Selenium and Iodine Biofortification Interacting with Supplementary Blue Light to Enhance the Growth Characteristics, Pigments, Trigonelline and Seed Yield of Fenugreek (Trigonella foenum-gracum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions
2.2. Morphological Traits
2.3. Physiological and Biochemical Traits
2.3.1. Leaf Relative Water Content (RWC)
- Fw: Leaf fresh weight.
- Dw: Leaf dry weight.
- Sw: Leaf saturated weight.
2.3.2. Total Chlorophyll and Carotenoids
2.3.3. Total Anthocyanin
2.3.4. Trigonelline Content of Shoot and Seed
2.4. Data Analysis
3. Results
3.1. Plant Growth and Seed Yield
3.1.1. Plant Length
3.1.2. Fresh Weight of Shoots
3.1.3. Dry Weight of Shoot
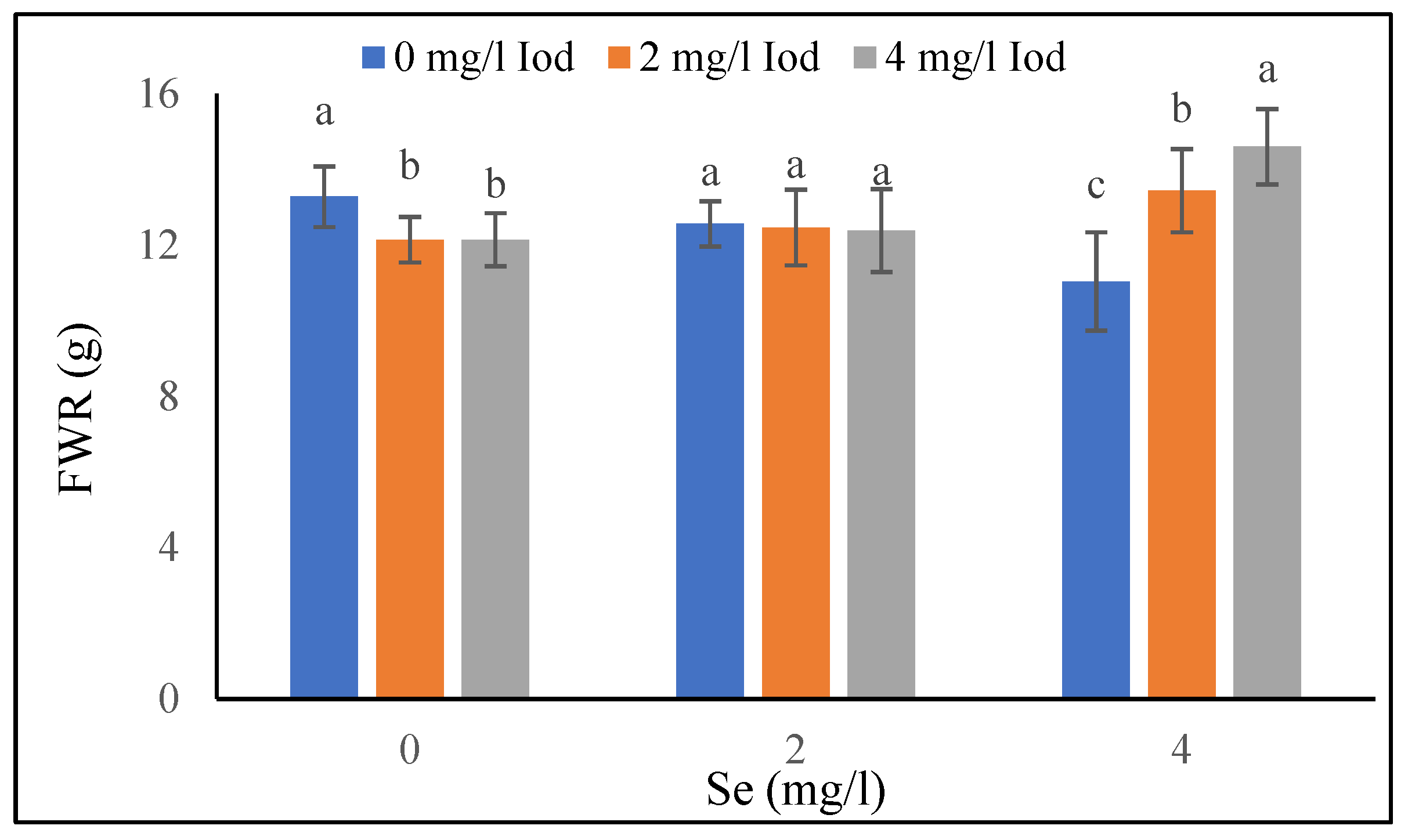
3.1.4. Fresh Weight of Roots
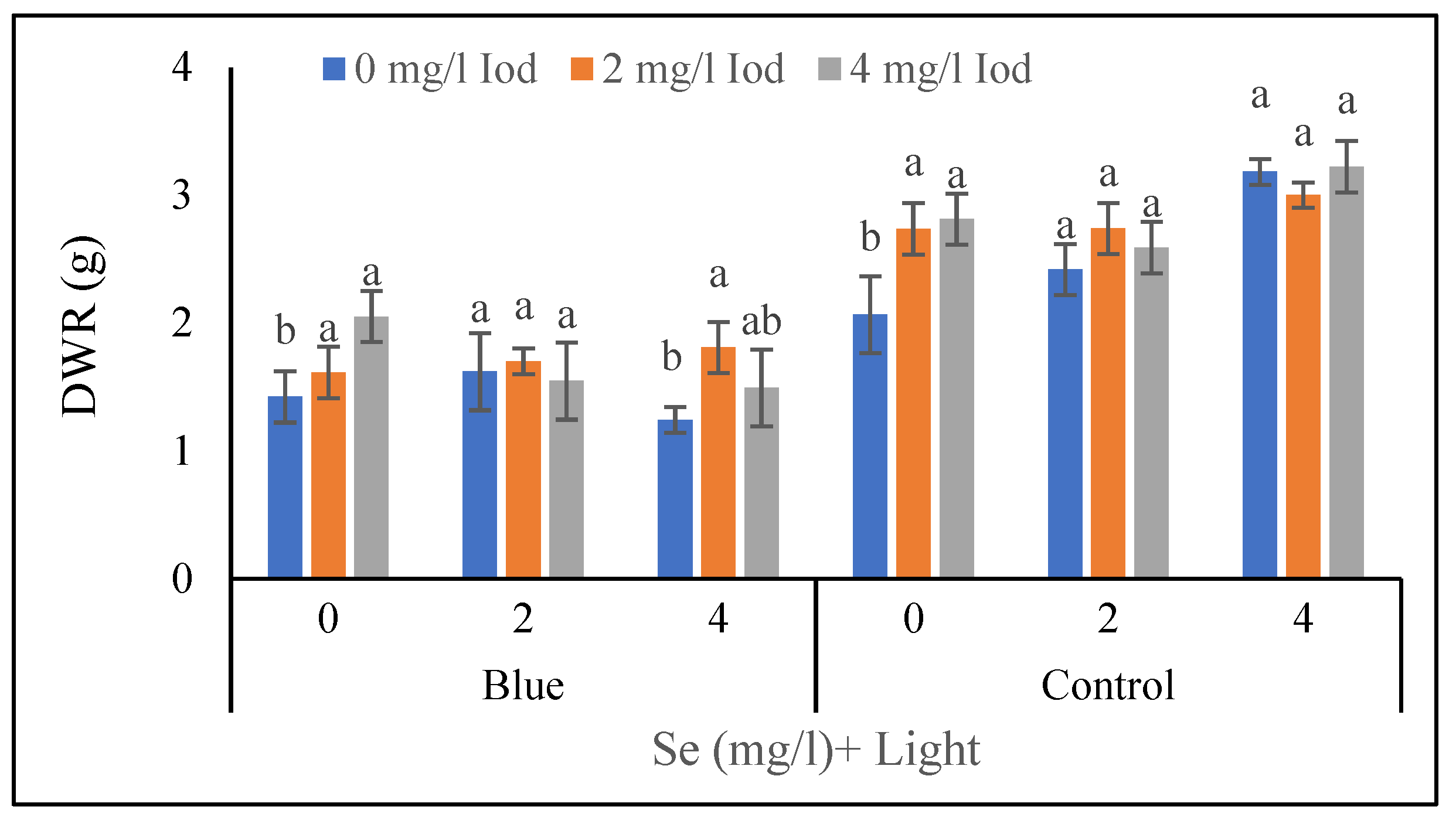
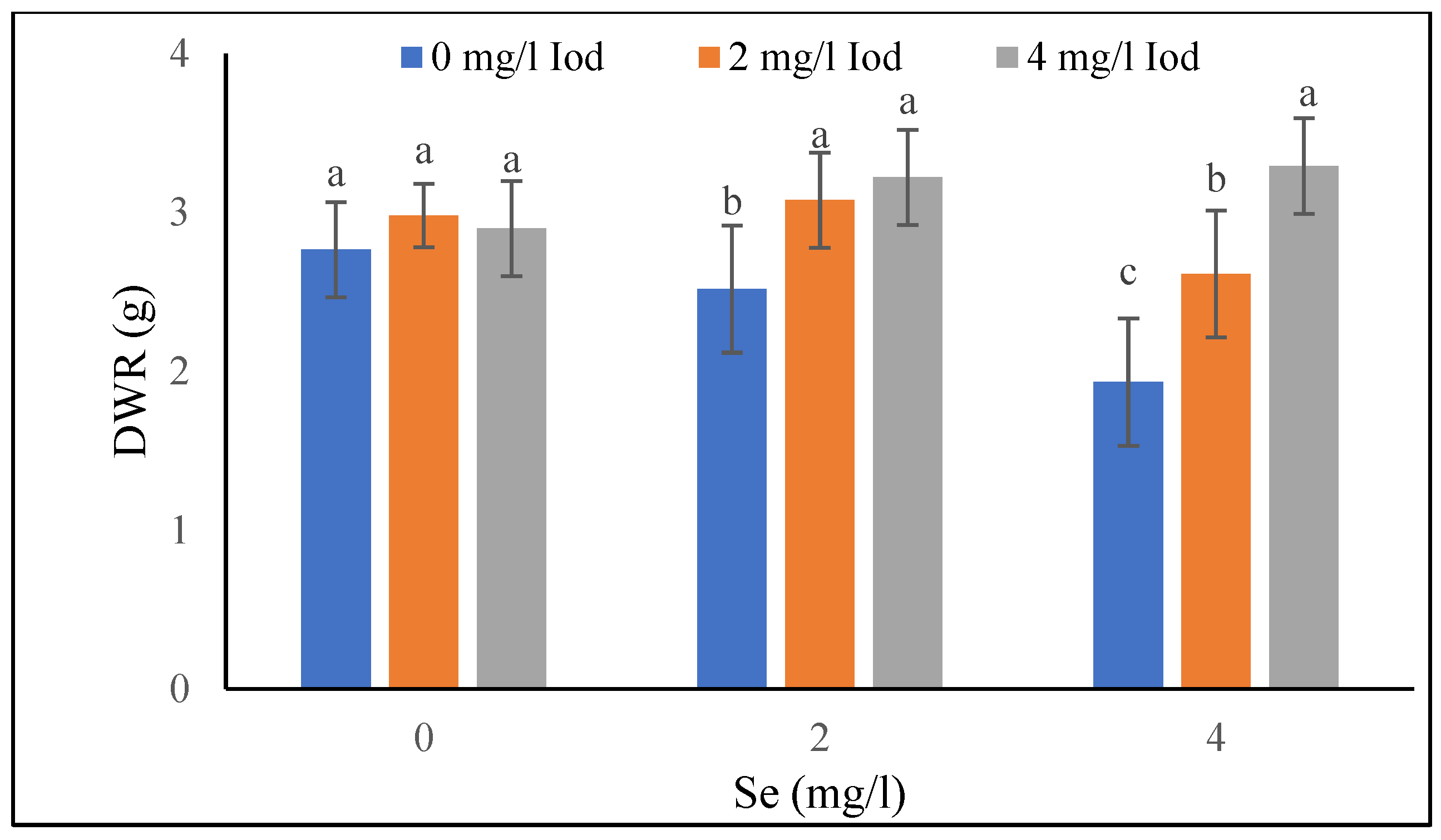
3.1.5. Dry Weight of Roots
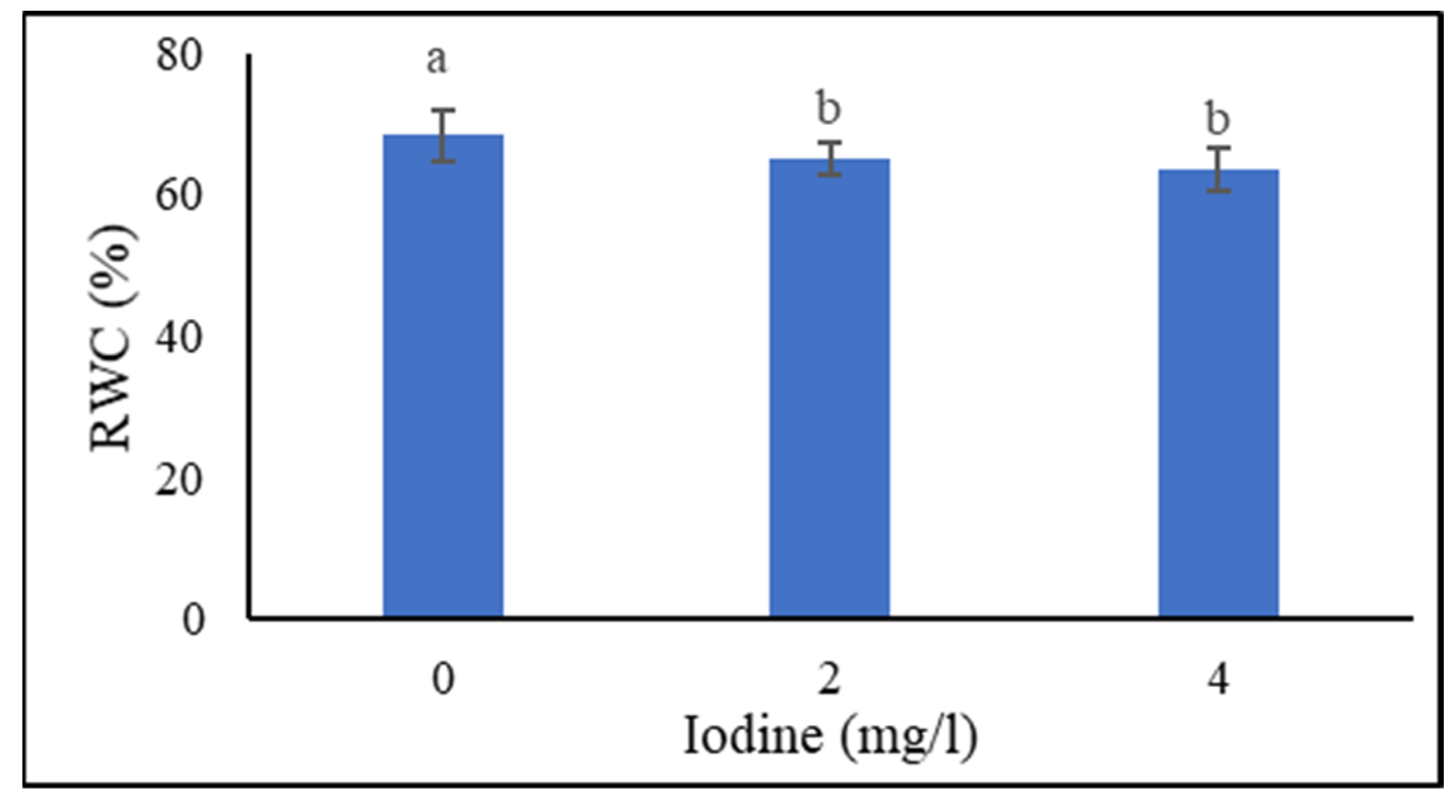
3.1.6. Relative Water Content (RWC) of Leaves
3.1.7. Seed Yield
3.2. Plant Pigments
3.2.1. Total Chlorophyll
3.2.2. Anthocyanin Content
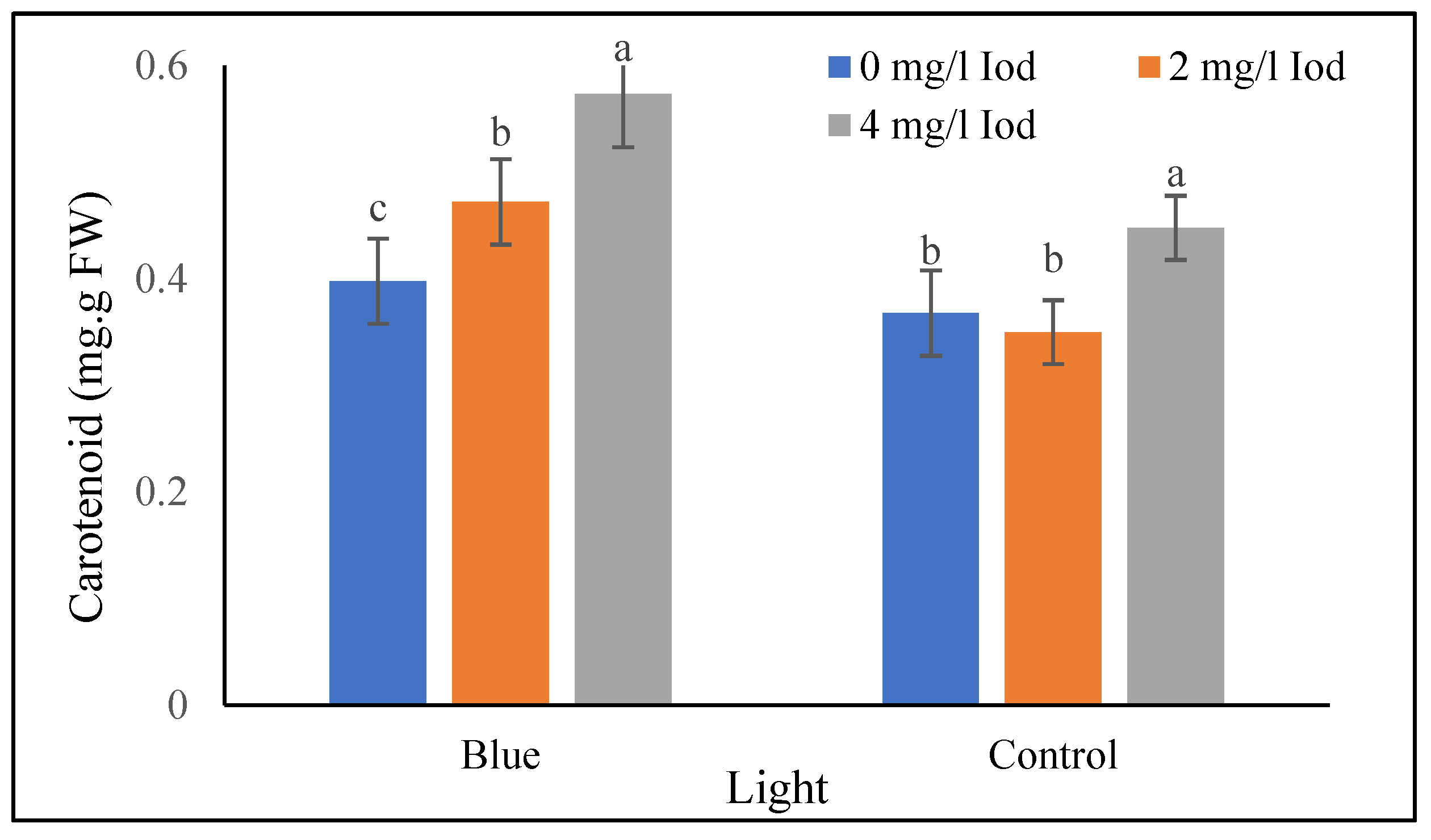
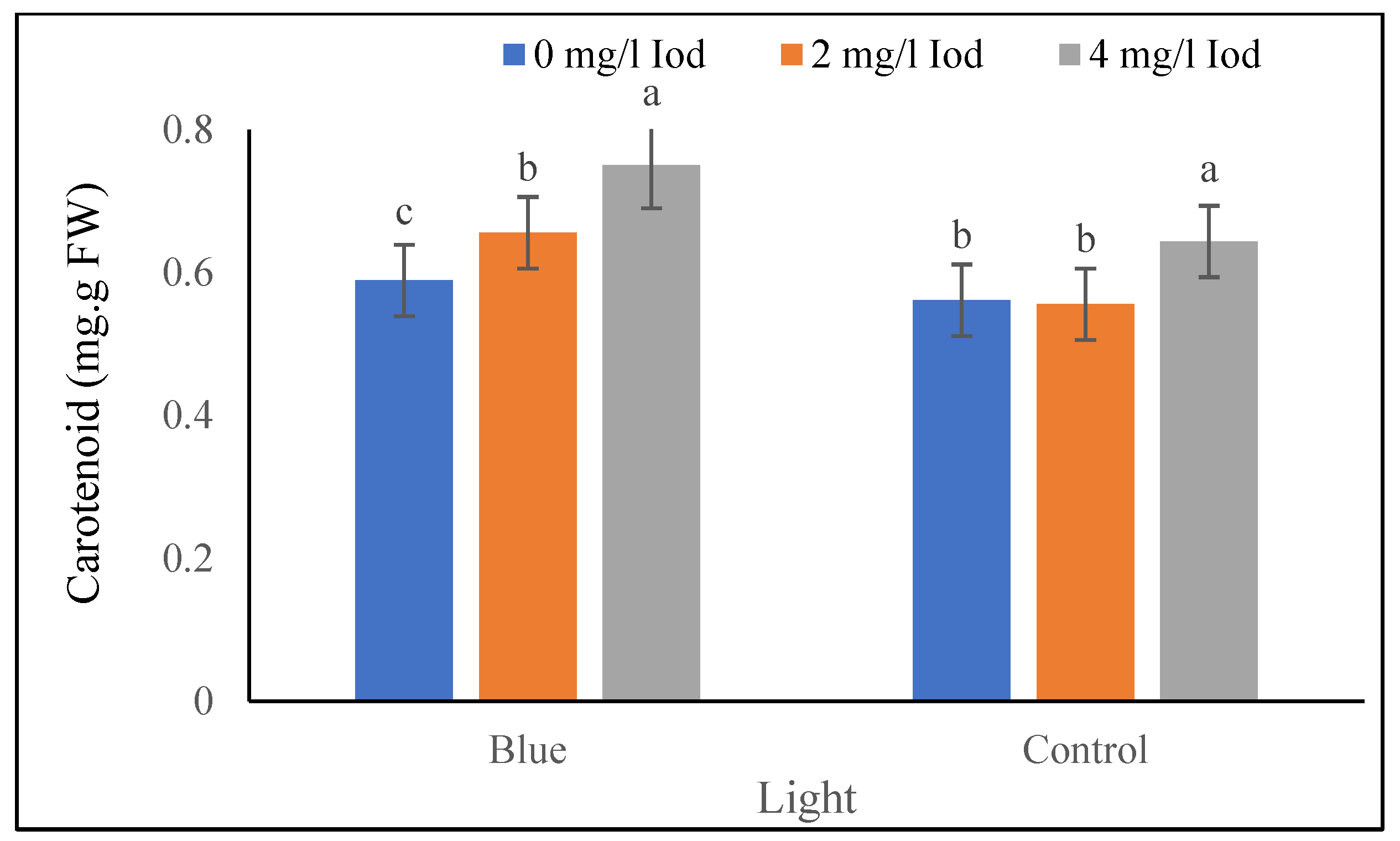
3.2.3. Carotenoid Content
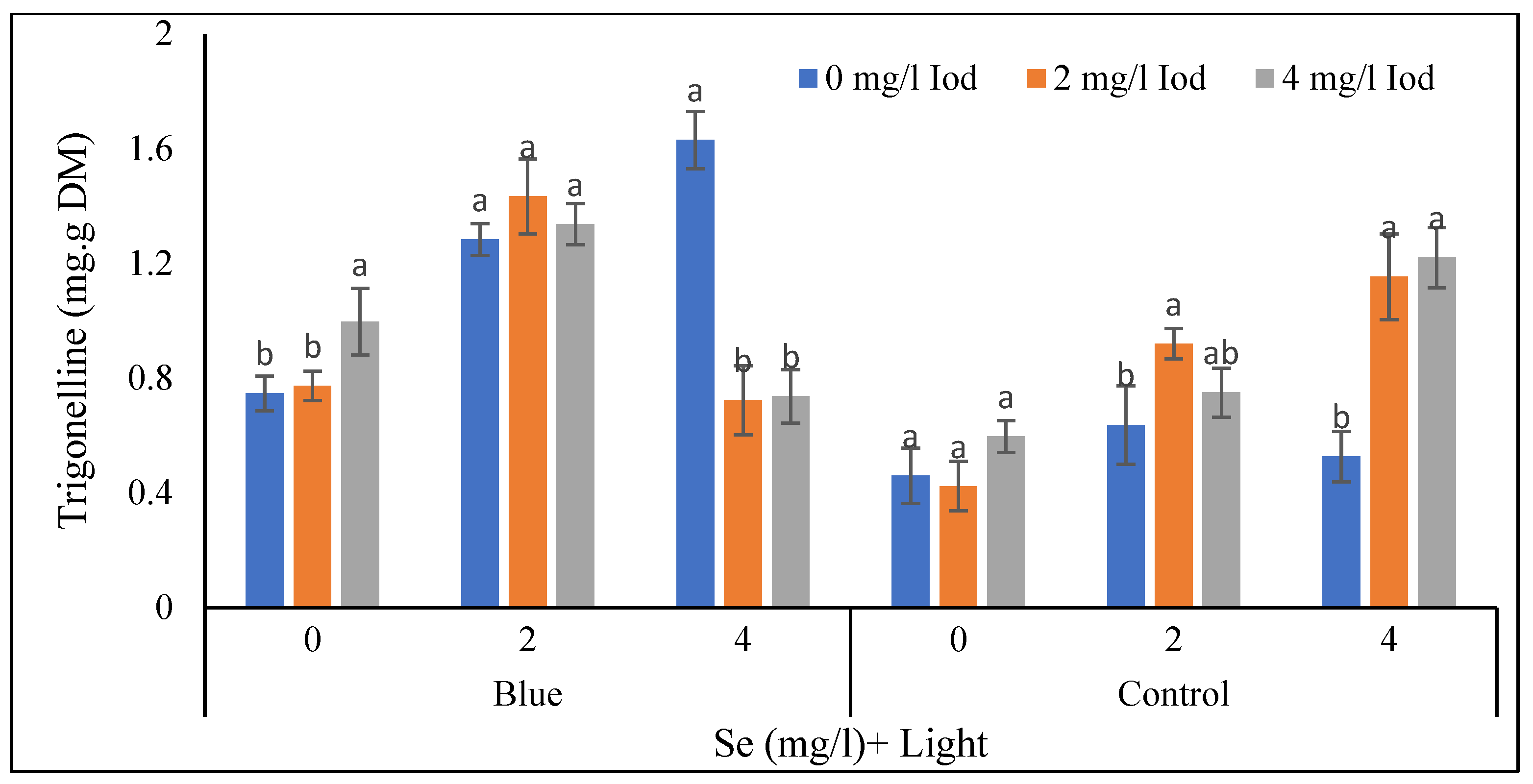
3.3. Trigonelline Content of Shoot and Seed
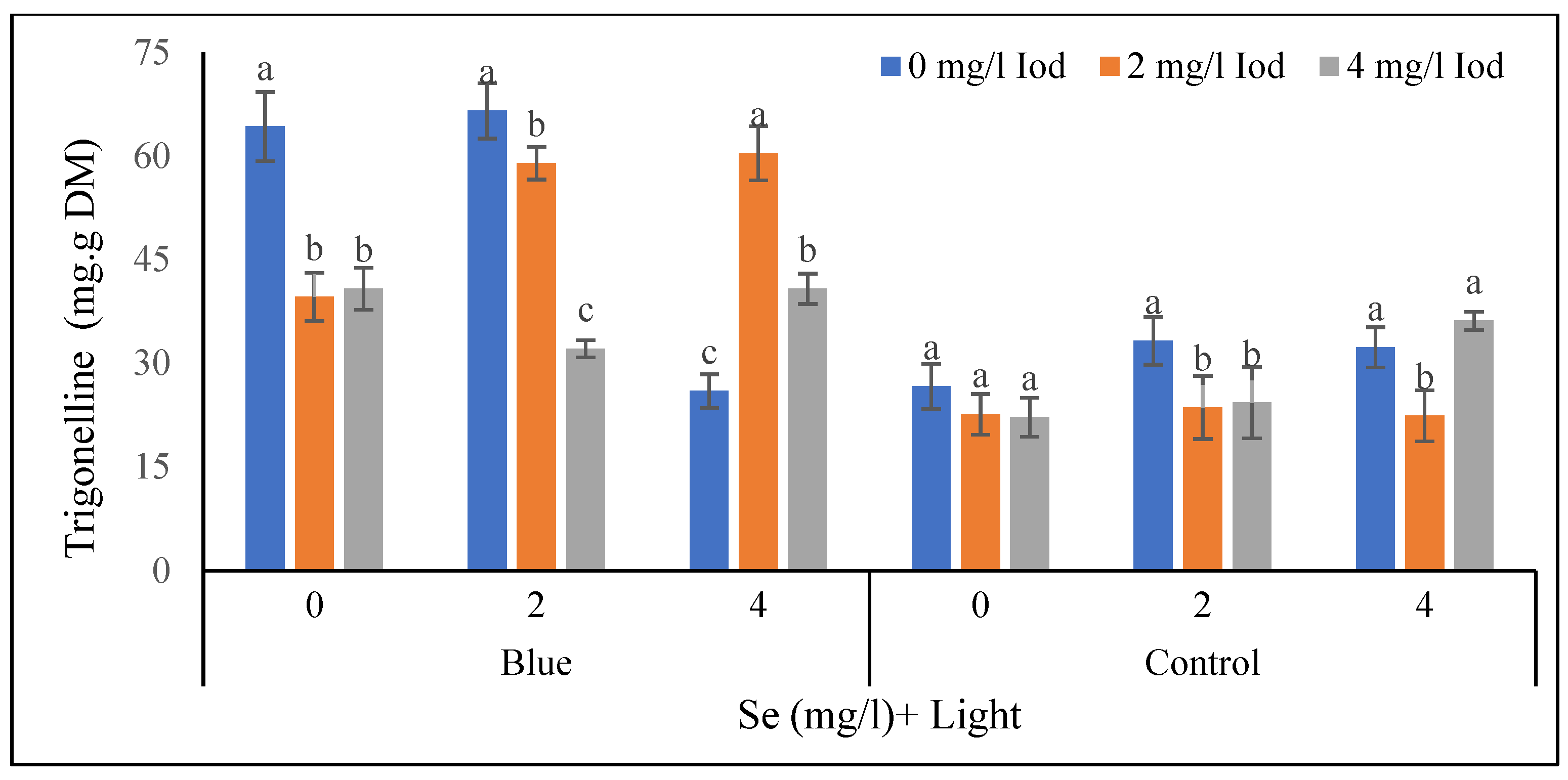
3.3.1. Shoot Trigonelline Content
3.3.2. Seed Trigonelline Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haouala, R.; Hawala, S.; El-Ayeb, A.; Khanfir, R.; Boughanmi, N. Aqueous and organic extracts of Trigonella foenum-graecum L. inhibit the mycelia growth of fungi. J. Environ. Sci. 2008, 20, 1453–1457. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kaur, M.; Sandhu, K.S. Antioxidant characterization and in vitro DNA damage protection potential of some Indian fenugreek (Trigonella foenum-graecum) cultivars: Effect of solvents. J. Food Sci. Technol. 