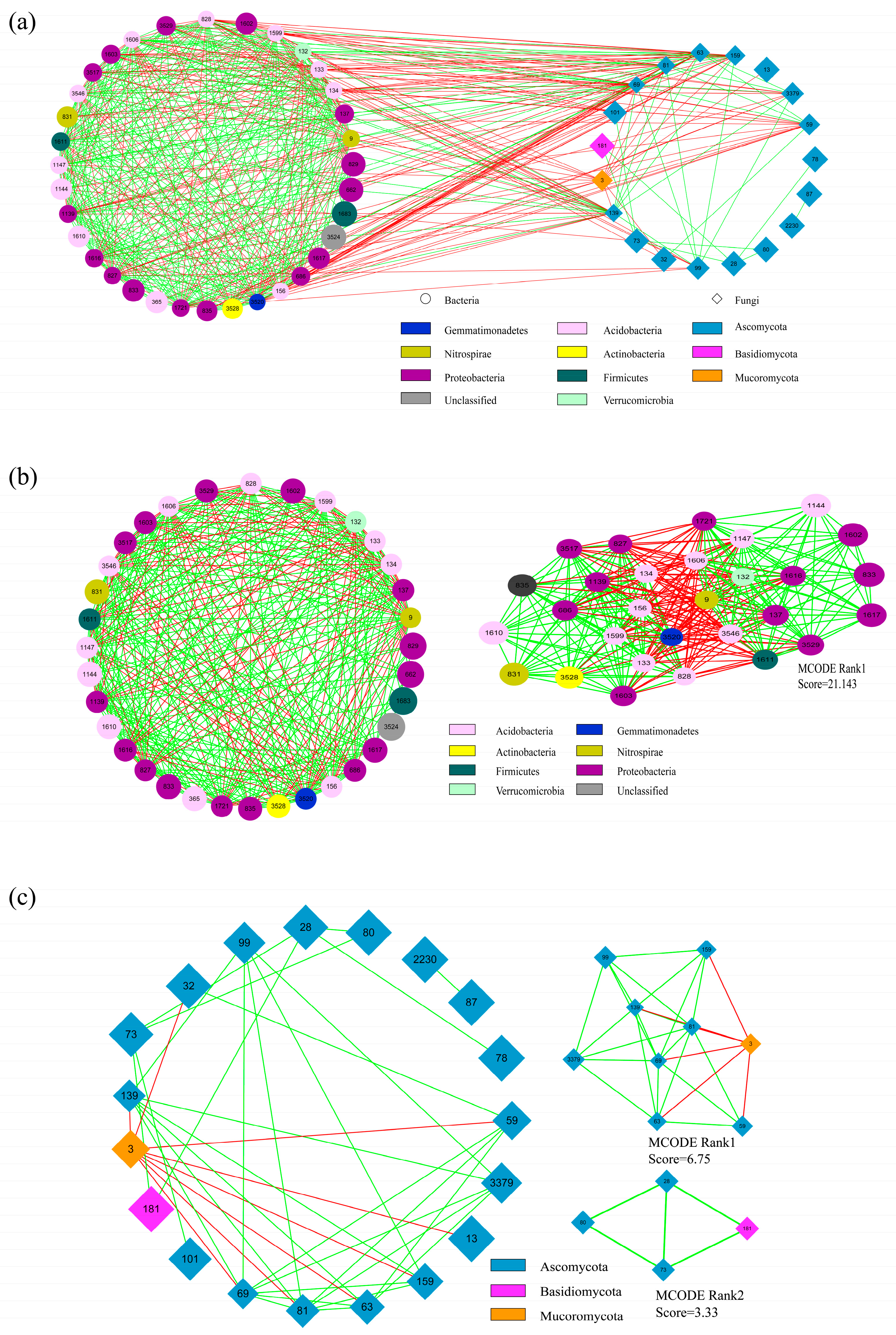
3.5. Co-Occurrence Network Analysis of Fungal and Bacterial Taxa in Rice Field Soils
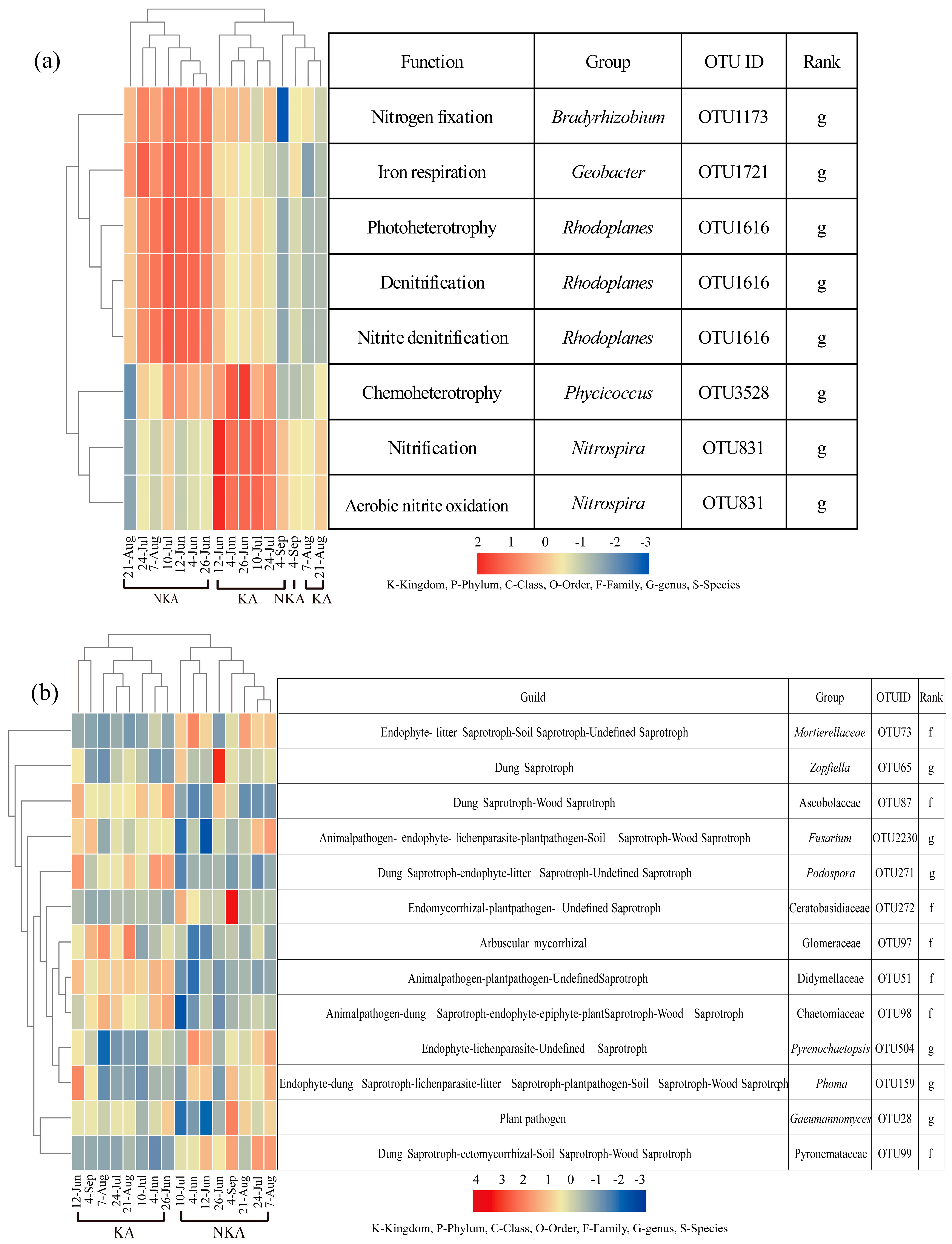
3.6. Correlational Analysis of Soil Respiration with Fungal and Bacterial Functional Groups
4. Discussion
4.1. Effects of the F/B on In Situ CO2 Fluxes
4.2. Effect of Fungal and Background Community Composition On Paddy Field In Situ CO2
4.3. Effects of Fungal and Bacterial Functional Groups on Paddy Field In Situ CO2 Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst Landscapes of China: Patterns, Ecosystem Processes and Services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Y.; Wu, F.; Liu, W.; Ning, Y.; Huang, Z.; Tang, S.; Liang, Y. Plant Adaptability in Karst Regions. J. Plant Res. 2021, 134, 889–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dreybrodt, W. Significance of the Carbon Sink Produced by H2O-Carbonate-CO2-Aquatic Phototroph Interaction on Land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Abbaszadeh, M.; Nasiri, M.; Riazi, M. Experimental Investigation of the Impact of Rock Dissolution on Carbonate Rock Properties in the Presence of Carbonated Water. Environ. Earth Sci. 2016, 75, 791. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Z.; Kaufmann, G. Sensitivity of the Global Carbonate Weathering Carbon-Sink Flux to Climate and Land-Use Changes. Nat. Commun. 2019, 10, 5749. [Google Scholar] [CrossRef]
- Dass, P.; Houlton, B.Z.; Wang, Y.; Warlind, D.; Morford, S. Bedrock Weathering Controls on Terrestrial Carbon-Nitrogen-Climate Interactions. Glob. Biogeochem. Cycle 2021, 35, e2020GB006933. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process Effect of Microalgal-Carbon Dioxide Fixation and Biomass Production: A Review. Renew. Sustain. Energ. Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Sha, Z.; Bai, Y.; Li, R.; Lan, H.; Zhang, X.; Li, J.; Liu, X.; Chang, S.; Xie, Y. The Global Carbon Sink Potential of Terrestrial Vegetation Can Be Increased Substantially by Optimal Land Management. Commun. Earth Environ. 2022, 3, 8. [Google Scholar] [CrossRef]
- Huang, W.; Han, T.; Liu, J.; Wang, G.; Zhou, G. Changes in Soil Respiration Components and Their Specific Respiration along Three Successional Forests in the Subtropics. Funct. Ecol. 2016, 30, 1466–1474. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-Associated Increases in the Global Soil Respiration Record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Su, Y.G.; Huang, G.; Lin, Y.J.; Zhang, Y.M. No Synergistic Effects of Water and Nitrogen Addition on Soil Microbial Communities and Soil Respiration in a Temperate Desert. Catena 2016, 142, 126–133. [Google Scholar] [CrossRef]
