Genome-Wide Syntenic and Evolutionary Analysis of 30 Key Genes Found in Ten Oryza Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequence Data
2.2. Phylogenetic Analysis
2.3. Synteny Analysis
2.4. Identification of Key Genes
3. Results
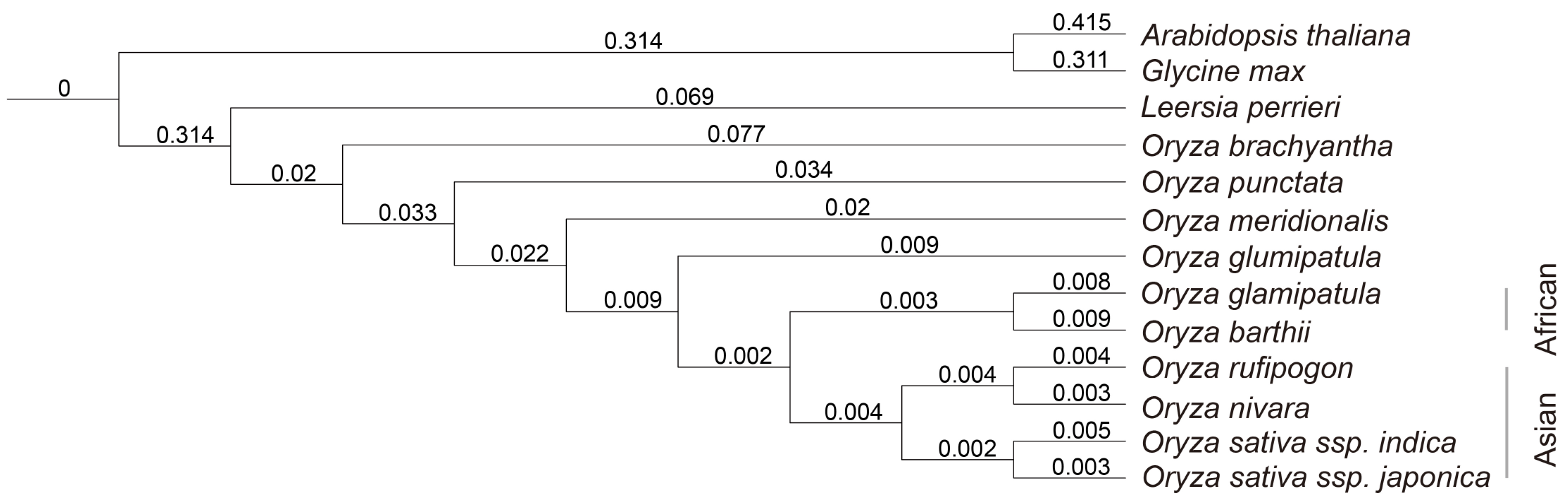
3.1. Phylogenetic Analysis of the Genus Oryza
3.2. Syntenic Analysis within the Asian Group
3.3. Identification of Orthologs and Paralogs of Target Genes
3.4. Syntenic Analysis of His1, qSW5/GW5, and GS3 in the HV Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rezvi, H.U.A.; Tahjib-Ul-Arif, M.; Azim, M.A.; Tumpa, T.A.; Tipu, M.M.H.; Najnine, F.; Dawood, M.F.A.; Skalicky, M.; Brestič, M. Rice and Food Security: Climate Change Implications and the Future Prospects for Nutritional Security. Food Energy Secur. 2023, 12, e430. [Google Scholar] [CrossRef]
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not Just a Grain of Rice: The Quest for Quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 13 June 2023).
- Carcea, M. Value of Wholegrain Rice in a Healthy Human Nutrition. Agriculture 2021, 11, 720. [Google Scholar] [CrossRef]
- Genevois, C.E.; Grenóvero, M.S.; de Escalada Pla, M.F. Use of Different Proportions of Rice Milling Fractions as Strategy for Improving Quality Parameters and Nutritional Profile of Gluten-Free Bread. JFST 2021, 58, 3913–3923. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Pasqualone, A.; Caponio, F. Potential Use of Plant-Based by-Products and Waste to Improve the Quality of Gluten-Free Foods. J. Sci. Food Agric. 2022, 102, 2199–2211. [Google Scholar] [CrossRef]
- World Development Indicators|DataBank. Available online: https://databank.worldbank.org/reports.aspx?source=World-Development-Indicators (accessed on 18 June 2023).
