Heterosis and Mixed Genetic Analysis of Flowering Traits in Cross Breeding of Day-Neutral Chrysanthemum (Asteraceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Investigation of Field Traits
2.3. Definition and Measurement Method of Abnormal (Crown) Bud
2.4. Definition and Calculation of the Photoperiod
2.5. Heterosis Analysis and Significance Test
2.6. Mixed Genetic Analysis
2.7. Screening Methods for Hybrid Progeny
3. Results
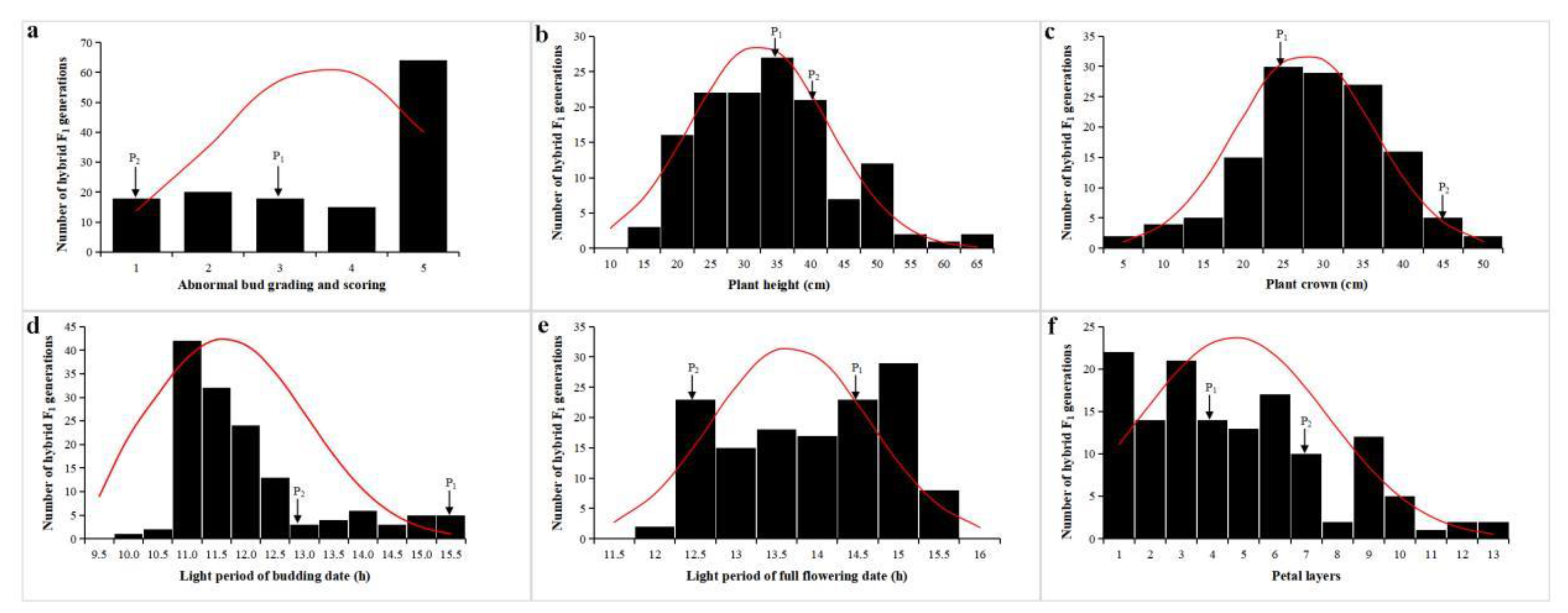
3.1. Phenotypic Distribution and Heterosis of Flowering-Related Traits of the F1 Population
3.2. Suitability Analysis of the Optimal Genetic Model for Flowering-Related Traits in Day-Neutral Chrysanthemums
3.3. Estimation of Genetic Parameters for Flowering-Related Traits in Day-Neutral Chrysanthemum
3.4. Correlation Analysis of Flowering Traits of Day-Neutral Chrysanthemum
3.5. Screening of Selected Day-Neutral Chrysanthemum Genotypes
4. Discussion
4.1. Establishment of Evaluation Criteria for Flowering Time of Day-Neutral Multiflora Chrysanthemum
4.2. Heterosis of Flowering-Related Traits in Day-Neutral Chrysanthemums
4.3. Genetic Effects of Flowering-Related Traits in Day-Neutral Chrysanthemums
4.4. Breeding Prospects of Day-Neutral Chrysanthemums
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, N.O.; Ascher, P.D. Selection of Day-neutral, Heat-delay-insensitive Dendranthem ×grandiflora Genotypes. J. Am. Soc. Hortic. Sci. 2001, 126, 710–721. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.B.; Zhang, Q.X. Advances and prospects in molecular breeding of day-neutral chrysanthemum. Mol. Plant Breed. 2010, 8, 350–358. (In Chinese) [Google Scholar]
- Anderson, N.O.; Ascher, P.D. Inheritance of Seed Set, Germination, and Day Neutrality/Heat Delay Insensitivity of Garden Chrysanthemums (Dendranthema ×grandiflora) under Glasshouse and Field Conditions. J. Am. Soc. Hortic. Sci. 2004, 129, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.L.; Zhou, X.Y.; Xi, L.; Li, J.X.; Zhao, R.Y.; Ma, N.; Zhao, L.J. Roles of DgBRC1 in Regulation of Lateral Branching in Chrysanthemum (Dendranthema ×grandiflora cv. Jinba). PLoS ONE 2013, 8, e61717. [Google Scholar] [CrossRef] [Green Version]
- Dierck, R.; Leus, L.; Dhooghe, E.; Van Huylenbroeck, J.; De Riek, J.; Van Der Straeten, D.; De Keyser, E. Branching gene expression during chrysanthemum axillary bud outgrowth regulated by strigolactone and auxin transport. Plant Growth Regul. 2018, 86, 23–36. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, L.; Song, A.P.; Li, S.; Liu, J.Y.; Zhao, W.Q.; Jia, D.W.; Guan, Y.X.; Zhao, K.K.; Chen, S.M.; et al. Dwarf and Robust Plant regulates plant height via modulating gibberellin biosynthesis in chrysanthemum. Plant Physiol. 2022, 190, 2484–2500. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.W.; Wen, X.H.; Fu, J.X.; Dai, S.L. ClCRY2 facilitates floral transition in Chrysanthemum lavandulifolium by affecting the transcription of circadian clock-related genes under short-day photoperiods. Hortic. Res. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.Q.; Ahmad, S.; Cheng, T.R.; Wang, J.; Pan, H.T.; Zhao, L.J.; Zhang, Q.X. Red to Far-Red Light Ratio Modulates Hormonal and Genetic Control of Axillary bud Outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Int. J. Mol. Sci. 2018, 19, 1590. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.J. The Lateral Bud Development Related Candidate Genes Research and Functional Verification of Inhibition of High Temperature on Cut Flower Chrysanthemum ‘SEI NO ISSEI’. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2019. [Google Scholar]
- Liu, W.X. Molecular Mechanism of Sucrose Regulates Axillary Bud. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2019. [Google Scholar]
- Ding, L.; Song, A.P.; Zhang, X.; Li, S.; Su, J.S.; Xia, W.K.; Zhao, K.K.; Zhao, W.Q.; Guan, Y.X.; Fang, W.M.; et al. The core regulatory networks and hub genes regulating flower development in Chrysanthemum morifolium. Plant Mol. Biol. 2020, 103, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Liu, P.S.; Yin, D.M. Heterosis and mixed genetic analysis for fluorescence related traits of chrysanthemum. J. Nanjing Agric. Univ. 2011, 34, 31–36. (In Chinese) [Google Scholar]
- Lyu, J.; Aiwaili, P.; Gu, Z.Y.; Xu, Y.J.; Zhang, Y.H.; Wang, Z.L.; Huang, H.F.; Zeng, R.H.; Ma, C.; Gao, J.P.; et al. Chrysanthemum MAF2 regulates flowering by repressing gibberellin biosynthesis in response to low temperature. Plant J. 2022, 112, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Shulga, O.A.; Mitiouchkina, T.Y.; Shchennikova, A.V.; Skryabin, K.G.; Dolgov, S.V. Overexpression of AP1-like genes from Asteraceae induces early-flowering in transgenic Chrysanthemum plants. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 553–560. [Google Scholar] [CrossRef]
- Yang, Y.J.; Ma, C.; Xu, Y.J.; Wei, Q.; Imtiaz, M.; Lan, H.B.; Gao, S.; Cheng, L.N.; Wang, M.Y.; Zhang, J.F.; et al. A Zinc Finger Protein Regulates Flowering Time and Abiotic Stress Tolerance in Chrysanthemum by Modulating Gibberellin Biosynthesis. Plant Cell 2014, 26, 2038–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Ma, C.; Xu, Y.J.; Wang, T.L.; Chen, Y.Y.; Lu, J.; Zhang, L.L.; Jiang, C.Z.; Hong, B.; Gao, J.P. Control of chrysanthemum flowering through integration with an aging pathway. Nat. Commun. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, A.; Narumi, T.; Li, T.P.; Kando, T.; Higuchi, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. CsFTL3, a chrysanthemum FLOWERING LOCUS T-like gene, is a key regulator of photoperiodic flowering in chrysanthemums. J. Exp. Bot. 2012, 63, 1461–1477. [Google Scholar] [CrossRef] [Green Version]
- Oda, A.; Higuchi, Y.; Hisamatsu, T. Photoperiod-insensitive floral transition in chrysanthemum induced by constitutive expression of chimeric repressor CsLHY-SRDX. Plant Sci. 2017, 259, 86–93. [Google Scholar] [CrossRef]