- Jian, J.; Steele, M.K.; Thomas, R.Q.; Day, S.D.; Hodges, S.C. Constraining Estimates of Global Soil Respiration by Quantifying Sources of Variability. Glob. Change Biol. 2018, 24, 4143–4159. [Google Scholar] [CrossRef]
- Moinet, G.Y.K.; Cieraad, E.; Hunt, J.E.; Fraser, A.; Turnbull, M.H.; Whitehead, D. Soil Heterotrophic Respiration Is Insensitive to Changes in Soil Water Content but Related to Microbial Access to Organic Matter. Geoderma 2016, 274, 68–78. [Google Scholar] [CrossRef]
- Ming, L.; Ekschmitt, K.; Bin, Z.; Holzhauer, S.I.J.; Zhong-pei, L.; Tao-lin, Z.; Rauch, S. Effect of Intensive Inorganic Fertilizer Application on Microbial Properties in a Paddy Soil of Subtropical China. Agric. Sci. China 2011, 10, 1758–1764. [Google Scholar] [CrossRef]
- Chen, X.; Xia, Y.; Rui, Y.; Ning, Z.; Hu, Y.; Tang, H.; He, H.; Li, H.; Kuzyakov, Y.; Ge, T.; et al. Microbial Carbon Use Efficiency, Biomass Turnover, and Necromass Accumulation in Paddy Soil Depending on Fertilization. Agric. Ecosyst. Environ. 2020, 292, 106816. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.; Wu, B.; Zhang, Y.; Chen, W.; Wang, X.; Yu, H.; He, G. Temperature Response of Soil Respiration in a Chinese Pine Plantation: Hysteresis and Seasonal vs. Diel Q10. PLoS ONE 2013, 8, e57858. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Yang, Z.; Yu, T.; Yang, Q.; Wen, Y.; Wu, T. Potential Ecological Risk Assessment of Heavy Metals in the Fe-Mn Nodules in the Karst Area of Guangxi, Southwest China. Bull. Environ. Contam. Toxicol. 2021, 106, 51–56. [Google Scholar] [CrossRef]
- Miao, X.; Hao, Y.; Liu, H.; Xie, Z.; Miao, D.; He, X. Effects of Heavy Metals Speciations in Sediments on Their Bioaccumulation in Wild Fish in Rivers in Liuzhou—A Typical Karst Catchment in Southwest China. Ecotox. Environ. Saf. 2021, 214, 112099. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of Soil Carbon Dioxide Fluxes, Soil Organic Carbon and Microbial Biomass Carbon to Biochar Amendment: A Meta-Analysis. GCB Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Pierce, E.C.; Morin, M.; Little, J.C.; Liu, R.B.; Tannous, J.; Keller, N.P.; Pogliano, K.; Wolfe, B.E.; Sanchez, L.M.; Dutton, R.J. Bacterial-Fungal Interactions Revealed by Genome-Wide Analysis of Bacterial Mutant Fitness. Nat. Microbiol. 