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding Crops to Feed 10 Billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The Rice Genome Revolution: From an Ancient Grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Morishima, H.; Kadowaki, K. Diversity in the Oryza Genus. Curr. Opin. Plant Biol. 2003, 6, 139–146. [Google Scholar] [CrossRef]
- Harlan, J.R. Crops and Man; American Society of Agronomy: Madison, WI, USA, 1992. [Google Scholar]
- Li, Z.M.; Zheng, X.M.; Ge, S. Genetic Diversity and Domestication History of African Rice (Oryza Glaberrima) as Inferred from Multiple Gene Sequences. Theor. Appl. Genet. 2011, 123, 21–31. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, X.; Luo, J.; Gaut, B.S.; Ge, S. Multilocus Analysis of Nucleotide Variation of Oryza Sativa and Its Wild Relatives: Severe Bottleneck during Domestication of Rice. Mol. Biol. Evol. 2007, 24, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, R.; Singh, B.; Mondal, T.K.; Bisht, D.S. Current Status of Genomic Resources on Wild Relatives of Rice. Breed. Sci. 2020, 70, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Quan, R.; Wang, J.; Hui, J.; Bai, H.; Lyu, X.; Zhu, Y.; Zhang, H.; Zhang, Z.; Li, S.; Huang, R. Improvement of Salt Tolerance Using Wild Rice Genes. Front. Plant Sci. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Das-Chatterjee, A.; Goswami, L.; Maitra, S.; Dastidar, K.G.; Ray, S.; Majumder, A.L. Introgression of a Novel Salt-Tolerant L-Myo-Inositol 1-Phosphate Synthase from Porteresia Coarctata (Roxb.) Tateoka (PcINO1) Confers Salt Tolerance to Evolutionary Diverse Organisms. FEBS Lett. 2006, 580, 3980–3988. [Google Scholar] [CrossRef] [Green Version]
- Jeung, J.U.; Kim, B.R.; Cho, Y.C.; Han, S.S.; Moon, H.P.; Lee, Y.T.; Jena, K.K. A Novel Gene, Pi40(t), Linked to the DNA Markers Derived from NBS-LRR Motifs Confers Broad Spectrum of Blast Resistance in Rice. Theor. Appl. Genet. 2007, 115, 1163–1177. [Google Scholar] [CrossRef]
- Leach, D.R.F. Genetic Recombination; Blackwell Science Ltd.: Oxford, UK, 1996. [Google Scholar]
- Flint-Garcia, S.A.; Thornsberry, J.M.; Buckler, E.S. Structure of Linkage Disequilibrium in Plants. Annu. Rev. Plant Biol. 2003, 54, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Slatkin, M. Linkage Disequilibrium—Understanding the Evolutionary Past and Mapping the Medical Future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Rieseberg, L.H.; Linder, C.R.; Seiler, G.J. Chromosomal and Genic Barriers to Introgression in Helianthus. Genetics 1995, 141, 1163–1171. [Google Scholar] [CrossRef]
- Knoll, A.; Fauser, F.; Puchta, H. DNA Recombination in Somatic Plant Cells: Mechanisms and Evolutionary Consequences. Chromosome Res. 2014, 22, 191–201. [Google Scholar] [CrossRef]
- Xiao, N.; Pan, C.; Li, Y.; Wu, Y.; Cai, Y.; Lu, Y.; Wang, R.; Yu, L.; Shi, W.; Kang, H.; et al. Genomic Insight into Balancing High Yield, Good Quality, and Blast Resistance of Japonica Rice. Genome Biol. 2021, 22, 283. [Google Scholar] [CrossRef]
- Rangel, P.N.; Brondani, R.P.V.; Rangel, P.H.N.; Brondani, C. Agronomic and Molecular Characterization of Introgression Lines from the Interspecific Cross Oryza Sativa (BG90-2) x Oryza Glumaepatula (RS-16). Genet. Mol. Res. 2008, 7, 184–195. [Google Scholar] [CrossRef]
- Ali, M.L.; Sanchez, P.L.; Yu, S.; Lorieux, M.; Eizenga, G.C. Chromosome Segment Substitution Lines: A Powerful Tool for the Introgression of Valuable Genes from Oryza Wild Species into Cultivated Rice (O. Sativa). Rice 2010, 3, 218–234. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Chand, S.; Singh, N.K.; Sharma, T.R. Genome-Wide Distribution, Organisation and Functional Characterization of Disease Resistance and Defence Response Genes across Rice Species. PLoS ONE 2015, 10, e0125964. [Google Scholar] [CrossRef] [Green Version]