- Nakano, Y.; Takase, T.; Takahashi, S.; Sumitomo, K.; Higuchi, Y.; Hisamatsu, T. Chrysanthemum requires short-day repeats for anthesis: Gradual CsFTL3 induction through a feedback loop under short-day conditions. Plant Sci. 2019, 283, 247–255. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.M.; Chen, F.D.; Fang, W.M.; Li, F.T. A preliminary genetic linkage map of chrysanthemum (Chrysanthemum morifolium) cultivars using RAPD, ISSR and AFLP markers. Sci. Hortic. 2010, 125, 422–428. [Google Scholar] [CrossRef]
- Dugo, M.L.; Satovic, Z.; Millan, T.; Cubero, J.I.; Rubiales, D.; Cabrera, A.; Torres, A.M. Genetic mapping of QTLs controlling horticultural traits in diploid roses. Theor. Appl. Genet. 2005, 111, 511–520. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Gai, J.Y.; Yang, Y.H. The EIM algorithm in the joint segregation analysis of quantitative traits. Genet. Res. 2003, 81, 157–163. [Google Scholar] [CrossRef]
- Anbessa, Y.; Warkentin, T.; Vandenberg, A.; Ball, R. Inheritance of time to flowering in chickpea in a short-season temperate environment. J. Hered. 2006, 97, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.J.; Yu, S.X.; Ma, Q.X.; Fan, S.L.; Song, M.Z. Inheritance of time of flowering in upland cotton under natural conditions. Plant Breed. 2008, 127, 383–390. [Google Scholar] [CrossRef]
- Wang, X.D.; Zheng, M.; Liu, H.F.; Zhang, L.; Chen, F.; Zhang, W.; Fan, S.H.; Peng, M.L.; Hu, M.L.; Wang, H.Z.; et al. Fine-mapping and transcriptome analysis of a candidate gene controlling plant height in Brassica napus L. Biotechnol. Biofuels 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Khan, A.J.; Khattak, G.S.S.; Subhan, F. Genetic effects in controlling grain filling duration in wheat crosses. J. Anim. Plant Sci. 2014, 24, 803–813. [Google Scholar]
- Wang, Y.; Liu, Z.S.; Yang, X.Q.; Wang, Z.Y.; Ma, L.; Tu, H.X.; Ma, Y.; Zhou, J.T.; Zhang, J.; Wang, H.; et al. Inheritance analysis of fruit-related traits in Chinese cherry [Cerasus pseudocerasus (Lindl.) G.Don] breeding progenies. Sci. Hortic. 2023, 307, 111519. [Google Scholar] [CrossRef]
- Ye, H.X.; Lv, L.; Hai, R.; Hu, Y.Q.; Wang, B.L. Genetic analysis of fruit sugar content in melon (Cucumis melo L.) based on a mixed model of major genes and polygenes. J. Zhejiang Univ. (Agric. Life Sci.) 2019, 45, 391–400. (In Chinese) [Google Scholar]
- Kong, C.C.; Chen, G.; Yang, L.M.; Zhuang, M.; Zhang, Y.Y.; Wang, Y.; Ji, J.L.; Fang, Z.Y.; Lv, H.H. Germplasm screening and inheritance analysis of resistance to cabbage black rot in a worldwide collection of cabbage (Brassica oleracea var. capitata) resources. Sci. Hortic. 2021, 288, 110234. [Google Scholar] [CrossRef]
- Liang, D.N.; Hu, Q.J.; Xu, Q.; Qi, X.H.; Zhou, F.C.; Chen, X.H. Genetic inheritance analysis of melon aphid (Aphis gossypii Glover) resistance in cucumber (Cucumis sativus L.). Euphytica 2015, 205, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Song, X.B.; Kong, D.Y.; Dai, S.L. Genetic Analysis of Leaf Traits in Small-Flower Chrysanthemum (Chrysanthemum ×morifolium Ramat.). Agronomy 2020, 10, 697. [Google Scholar] [CrossRef]