2021, 6, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Feng, Q.; Sun, X.; Wang, H.; Gielen, G.; Wu, W. Rice (Oryza Sativa L.) Plantation Affects the Stability of Biochar in Paddy Soil. Sci. Rep. 2015, 5, 10001. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.M.; Achouak, W.; Abrouk, D.; Heulin, T.; Nesme, X.; Haichar, F.e.Z. Diversifying Anaerobic Respiration Strategies to Compete in the Rhizosphere. Front. Environ. Sci. 2018, 6, 139. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Hu, Y.; Zhou, P.; Wang, T.; Shibistova, O.; Guggenberger, G.; Su, Y.; Wu, J. Fate of Rice Shoot and Root Residues, Rhizodeposits, and Microbial Assimilated Carbon in Paddy Soil—Part 2: Turnover and Microbial Utilization. Plant Soil 2017, 416, 243–257. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Z.; Leng, M.; Xiao, X.; Wang, X.; Pan, F.; Li, Q. Investigation of Soil Bacterial Communities and Functionalities Within Typical Karst Paddy Field Soils in Southern China. Fresenius Environ. Bull. 2021, 30, 3537–3548. [Google Scholar]
- Xu, H.; Du, H.; Zeng, F.; Song, T.; Peng, W. Diminished Rhizosphere and Bulk Soil Microbial Abundance and Diversity across Succession Stages in Karst Area, Southwest China. Appl. Soil Ecol. 2021, 158, 103799. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Zhu, T.; Zhou, M. Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region. Forests 2021, 12, 221. [Google Scholar] [CrossRef]
- Cao, W.; Xiong, Y.; Zhao, D.; Tan, H.; Qu, J. Bryophytes and the Symbiotic Microorganisms, the Pioneers of Vegetation Restoration in Karst Rocky Desertification Areas in Southwestern China. Appl. Microbiol. Biotechnol. 2020, 104, 873–891. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, Z.; Leng, M.; Li, X.; Xiong, L. Comparison of Bacterial Community Structure and Functional Groups of Paddy Soil Aggregates Between Karst and Non-Karst Areas. Huan Jing Ke Xue = Huanjing Kexue 2022, 43, 3865–3875. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, W.; Sun, W.; Zheng, X. Net Primary Production of Chinese Croplands from 1950 to 1999. Ecol. Appl. 2007, 17, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Murase, J.; Lu, Y.H. Carbon Cycling in Rice Field Ecosystems in the Context of Input, Decomposition and Translocation of Organic Materials and the Fates of Their End Products (CO2 and CH4). Soil Biol. Biochem. 2004, 36, 1399–1416. [Google Scholar] [CrossRef]