- Ganie, S.A.; Pani, D.R.; Mondal, T.K. Genome-Wide Analysis of DUF221 Domain-Containing Gene Family in Oryza Species and Identification of Its Salinity Stress-Responsive Members in Rice. PLoS ONE 2017, 12, e0182469. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Rational Design of High-Yield and Superior-Quality Rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef]
- Kraehmer, H.; van Almsick, A.; Beffa, R.; Dietrich, H.; Eckes, P.; Hacker, E.; Hain, R.; Strek, H.J.; Stuebler, H.; Willms, L. Herbicides as Weed Control Agents: State of the Art: II. Recent Achievements. Plant Physiol. 2014, 166, 1132–1148. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.; Waters, D.L.E. The Gene for Fragrance in Rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- Bandi, V.K. SynVisio: A Multiscale Tool to Explore Genomic Conservation. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2020. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, Y.; Oono, Y.; Wakimoto, H.; Ogata, J.; Kanamori, H.; Sasaki, H.; Mori, S.; Matsumoto, T.; Itoh, T. TENOR: Database for Comprehensive MRNA-Seq Experiments in Rice. PCP 2016, 57, e7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Liang, Q.; Li, C.; Fu, S.; Kundu, J.K.; Zhou, X.; Wu, J. Transcriptome Analysis of Rice Reveals the LncRNA–MRNA Regulatory Network in Response to Rice Black-Streaked Dwarf Virus Infection. Viruses 2020, 12, 951. [Google Scholar] [CrossRef]
- Yang, D.; Li, S.; Xiao, Y.; Lu, L.; Zheng, Z.; Tang, D.; Cui, H. Transcriptome Analysis of Rice Response to Blast Fungus Identified Core Genes Involved in Immunity. Plant Cell Environ. 2021, 44, 3103–3121. [Google Scholar] [CrossRef]
- Tran, T.T.; Pérez-Quintero, A.L.; Wonni, I.; Carpenter, S.C.D.; Yu, Y.; Wang, L.; Leach, J.E.; Verdier, V.; Cunnac, S.; Bogdanove, A.J.; et al. Functional Analysis of African Xanthomonas Oryzae Pv. Oryzae TALomes Reveals a New Susceptibility Gene in Bacterial Leaf Blight of Rice. PLoS Pathog. 2018, 14, e1007092. [Google Scholar] [CrossRef]
- Ram, S.G.; Thiruvengadam, V.; Vinod, K.K. Genetic Diversity among Cultivars, Landraces and Wild Relatives of Rice as Revealed by Microsatellite Markers. J. Appl. Genet. 2007, 48, 337–345. [Google Scholar] [CrossRef]
- Oka, H.I. Origin of Cultivated Rice; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Guo, J.; Xu, X.; Li, W.; Zhu, W.; Zhu, H.; Liu, Z.; Luan, X.; Dai, Z.; Liu, G.; Zhang, Z.; et al. Overcoming Inter-Subspecific Hybrid Sterility in Rice by Developing Indica-Compatible Japonica Lines. Sci. Rep. 2016, 6, 26878. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Chen, J.; Ding, J.; Zhang, Q. Advances in the Understanding of Inter-Subspecific Hybrid Sterility and Wide-Compatibility in Rice. Chin. Sci. Bull. 2009, 54, 2332–2341. [Google Scholar] [CrossRef]
- Zhang, L.; Reifová, R.; Halenková, Z.; Gompert, Z. How Important Are Structural Variants for Speciation? Genes 2021, 12, 1084. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Grover, A.; Sharma, P.C. Genome-Wide Analysis of Conservation and Divergence of Microsatellites in Rice. Mol. Genet. Genom. 2009, 282, 205–215. [Google Scholar] [CrossRef]
- Kim, H.; Hurwitz, B.; Yu, Y.; Collura, K.; Gill, N.; SanMiguel, P.; Mullikin, J.C.; Maher, C.; Nelson, W.; Wissotski, M.; et al. Construction, Alignment and Analysis of Twelve Framework Physical Maps That Represent the Ten Genome Types of the Genus Oryza. Genome Biol. 2008, 9, R45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zheng, H.G.; Aarti, A.; Pantuwan, G.; Nguyen, T.T.; Tripathy, J.N.; Sarial, A.K.; Robin, S.; Babu, R.C.; Nguyen, B.D.; et al. Locating Genomic Regions Associated with Components of Drought Resistance in Rice: Comparative Mapping within and across Species. Theor. Appl. Genet. 2001, 103, 19–29. [Google Scholar] [CrossRef]