2020, 57, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.M.; Shyamala, B.N.; Naik, J.P.; Sulochanamma, G.; Srinivas, P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. LWT-Food Sci. Technol. 2011, 44, 451–456. [Google Scholar] [CrossRef]
- Chatterjee, S.; Variyar, P.S.; Sharma, A. Bioactive lipid constituents of fenugreek. Food Chem. 2010, 119, 349–353. [Google Scholar] [CrossRef]
- Leela, N.K.; Shafeekh, K.M. Fenugreek. In Chemistry of Spices; CABI: Wallingford, UK, 2008; pp. 242–259. [Google Scholar]
- Priya, V.; Jananie, R.K.; Vijayalakshmi, K. GC/MS determination of bioactive components of Trigonella foenum grecum. J. Chem. Pharm. Res. 2011, 3, 35–40. [Google Scholar]
- Ashihara, H.; Ludwig, I.A.; Katahira, R.; Yokota, T.; Fujimura, T.; Crozier, A. Trigonelline and related nicotinic acid metabolites: Occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 2015, 14, 765–798. [Google Scholar] [CrossRef]
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.-D.A.; Shams, M.S.; Domokos-Szabolcsy, E. Selenium and Its Role in Higher Plants; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–296. [Google Scholar]
- Jerše, A.; Kacjan Maršić, N.; Kroflič, A.; Germ, M.; Šircelj, H.; Stibilj, V. Is foliar enrichment of pea plants with iodine and selenium appropriate for production of functional food. Food Chem. 2018, 267, 368–375. [Google Scholar] [CrossRef]
- Santhosh, K.B.; Priyadarsini, K.I. Selenium nutrition: How important is it? Biomed. Prev. Nutr. 2014, 4, 333–341. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Chudinova, E.; Astarkhanova, T.; Pakina, E. In Vitro Evaluation of Antibacterial and Antifungal Activity of Biogenic Silver and Copper Nanoparticles: The First Report of Applying Biogenic Nanoparticles against Pilidium concavum and Pestalotia sp. Fungi. Molecules 2021, 26, 5402. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Krzepiłko, A.; Prażak, R.; Skwaryło-Bednarz, B.; Molas, J. Agronomic biofortifcation as a means of enriching plant foodstufs with iodine. Acta Agrobot. 2019, 72, 1–9. [Google Scholar] [CrossRef]
- Zargar, M.; Najafi, H.; Fakhri, K.; Mafakheri, S.; Sarajuoghi, M. Agronomic evaluation of mechanical and chemical weed management for reducing use of herbicides in single vs. twinrow sugarbeet. Res. Crops 2011, 12, 173–178. [Google Scholar]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant. Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Ferrarese, M.; Sourestani, M.; Quattrini, E.; Schiavi, M.; Ferrante, A. Biofortification of spinach plants applying selenium in the nutrient solution of floating system. Veg. Crop. Res. Bull. 2012, 76, 127–136. [Google Scholar] [CrossRef]
- Sindelařova, K.; Szakova, J.; Tremlova, J.; Mestek, O.; Praus, L.; Kaňa, A.; Najmanova, J.; Tlustos, P. The response of broccoli (Brassica oleracea convar. italica) varieties on foliar application of selenium: Uptake, translocation, and speciation. Food Addit. Contam. Part. A-Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 2027–2038. [Google Scholar]
- Mechora, P.; Stibilj, V.; Kreft, I.; Germ, M. The physiology and biochemical tolerance of cabbage to Se (VI) addition to the soil and by foliar spraying. J. Plant. Nutr. 2014, 37, 2157–2169. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Pakina, E.; Zargar, M. Evaluation of Phoma sp. Biomass as an Endophytic Fungus for Synthesis of Extracellular Gold Nanoparticles with Antibacterial and Antifungal Properties. Molecules. 2022, 27, 1181. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Shi, R.; Song, S.; Zhang, Y.; Su, W.; Liu, H. The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprout. Molecules 2020, 25, 4788. [Google Scholar] [CrossRef]