- Mandal, B.; Majumder, B.; Adhya, T.K.; Bandyopadhyay, P.K.; Gangopadhyay, A.; Sarkar, D.; Kundu, M.C.; Choudhury, S.G.; Hazra, G.C.; Kundu, S.; et al. Potential of Double-Cropped Rice Ecology to Conserve Organic Carbon under Subtropical Climate. Glob. Change Biol. 2008, 14, 2139–2151. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Xiong, L.; Tong, L.; Zhu, H.; Zhang, X.; Qin, G. Effects of Land Reclamation on Soil Bacterial Community and Potential Functions in Bauxite Mining Area. Int. J. Environ. Res. Public Health 2022, 19, 16921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D.; Shi, X.; Weindorf, D.C.; Zhao, L.; Ding, W.; Wang, H.; Pan, J.; Li, C. Simulation of Global Warming Potential (GWP) from Rice Fields in the Tai-Lake Region, China by Coupling 1:50,000 Soil Database with DNDC Model. Atmos. Environ. 2009, 43, 2737–2746. [Google Scholar] [CrossRef]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilae, J.; Marchesi, J.R.; Smidt, H.; de Vos, W.M.; Ross, R.P.; O’Toole, P.W. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality Control and Preprocessing of Metagenomic Datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Cannon, A.J. Multivariate Quantile Mapping Bias Correction: An N-Dimensional Probability Density Function Transform for Climate Model Simulations of Multiple Variables. Clim. Dyn. 2018, 50, 31–49. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Assenov, Y.; Domingues, F.S.; Albrecht, M. Topological Analysis and Interactive Visualization of Biological Networks and Protein Structures. Nat. Protoc. 2012, 7, 670–685. [Google Scholar] [CrossRef]
- Zallot, R.; Oberg, N.; Gerlt, J.A. The EFI Web Resource for Genomic Enzymology Tools: Leveraging Protein, Genome, and Metagenome Databases to Discover Novel Enzymes and Metabolic Pathways. Biochemistry 2019, 58, 4169–4182. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, M.; Yuan, H.; Liang, C.; Zhu, Z.; Xu, J.; Kuzyakov, Y.; Wu, J.; Ge, T.; Tang, C. Rice Rhizodeposition Promotes the Build-up of Organic Carbon in Soil via Fungal Necromass. Soil Biol. Biochem. 2021, 160, 108345. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Wang, X.; Ma, X.; Jiang, L.; Zhu, J.; Gao, J.; Song, C. Microbial Abundance as an Indicator of Soil Carbon and Nitrogen Nutrient in Permafrost Peatlands. Ecol. Indic. 2020, 115, 106362. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Polyanskaya, L.M.; Stolnikova, E.V.; Zvyagintzev, D.G. Fungal to Bacterial Biomass Ratio in the Forests Soil Profile. Biol. Bull 2010, 37, 254–262. [Google Scholar] [CrossRef]