- Song, X.B.; Zhao, X.G.; Fan, G.X.; Gao, K.; Dai, S.L.; Zhang, M.M.; Ma, C.F.; Wu, X.Y. Genetic analysis of the corolla tube merged degree and the relative number of ray florets in chrysanthemum (Chrysanthemum ×morifolium Ramat.). Sci. Hortic. 2018, 242, 214–224. [Google Scholar] [CrossRef]
- Tang, H.Q.; Zhang, F.; Chen, F.D.; Fang, W.M.; Wang, C.C.; Chen, S.M. Heterosis and Mixed Genetic Analysis of Inflorescence Traits of Anemone-typed Chrysanthemum. Acta Hortic. Sin. 2015, 42, 907–916. (In Chinese) [Google Scholar]
- Fu, X.; Su, J.S.; Li, Y.Y.; Zhang, F.; Fang, W.M.; Chen, S.M.; Chen, F.D.; Guan, Z.Y. Genetic Variation and QTL Mapping for Aphid Resistance in an F1 Population of Chrysanthemum. Acta Hortic. Sin. 2019, 46, 1351–1358. (In Chinese) [Google Scholar]
- Zhao, Q.R.; Zhong, X.H.; Zhang, F.; Fang, W.M.; Chen, F.D.; Teng, N.J. Heterosis and Mixed Genetic Analysis of Green-Center Trait of Spray Cut Chrysanthemum. Sci. Agric. Sin. 2018, 51, 964–976. [Google Scholar]
- Peng, H.; Chen, F.D.; Fang, W.M.; Jiang, J.F.; Chen, S.M.; Guan, Z.Y.; Liao, Y. Heterosis and Mixed Genetic Analysis of Branch Traits of Cut Chrysanthemum. Acta Hortic. Sin. 2013, 40, 1327–1336. (In Chinese) [Google Scholar]
- Chi, T.H.; Xu, T.T.; Liu, Y.X.; Ma, J.; Guan, Z.Y.; Fang, W.M.; Chen, F.D.; Zhang, F. Genetic Variation for Cold Tolerance in an Interspecific C. dichrum × C. nankingense Population. J. Nucl. Agric. Sci. 2018, 32, 2298–2304. (In Chinese) [Google Scholar]
- Zhang, F.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Li, F.T. Heterosis and Mixed Genetic Analysis of Inflorescence Traits of Chrysanthemum. Sci. Agric. Sin. 2010, 43, 2953–2961. [Google Scholar]
- Zhang, F.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Li, F.T. Heterosis and Major Gene Plus Polygene Mixed Genetic Analysis for Vegetative Traits in Chrysanthemum. Sci. Silvae Sin. 2011, 47, 46–52. [Google Scholar]
- Zhang, M.M.; Huang, H.; Wang, Q.; Dai, S. Cross Breeding New Cultivars of Early-flowering Multiflora Chrysanthemum Based on Mathematical Analysis. Hortscience 2018, 53, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Hou, M. The formation and countermeasures of abnormal bud. Flower Plant Penjing 2007, 9, 20. (In Chinese) [Google Scholar]
- Cockshull, K.E.; Kofranek, A.M. Long-day flower initiation by Chrysanthemum. Hortscience 1985, 20, 296–298. [Google Scholar] [CrossRef]
- Li, B.L.; Wu, R. Heterosis and genotype environment interactions of juvenile aspens in two contrasting sites. Can. J. For. Res. 1997, 73, 3671–3675. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.Y. Segregation analysis of genetic system of quantitative traits in plants. Hereditas 2005, 27, 130–136. (In Chinese) [Google Scholar]