- Alsina, I.; Dubova, L.; Smiltina, Z.; Stroksa, L.; Duma, M. The effect of selenium on yield quality of lettuce. Acta Hortic. 2012, 939, 269–275. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Ghasemi, K.; Pirdashti, H.; Asgharzadeh, R. Effect of selenium enrichment on the growth, photosynthesis and mineral nutrition of broccoli. Not. Sci. Biol. 2016, 8, 199–203. [Google Scholar] [CrossRef]
- Oraghi Ardebili, Z.; Oraghi Ardebili, N.; Jalili, S.; Safiallah, S. The modified qualities of basil plants by selenium and/or ascorbic acid. Turk. J. Bot. 2015, 39, 401–407. [Google Scholar] [CrossRef]
- Lee, G.J.; Kang, B.K.; Kim, T.I.; Kim, T.J.; Kim, J.H. Effects of different selenium concentrations of the nutrient solution on the growth and quality of tomato fruit in hydroponics. Acta Hortic. 2007, 761, 443–448. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; D’Imperio, M.; Santamaria, P.; Serio, F. Iodine biofortification of four Brassica genotypes is effective already at low rates of potassium iodate. Nutrients 2019, 11, 451. [Google Scholar] [CrossRef]
- He, D.; Kozai, T.; Niu, G.; Zhang, X. Light-emitting diodes for horticulture. In Solid State Lighting Technology and Application Series; Springer: Cham, Switzerland, 2019; pp. 513–547. [Google Scholar]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Shin, Y.S.; Lee, M.J.; Lee, E.S. Effect of light emitting diodes treatment on growth and mineral contents of lettuce (Lactuca sativa L. Chung Chi Ma). Korean J. Org. Agric. 2013, 21, 659–668. [Google Scholar] [CrossRef]