- Thiet, R.K.; Frey, S.D.; Six, J. Do Growth Yield Efficiencies Differ between Soil Microbial Communities Differing in Fungal: Bacterial Ratios? Reality Check and Methodological Issues. Soil Biol. Biochem. 2006, 38, 837–844. [Google Scholar] [CrossRef]
- Jianhua, C.; Daoxian, Y.; Groves, C.; Fen, H.; Hui, Y.; Qian, L. Carbon Fluxes and Sinks: The Consumption of Atmospheric and Soil CO2 by Carbonate Rock Dissolution. Acta Geol. Sin.-Engl. Ed. 2012, 86, 963–972. [Google Scholar] [CrossRef]
- Zhong, L.; Bowatte, S.; Newton, P.C.D.; Hoogendoorn, C.J.; Luo, D. An Increased Ratio of Fungi to Bacteria Indicates Greater Potential for N2O Production in a Grazed Grassland Exposed to Elevated CO2. Agric. Ecosyst. Environ. 2018, 254, 111–116. [Google Scholar] [CrossRef]
- Laughlin, R.J.; Rutting, T.; Mueller, C.; Watson, C.J.; Stevens, R.J. Effect of Acetate on Soil Respiration, N2O Emissions and Gross N Transformations Related to Fungi and Bacteria in a Grassland Soil. Appl. Soil Ecol. 2009, 42, 25–30. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.J.; Yuan, D.X.; Xu, H.B.; Yang, Y. Bacteria Biomass and Carbonic Anhydrase Activity in Some Karst Areas of Southwest China. J. Asian Earth Sci. 2004, 24, 145–152. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Haemmerle, I.; Ellersdorfer, G.; Boeck, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The Effect of Resource Quantity and Resource Stoichiometry on Microbial Carbon-Use-Efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; McCormack, M.L.; Ma, C.; Guo, D. Similar Below-Ground Carbon Cycling Dynamics but Contrasting Modes of Nitrogen Cycling between Arbuscular Mycorrhizal and Ectomycorrhizal Forests. New Phytol. 2017, 213, 1440–1451. [Google Scholar] [CrossRef]
- Craig, M.E.; Turner, B.L.; Liang, C.; Clay, K.; Johnson, D.J.; Phillips, R.P. Tree Mycorrhizal Type Predicts Within-Site Variability in the Storage and Distribution of Soil Organic Matter. Glob. Change Biol. 2018, 24, 3317–3330. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, C.; Bao, X.; Wang, P.; Li, X.; Yang, S.; Ding, G.; Christie, P.; Li, L. Crop Diversity Facilitates Soil Aggregation in Relation to Soil Microbial Community Composition Driven by Intercropping. Plant Soil 2019, 436, 173–192. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, W.; Hu, P.; Xiao, D.; Yang, R.; Ye, Y.; Wang, K. The Formation of Large Macroaggregates Induces Soil Organic Carbon Sequestration in Short-Term Cropland Restoration in a Typical Karst Area. Sci. Total Environ. 2021, 801, 149588. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the Core Arabidopsis Thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ma, J.; Yang, C.