- Cook, D.E.; Lee, T.G.; Guo, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; et al. Copy Number Variation of Multiple Genes at Rhg1 Mediates Nematode Resistance in Soybean. Science 2012, 338, 1206–1209. [Google Scholar] [CrossRef] [Green Version]
- Bakker, E.G.; Toomajian, C.; Kreitman, M.; Bergelson, J. A Genome-Wide Survey of R Gene Polymorphisms in Arabidopsis. Plant Cell 2006, 18, 1803–1818. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Ren, J.; Peng, Z.; Umana, A.A.; Le, H.; Danilova, T.; Fu, J.; Wang, H.; Robertson, A.; Hulbert, S.H.; et al. Analysis of Extreme Phenotype Bulk Copy Number Variation (XP-CNV) Identified the Association of Rp1 with Resistance to Goss’s Wilt of Maize. Front. Plant Sci. 2018, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Dolatabadian, A.; Yuan, Y.; Bayer, P.E.; Petereit, J.; Severn-Ellis, A.; Tirnaz, S.; Patel, D.; Edwards, D.; Batley, J. Copy Number Variation among Resistance Genes Analogues in Brassica Napus. Genes 2022, 13, 2037. [Google Scholar] [CrossRef]
- Kwon, O.D.; Shin, S.H.; An, K.N.; Lee, Y.; Min, H.K.; Park, H.G.; Shin, H.R.; Jung, H.I.; Kuk, Y.I. Response of Phytotoxicity on Rice Varieties to HPPD-inhibiting Herbicides in Paddy Rice Fields. Korean J. Weed Sci. 2012, 32, 240–255. [Google Scholar] [CrossRef] [Green Version]
- Young, M.L.; Norsworthy, J.K.; Scott, R.C.; Bond, J.A.; Heiser, J. Benzobicyclon as a Post-Flood Option for Weedy Rice Control. Weed Technol. 2018, 32, 371–378. [Google Scholar] [CrossRef]
- Zuo, J.; Li, J. Molecular Genetic Dissection of Quantitative Trait Loci Regulating Rice Grain Size. Annu. Rev. Genet. 2014, 48, 99–118. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, G.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.; Zeng, L.; Xu, E.; Xu, J.; et al. Copy Number Variation at the GL7 Locus Contributes to Grain Size Diversity in Rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef]
- Mi, J.; Lei, Y.; Kim, S.-R.; Prahalada, G.D.; Ouyang, Y.; Mou, T. An Effective Strategy for Fertility Improvement of Indica-Japonica Hybrid Rice by Pyramiding S5-n, F5-n, and Pf12-j. Mol. Breed. 2019, 39, 138. [Google Scholar] [CrossRef]



| (A) Numbers of Synteny Blocks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obra | Opun | Omer | Oglu | Ogla | Obar | Oruf | Oniv | Oind | Ojap | |
| Obra | 115 | 353 | 486 | 378 | 344 | 300 | 331 | 645 | 440 | 305 |
| Opun | 159 | 551 | 458 | 410 | 379 | 420 | 745 | 515 | 394 | |
| Omer | 137 | 595 | 510 | 513 | 571 | 823 | 658 | 523 | ||
| Oglu | 150 | 427 | 404 | 436 | 824 | 509 | 421 | |||
| Ogla | 134 | 337 | 387 | 710 | 487 | 358 | ||||
| Obar | 140 | 350 | 729 | 419 | 328 | |||||
| Oruf | 149 | 818 | 448 | 342 | ||||||
| Oniv | 160 | 901 | 706 | |||||||
| Oind | 155 | 443 | ||||||||
| Ojap | 155 | |||||||||
| (B) Average number of genes per synteny block | ||||||||||
| Obra | Opun | Omer | Oglu | Ogla | Obar | Oruf | Oniv | Oind | Ojap | |
| Obra | 24.5 | 68.9 | 40.9 | 63.1 | 63.4 | 78.2 | 73.4 | 34.9 | 61.6 | 80.7 |
| Opun | 21.4 | 40.4 | 58.9 | 58.6 | 69.5 | 65.6 | 34.4 | 49.6 | 67.8 | |
| Omer | 14.8 | 39.9 | 40.8 | 45.2 | 43.0 | 27.5 | 34.7 | 42.9 | ||
| Oglu | 20.4 | 61.4 | 74.7 | 72.5 | 35.7 | 57.3 | 65.9 | |||
| Ogla | 19.8 | 79.0 | 70.9 | 35.6 | 53.5 | 71.2 | ||||
| Obar | 20.3 | 90.1 | 39.9 | 69.4 | 83.4 | |||||
| Oruf | 21.3 | 38.4 | 70.1 | 84.4 | ||||||
| Oniv | 14.1 | 32.3 | 37.5 | |||||||
| Oind | 16.9 | 62.3 | ||||||||
| Ojap | 22.0 | |||||||||