- Terfa, M.T.; Solhaug, K.A.; Gislerød, H.R.; Olsen, J.E.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa × hybrida but does not affect time to flower opening. Physiol. Plant. 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Zargar, M.; Astarkhanova, T.; Pakina, E.; Ladan, S.; Lyashko, M.; Shkurkin, S.I. Facile Biogenic Synthesis and Characterization of SevenMetal-Based Nanoparticles Conjugated with Phytochemical Bioactives Using Fragaria ananassa Leaf Extract. Molecules 2021, 26, 3025. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L. A non-destructive method to determine chlorophyll content of leaves. Photosynthetica 1992, 26, 469–473. [Google Scholar]
- Xu, J.; Zhang, M.; Liu, X.; Lu, G.; Chi, J.; Sun, L. Extraction and antioxidation of anthocyanin of black soybean seed coat. Trans. Chin. Soc. Agric. Eng. 2005, 21, 161–164. [Google Scholar]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Badi, H.N.; Mehrafarin, A.; Mustafavi, S.H.; Labbafi, M. Exogenous arginine improved fenugreek sprouts growth and trigonelline production under salinity condition. Ind. Crops Prod. 2018, 122, 609–616. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LEDs light effect on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–229. [Google Scholar] [CrossRef]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light Quality Affects Photosynthesis and Leaf Anatomy of Birch Plantlets In Vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Duborská, E.; Šebesta, M.; Matulová, M.; Zvěřina, O.; Urík, M. Current Strategies for Selenium and Iodine Biofortification in Crop Plants. Nutrients 2022, 14, 4717. [Google Scholar] [CrossRef]