-H.; Ibekwe, A.M. Soil Salinity, PH, and Indigenous Bacterial Community Interactively Influence the Survival of E. Coli O157:H7 Revealed by Multivariate Statistics. Environ. Sci. Pollut. Res. 2021, 28, 5575–5586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cong, J.; Lu, H.; Li, G.; Qu, Y.; Su, X.; Zhou, J.; Li, D. Community Structure and Elevational Diversity Patterns of Soil Acidobacteria. J. Environ. Sci. 2014, 26, 1717–1724. [Google Scholar] [CrossRef]
- Dunbar, J.; Gallegos-Graves, L.V.; Steven, B.; Mueller, R.; Hesse, C.; Zak, D.R.; Kuske, C.R. Surface Soil Fungal and Bacterial Communities in Aspen Stands Are Resilient to Eleven Years of Elevated CO2 and O3. Soil Biol. Biochem. 2014, 76, 227–234. [Google Scholar] [CrossRef]
- Bryant, D.A.; Costas, A.M.G.; Maresca, J.A.; Chew, A.G.M.; Klatt, C.G.; Bateson, M.M.; Tallon, L.J.; Hostetler, J.; Nelson, W.C.; Heidelberg, J.F.; et al. Candidatus Chloracidobacterium Thermophilum: An Aerobic Phototrophic Acidobacterium. Science 2007, 317, 523–526. [Google Scholar] [CrossRef]
- Mehrani, M.-J.; Sobotka, D.; Kowal, P.; Ciesielski, S.; Makinia, J. The Occurrence and Role of Nitrospira in Nitrogen Removal Systems. Bioresour. Technol. 2020, 303, 122936. [Google Scholar] [CrossRef]
- Hu, H.-W.; He, J.-Z. Comammox-a Newly Discovered Nitrification Process in the Terrestrial Nitrogen Cycle. J. Soils Sediments 2017, 17, 2709–2717. [Google Scholar] [CrossRef]
- Palomo, A.; Pedersen, A.G.; Fowler, S.J.; Dechesne, A.; Sicheritz-Pontén, T.; Smets, B.F. Comparative Genomics Sheds Light on Niche Differentiation and the Evolutionary History of Comammox Nitrospira. ISME J. 2018, 12, 1779–1793. [Google Scholar] [CrossRef]
- Koch, H.; van Kessel, M.A.H.J.; Lucker, S. Complete Nitrification: Insights into the Ecophysiology of Comammox Nitrospira. Appl. Microbiol. Biotechnol. 2019, 103, 177–189. [Google Scholar] [CrossRef]
- Baranova, A.A.; Rogozhin, E.A.; Georgieva, M.L.; Bilanenko, E.N.; Kul’ko, A.B.; Yakushev, A.V.; Alferova, V.A.; Sadykova, V.S. Antimicrobial Peptides Produced by Alkaliphilic Fungi Emericellopsis Alkalina: Biosynthesis and Biological Activity Against Pathogenic Multidrug-Resistant Fungi. Appl. Biochem. Microbiol. 2019, 55, 145–151. [Google Scholar] [CrossRef]