- Stone, M. An asymptotic equivalence of choice of model by cross-validation and Akaike’s criterion. J. R. Stat. Soc. 1977, 39, 44–47. [Google Scholar] [CrossRef]
- Chen, J.Y.; Deng, C.Z. Using the hundred-mask system to select super-trees in Camellia Chrysantha (Hu) Tuyama, C. tunghinensis Chang and C. pubipetala Y. wan et S. Z. Huang. J. Beijing For. Univ. 1986, 3, 35–43. (In Chinese) [Google Scholar]
- International Union for the Protection of New Varieties of Plants (UPOV). Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability Chrysanthemum (Chrysanthemum × morifolium Ramat.); UPOV: Geneva, Switzerland, 2010. [Google Scholar]
- Chen, S.L.; Li, Y.R.; Cheng, Z.S.; Liao, B.S.; Lei, Y.; Liu, J.S. Heterosis and Genetic Analysis of Oil Content in Peanut Using Mixed Model of Major Gene and Polygene. Sci. Agric. Sin. 2009, 42, 3048–3057. [Google Scholar]
- Cheng, H.; Yu, Y.; Zhai, Y.W.; Wang, L.J.; Wang, L.K.; Chen, S.M.; Chen, F.D.; Jiang, J.F. An ethylene-responsive transcription factor and a B-box protein coordinate vegetative growth and photoperiodic flowering in chrysanthemum. Plant Cell Environ. 2023, 46, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.X.; Ding, L.; Jiang, J.F.; Shentu, Y.Y.; Zhao, W.Q.; Zhao, K.K.; Zhang, X.; Song, A.P.; Chen, S.M.; Chen, F.D. Overexpression of the CmJAZ1-like gene delays flowering in Chrysanthemum morifolium. Hortic. Res. 2021, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.X.; Ding, L.; Jiang, J.F.; Jia, D.W.; Li, S.; Jin, L.; Zhao, W.Q.; Zhang, X.; Song, A.P.; Chen, S.M.; et al. The TIFY family protein CmJAZ1-like negatively regulates petal size via interaction with the bHLH transcription factor CmBPE2 in Chrysanthemum morifolium. Plant J. 2022, 112, 1489–1506. [Google Scholar] [CrossRef]
- Jiang, J.F.; Chen, F.D.; Guo, W.M. Heredity of several characters in Dendranthema grandiflora with small florence. J. Nanjing Agric. Univ. 2003, 26, 11–15. (In Chinese) [Google Scholar]
- Anderson, N.O.; Widmer, R.E.; Ascher, P.D. Greenhouse Potted Chrysanthemum, Mn. Sel’n. 83-267-3. U.S. Plant Patent No. 6884, 1988. [Google Scholar]
| Hybrid Parents | Plant Height (cm) | Plant Crown Width (cm) | Photoperiod at Flower Bud Initiation (Visible Bud Date) (h:min:s) | Photoperiod at Full Flowering (Anthesis) Date (h:min:s) | No. of Petal Layers |
|---|---|---|---|---|---|
| 82-81-19 (female) | 25 | 17 | 14:13:03 | 13:45:45 | 4 (quadriplex) |
| 388Q-76 (male) | 37 | 38 | 12:15:56 | 10:36:50 | 7 (septiplex) |
| Plant Traits | Measuring Methods |
|---|---|
| Plant height (cm) | The distance from the base of the stem to the highest point of inflorescence |
| Plant crown width (cm) | The diameter of its development is measured from the top surface of the plant |
| Budding date (VBD) | The specific time when the bud size is about 5 mm |
| Full flowering date (anthesis) | The specific time when 50% of the buds reach full flowering (anthesis) |