- Son, K.H.; Park, J.H.; Kim, D.; Oh, M.M. Leaf shape, growth, and phytochemicals in two leaf lettuce cultivars grown under monochromatic light-emitting diodes. Korean J. Hortic. Sci. Technol. 2012, 30, 664–672. [Google Scholar] [CrossRef]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in laves of cabbage seedlings. Acta Hortic. 2011, 907, 179–184. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hort. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Xin, J.; Liu, H.; Song, S.; Chen, R.; Sun, G. Growth and quality of Chinese kale grown under different LEDs. Agric. Sci. Technol. 2015, 16, 68–69. [Google Scholar]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Farrokhzad, Y.; Babaei, A.; Yadollahi, A.; Kashkooli, A.B.; Mokhtassi-Bidgoli, A. In vitro rooting, plant growth, monosaccharide profile and anatomical analysis of Phalaenopsis regenerants under different regions of visible light. S. Afr. J. Bot. 2022, 149, 622–631. [Google Scholar] [CrossRef]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Silva, V.M.; Montanha, G.S.; Dos Reis, A.R. Selenium increases photosynthetic capacity, daidzein biosynthesis, nodulation and yield of peanuts plants (Arachis hypogaea L.). Plant Physiol. Biochem. 2022, 190, 231–239. [Google Scholar] [CrossRef]
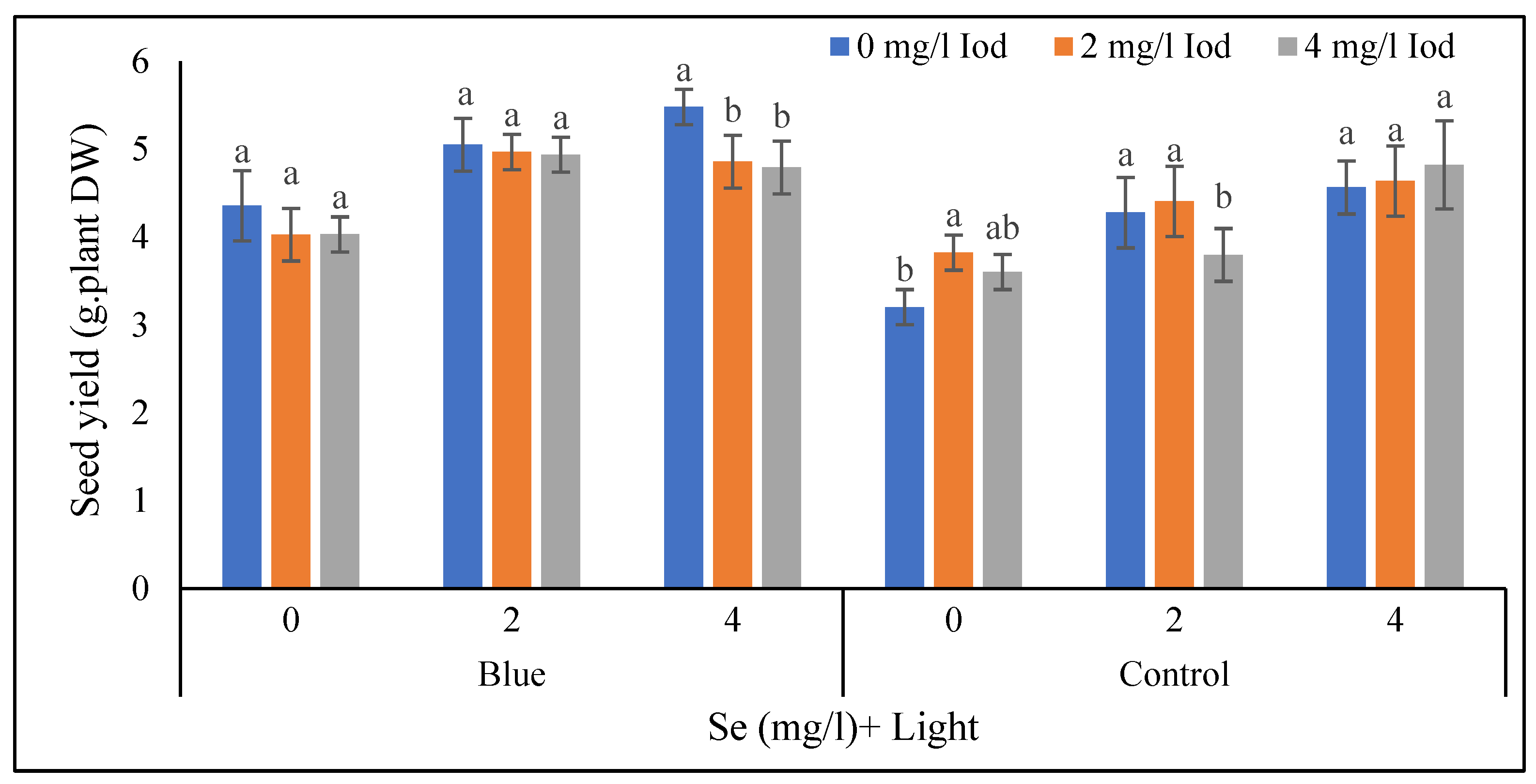


| Light | Selenium | Plant Height (cm) | Chlorophyll (Mg·g FW) | RWC (%) |
|---|---|---|---|---|
| Blue | 0 | 12.44 ± 1.1 b | 3.84 ± 0.2 c | 64.88 ± 1.9 c |
| 2 | 15.77 ± 1.1 a | 4.76 ± 0.2 b | 65.11 ± 2.2 b | |
| 4 | 15.00 ± 1.0 a | 5.85 ± 0.2 a | 72.66 ± 3.3 a | |
| Control | 0 | 18.66 ± 1.2 a | 2.71 ± 0.3 c | 64.55 ± 2.4 c |
| 2 | 18.22 ± 1.1 a | 3.39 ± 0.2 b | 61.55 ± 2.7 b | |
| 4 | 19.44 ± 1.0 a | 4.20 ± 0.2 a | 65.55 ± 2.2 a | |
| LSD | 1.3 | 0.2 | 4.0 |
| Light | Plant Height (cm) | FWS (gr) | RWC (%) | Chlorophyll (Mg·g FW) | Anthocyanin (Ug·g FW) |
|---|---|---|---|---|---|
| Blue | 29.50 ± 2.0 b | 75.99 ± 2.6 a | 54.07 ± 2.8 a | 3.49 ± 0.3 a | 8.10 ± 0.5 a |