- Goncalves, M.F.M.; Vicente, T.F.L.; Esteves, A.C.; Alves, A. Novel Halotolerant Species of Emericellopsis and Parasarocladium Associated with Macroalgae in an Estuarine Environment. Mycologia 2020, 112, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, L.V.; Orleanskii, V.K.; Gerasimenko, L.M.; Ushatinskaya, G.T. The Role of Cyanobacteria in Crystallization of Magnesium Calcites. Paleontol. J. 2006, 40, 125–133. [Google Scholar] [CrossRef]
- de Oliveira, T.B.; de Lucas, R.C.; de Almeida Scarcella, A.S.; Contato, A.G.; Pasin, T.M.; Martinez, C.A.; Teixeira de Moraes Polizeli, M.d.L. Fungal Communities Differentially Respond to Warming and Drought in Tropical Grassland Soil. Mol. Ecol. 2020, 29, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Selim, H.M.M.; Gomaa, N.M.; Essa, A.M.M. Application of Endophytic Bacteria for the Biocontrol of Rhizoctonia solani (Cantharellales: Ceratobasidiaceae) Damping-off Disease in Cotton Seedlings. Biocontrol Sci. Technol. 2017, 27, 81–95. [Google Scholar] [CrossRef]
- Qu, T.; Du, X.; Peng, Y.; Guo, W.; Zhao, C.; Losapio, G. Invasive Species Allelopathy Decreases Plant Growth and Soil Microbial Activity. PLoS ONE 2021, 16, e0246685. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zheng, L.; Yang, X.; Liu, H.; Guo, J. Isolation and Characterization of an Antifungal Protein from Bacillus Licheniformis HS10. Biochem. Biophys. Res. Commun. 2014, 454, 48–52. [Google Scholar] [CrossRef]
- van Erven, G.; Kleijn, A.F.; Patyshakuliyeva, A.; Di Falco, M.; Tsang, A.; de Vries, R.P.; van Berkel, W.J.H.; Kabel, M.A. Evidence for Ligninolytic Activity of the Ascomycete Fungus Podospora Anserina. Biotechnol. Biofuels 2020, 13, 75. [Google Scholar] [CrossRef]
- Malagnac, F.; Lalucque, H.; Lepere, G.; Silar, P. Two NADPH Oxidase Isoforms Are Required for Sexual Reproduction and Ascospore Germination in the Filamentous Fungus Podospora Anserina. Fungal Genet. Biol. 2004, 41, 982–997. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.-J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial Diversity Drives Carbon Use Efficiency in a Model Soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, C.; Stahr, K.; Kuzyakov, Y.; Wei, X. The Effect of Microorganisms on Soil Carbonate Recrystallization and Abiotic CO2 Uptake of Soil. Catena 2020, 192, 104592. [Google Scholar] [CrossRef]