| (C) Total length of synteny blocks, including duplication (kbp) | ||||||||||
| Obra | Opun | Omer | Oglu | Ogla | Obar | Oruf | Oniv | Oind | Ojap | |
| Obra | 102,259 | 482,879 | 424,226 | 470,842 | 431,825 | 535,282 | 459,779 | 431,573 | 421,875 | 461,883 |
| Opun | 165,853 | 601,179 | 688,557 | 524,906 | 557,918 | 744,092 | 567,689 | 563,041 | 682,892 | |
| Omer | 95,698 | 599,219 | 467,896 | 491,808 | 597,297 | 552,403 | 483,530 | 598,173 | ||
| Oglu | 135,462 | 522,483 | 563,117 | 688,523 | 640,215 | 620,922 | 675,950 | |||
| Ogla | 101,031 | 511,157 | 509,612 | 492,492 | 483,220 | 513,075 | ||||
| Obar | 107,814 | 551,033 | 527,107 | 511,753 | 545,746 | |||||
| Oruf | 118,095 | 571,611 | 584,100 | 662,749 | ||||||
| Oniv | 80,763 | 513,998 | 616,664 | |||||||
| Oind | 89,577 | 577,416 | ||||||||
| Ojap | 141,898 | |||||||||
| Gene Name | Obra | Opun | Omer | Oglu | Ogla | Obar | Oruf | Oniv | Oind | Ojap | sum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| His1 | 2 | 7 | 4 | 2 | 6 | 4 | 3 | 5 | 3 | 7 | 43 | HV |
| GS3 | 2 | 3 | 2 | 3 | 1 | 3 | 3 | 3 | 5 | 3 | 28 | |
| qSW5/GW5 | 1 | 2 | 2 | 3 | 3 | 1 | 4 | 4 | 4 | 3 | 27 | |
| TAC1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 13 | SD |
| PUL | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 11 | |
| SSIII-1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | |
| SSI | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 11 | |
| Gn1a | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| OsAGPS2a | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 19 | SL |
| SBE3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 18 | |
| BADH2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 17 | |
| SSII-2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |
| HD1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | |
| Ghd7 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |
| GBSSII | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Ghd8 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | |
| DPE1, OsDPE1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | |
| SSIV-1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | NV |
| SSIV-2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| AGPL1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| AGPL4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| WX1, GBSSI | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| sd1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| OsAGPL1, OsAPL3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| ALK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| SCM2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| BEI, SBE1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| SSIII-2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| OsPHS8, PHS8, ISA1, OsISA1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| OsSSII-1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Lim, I.; Ha, J. Genome-Wide Syntenic and Evolutionary Analysis of 30 Key Genes Found in Ten Oryza Species. Agronomy 2023, 13, 2100. https://doi.org/10.3390/agronomy13082100
Cho Y, Lim I, Ha J. Genome-Wide Syntenic and Evolutionary Analysis of 30 Key Genes Found in Ten Oryza Species. Agronomy. 2023; 13(8):2100. https://doi.org/10.3390/agronomy13082100
Chicago/Turabian StyleCho, Yeonghun, Insu Lim, and Jungmin Ha. 2023. "Genome-Wide Syntenic and Evolutionary Analysis of 30 Key Genes Found in Ten Oryza Species" Agronomy 13, no. 8: 2100. https://doi.org/10.3390/agronomy13082100
APA StyleCho, Y., Lim, I., & Ha, J. (2023). Genome-Wide Syntenic and Evolutionary Analysis of 30 Key Genes Found in Ten Oryza Species. Agronomy, 13(8), 2100. https://doi.org/10.3390/agronomy13082100