| Control | 34.37 ± 2.2 a | 69.20 ± 2.5 b | 46.63 ± 3.6 b | 2.69 ± 0.3 b | 6.92 ± 0.5 b |
| LSD | 1.3 | 1.8 | 1.8 | 0.2 | 0.3 |
| Selenium | Carotenoids (Mg·g FW) | Anthocyanin (Ug·g FW) | Plant Height (cm) | FWS (g) | Chlorophyll (Mg·g FW) | RWC (%) | Anthocyanin (Ug·g FW) |
|---|---|---|---|---|---|---|---|
| DAP40 | DAP80 | ||||||
| 0 | 0.34 ± 0.04 c | 3.68 ± 0.2 c | 28.50 ± 1.7 c | 69.50 ± 2.3 b | 2.67 ± 0.3 c | 44.16 ± 3.5 b | 6.54 ± 0.4 c |
| 2 | 0.41 ± 0.04 b | 5.46 ± 0.3 b | 31.72 ± 2.1 b | 71.24 ± 2.9 b | 3.02 ± 0.3 b | 52.66 ± 2.4 a | 7.63 ± 0.4 b |
| 4 | 0.54 ± 0.05 a | 5.85 ± 0.3 a | 35.5 ± 2.0 a | 76.85 ± 3.0 a | 3.59 ± 0.3 a | 54.22 ± 3.0 a | 8.37 ± 0.5 a |
| LSD | 0.03 | 0.2 | 1.5 | 2.1 | 0.2 | 2.2 | 0.4 |
| Light | FWS (g) | DWS (g) | FWR (g) | Anthocyanin (Ug·g FW) |
|---|---|---|---|---|
| Blue | 55.91 ± 2.6 a | 11.44 ± 0.5 a | 8.29 ± 0.4 b | 5.24 ± 0.4 a |
| Control | 50.53 ± 2.8 b | 9.55 ± 0.6 b | 11.31 ± 0.5 a | 4.76 ± 0.3 b |
| LSD | 1.7 | 0.5 | 0.4 | 0.2 |
| Selenium | I | FWS (g) | DWS (g) | Chlorophyll (Mg·g FW) |
|---|---|---|---|---|
| 0 | 0 | 44.73 ± 3.8 b | 10.25 ± 0.5 ab | 3.11 ± 0.3 ab |
| 2 | 53.06 ± 2.0 a | 9.72 ± 0.7 b | 3.27 ± 0.3 a | |
| 4 | 51.47 ± 1.9 a | 11.30 ± 0.7 a | 2.76 ± 0.2 b | |
| 2 | 0 | 54.17 ± 2.0 a | 10.97 ± 0.7 ab | 3.84 ± 0.4 b |
| 2 | 52.96 ± 1.9 a | 11.25 ± 0.7 a | 3.84 ± 0.4 b | |
| 4 | 52.80 ± 1.7 a | 10.15 ± 0.7 b | 4.54 ± 0.3 a | |
| 4 | 0 | 57.63 ± 3.0 a | 10.70 ± 0.7 a | 4.78 ± 0.5 a |
| 2 | 56.57 ± 2.0 a | 10.03 ± 0.9 a | 5.12 ± 0.5 a | |
| 4 | 55.61 ± 3.5 a | 10.12 ± 1.1 a | 5.17 ± 0.6 a | |
| LSD | 2.1 | 1.1 | 0.45 |
| Light | Selenium | DWS (g) | FWR (g) | DWR (g) |
|---|---|---|---|---|
| Blue | 0 | 22.78 ± 1.3 a | 11.48 ± 0.4 a | 2.53 ± 0.2 a |
| 2 | 20.72 ± 1.7 b | 11.08 ± 0.5 a | 2.42 ± 0.1 a | |