- Luecker, S.; Wagner, M.; Maixner, F.; Pelletier, E.; Koch, H.; Vacherie, B.; Rattei, T.; Damste, J.S.S.; Spieck, E.; Le Paslier, D.; et al. A Nitrospira Metagenome Illuminates the Physiology and Evolution of Globally Important Nitrite-Oxidizing Bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 13479–13484. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification Driven by Bacteria and Not Archaea in Nitrogen-Rich Grassland Soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.T.; Jones, D.L. PH Regulation of Carbon and Nitrogen Dynamics in Two Agricultural Soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; He, Y.; Brookes, P.C.; Xu, J. Elevated Temperature Increased Nitrification Activity by Stimulating AOB Growth and Activity in an Acidic Paddy Soil. Plant Soil 2019, 445, 71–83. [Google Scholar] [CrossRef]

| Site | pH (H2O) | SOC /g·kg−1 | DOC /ug·L−1 | TN /g·kg−1 | AN /mg·kg−1 | TP /g·kg−1 | AP /mg·kg−1 | C/N /g·kg−1 | CEC /cmol·kg−1 | Ca2+ /cmol·kg−1 | Mg2+ /cmol·kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Karst area | 7.40 ± 0.18 a | 25.15 ± 1.03 a | 261.62 ± 9.22 a | 1.74 ± 0.09 a | 90.21 ± 1.24 a | 1.24 ± 0.02 a | 22.04 ± 1.63 a | 12..46 ± 0.11 a | 13.79 ± 0.42 a | 3.89 ± 0.04 a | 1.20 ± 0.01 a |
| Non-karst area | 5.76 ± 0.15 b | 13.86 ± 1.61 b | 202.78 ± 20.46 b | 1.50 ± 0.04 b | 85.24 ± 0.09 b | 0.49 ± 0.01 b | 18.63 ± 0.57 b | 9.23 ± 0.47 b | 6.09 ± 0.17 b | 2.36 ± 0.01 b | 0.48 ± 0.03 b |
| Microbial Taxa | Groups | Region | Percentage | Correlation Coefficient |
|---|---|---|---|---|
| Bacteria | Acidobacteria Gp6 | KA | 7.05% | −0.565 * |
| Fungi | Stellatospora | NKA | 0.07% | 0.571 * |
| Functional groups of fungi | Plant pathogen | KA | 2.61% | 0.582 * |
| Endomycorrhizal-plant pathogen-undefined saprotroph | NKA | 0.26% | 0.530 * | |
| Dung saprotroph-endophyte-litter saprotroph-undefined saprotroph | KA | 0.88% | −0.568 * | |
| Functional groups of bacteria | Aerobic nitrite oxidizers (Nitrifiers) | KA | 2.13% | −0.545 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Jin, Z.; Yuan, W.; Chen, W.; Li, X.; Xiong, L.; Cheng, G. Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas. Agronomy 2023, 13, 2001. https://doi.org/10.3390/agronomy13082001
Zhou J, Jin Z, Yuan W, Chen W, Li X, Xiong L, Cheng G. Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas. Agronomy. 2023; 13(8):2001. https://doi.org/10.3390/agronomy13082001
Chicago/Turabian StyleZhou, Junbo, Zhenjiang Jin, Wu Yuan, Weijian Chen, Xuesong Li, Liyuan Xiong, and Guanwen Cheng. 2023. "Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas" Agronomy 13, no. 8: 2001. https://doi.org/10.3390/agronomy13082001
APA StyleZhou, J., Jin, Z., Yuan, W., Chen, W., Li, X., Xiong, L., & Cheng, G. (2023). Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas. Agronomy, 13(8), 2001. https://doi.org/10.3390/agronomy13082001