| 4 | 20.10 ± 0.6 b | 11.34 ± 0.3 a | 1.95 ± 0.2 b | |
| Control | 0 | 16.91 ± 0.4 a | 13.56 ± 0.4 b | 3.22 ± 0.2 a |
| 2 | 17.53 ± 0.9 a | 13.86 ± 1.0 b | 3.45 ± 0.4 a | |
| 4 | 17.06 ± 0.7 a | 14.70 ± 1.0 a | 3.27 ± 0.3 a | |
| LSD | 0.9 | 0.4 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezani, S.; Yousefshahi, B.; Farrokhzad, Y.; Ramezan, D.; Zargar, M.; Pakina, E. Selenium and Iodine Biofortification Interacting with Supplementary Blue Light to Enhance the Growth Characteristics, Pigments, Trigonelline and Seed Yield of Fenugreek (Trigonella foenum-gracum L.). Agronomy 2023, 13, 2070. https://doi.org/10.3390/agronomy13082070
Ramezani S, Yousefshahi B, Farrokhzad Y, Ramezan D, Zargar M, Pakina E. Selenium and Iodine Biofortification Interacting with Supplementary Blue Light to Enhance the Growth Characteristics, Pigments, Trigonelline and Seed Yield of Fenugreek (Trigonella foenum-gracum L.). Agronomy. 2023; 13(8):2070. https://doi.org/10.3390/agronomy13082070
Chicago/Turabian StyleRamezani, Sadrollah, Behnaz Yousefshahi, Yusuf Farrokhzad, Dariush Ramezan, Meisam Zargar, and Elena Pakina. 2023. "Selenium and Iodine Biofortification Interacting with Supplementary Blue Light to Enhance the Growth Characteristics, Pigments, Trigonelline and Seed Yield of Fenugreek (Trigonella foenum-gracum L.)" Agronomy 13, no. 8: 2070. https://doi.org/10.3390/agronomy13082070
APA StyleRamezani, S., Yousefshahi, B., Farrokhzad, Y., Ramezan, D., Zargar, M., & Pakina, E. (2023). Selenium and Iodine Biofortification Interacting with Supplementary Blue Light to Enhance the Growth Characteristics, Pigments, Trigonelline and Seed Yield of Fenugreek (Trigonella foenum-gracum L.). Agronomy, 13(8), 2070. https://doi.org/10.3390/agronomy13082070