| No. of petal layers | The total number of petal (ray floret) whorls in the capitulum |
| Grade | Number of Abnormal Buds/Plant | Bud Development Description | Score |
|---|---|---|---|
| I | 0–10 | The buds have developed normally | 1 |
| II | 10–20 | The buds have developed normally | 2 |
| III | 20–30 | Less than 1/3 of the buds are abnormally developed | 3 |
| IV | 30–40 | More than 2/3 of the buds are abnormally developed | 4 |
| V | >40 | All buds are abnormally developed | 5 |
| Traits | Total Points | Traits and Scores | |||||
|---|---|---|---|---|---|---|---|
| Plant type | Plant height (cm) | 15 | >40.0 (15) | 30.0–40.0 (12) | 25.0–30.0 (9) | 15.0–25.0 (6) | <15.0 (3) |
| Plant crown width (cm) | 15 | >40.0 (15) | 30.0–40.0 (12) | 25.0–30.0 (9) | 15.0–25.0 (6) | <15.0 (3) | |
| Flowering date | Abnormal (crown) bud | 20 | 1 (20) | 2 (16) | 3 (10) | 4 (5) | 5 (0) |
| Budding date (VBD) | 20 | Before 5.10 (20) | 5.11–5.31 (15) | 6.1–6.15 (10) | 6.16–7.15 (5) | After 7.16 (3) | |
| Full flowering date (anthesis) | 20 | Before 7.15 (20) | 7.16–8.15 (15) | 8.16–9.1 (10) | 9.1–9.15 (5) | After 9.16 (3) | |
| Petal type | No. of petal layers | 10 | ≥10 (10) | 7–9 (8) | 4–6 (6) | 2–3 (3) | 1 (1) |
| Traits | Maximum | Minimum | Range | Mean | Standard Deviation (SD) | Coefficient of Variation (CV) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| Abnormal (crown) buds | 5 | 1 | 4 | 2.18 | 1.45 | 66% | −0.79 | 0.84 |
| Plant height (cm) | 76 | 14 | 62 | 33.58 | 11.69 | 34% | 0.73 | 0.75 |
| Plant crown width (cm) | 49 | 5 | 44 | 28.04 | 8.65 | 30% | −0.01 | −0.25 |
| Daylength at budding date (VBD) (h) | 16.1 | 9.8 | 3.3 | 14.66 | 7.25 | 49% | −0.23 | −1.33 |
| Daylength at full flowering date (h) | 16.1 | 12.0 | 4.1 | 12.5 | 8.22 | 65% | 1.38 | 1.18 |
| No. of petal layers | 24 | 1 | 23 | 5.28 | 4.48 | 84% | 3.08 | 14.52 |
| Traits | Female | Male | Mid-Parent Value | F1 Segregating Population | |
|---|---|---|---|---|---|
| Mid-Parent Heterosis (h2), Hm | Rate of Mid-Parent Heterosis (h2), RHm | ||||
| Abnormal (crown) bud | 3 | 1 | 2 | 1.09 ** | 45.50% |
| Plant height (cm) | 35.00 ± 3.21 | 40.00 ± 5.17 | 37.50 | 0.89 ** | 2.00% |
| Plant crown width (cm) | 25.00 ± 4.61 | 45.00 ± 2.80 | 35.00 | 0.80 * | 2.00% |
| Daylength at budding date (h) | 15.50 ± 0.21 | 12.80 ± 0.84 | 14.20 | 0.92 ** | 6.00% |
| Daylength at full flowering date (h) | 14.50 ± 1.59 | 12.40 ± 0.64 | 13.45 | 0.92 ** | 6.00% |
| No. of petal layers | 4.00 ± 1.40 | 7.00 ± 0.70 | 5.50 | −0.02 | −0.30% |
| Model | Abnormal (Crown) Bud | Plant Height | Plant Crown Width | Daylength at Budding (VBD) Date | Daylength at Full Flowering Date (Anthesis) | No. of Petal Layers |
|---|---|---|---|---|---|---|
| A-0 | 484.6195 | 1065.4281 | 969.2 | 402.7887 | 435.7714 | 792.9763 |
| A-1 | 441.4022 | 1065.7991 | 971.9632 | 335.8692 | 378.5224 | 722.6394 |
| A-2 | 433.25 | 1067.4359 | 971.1954 | 339.7139 | 437.7773 | 794.9767 |
| A-3 | 488.6195 | 1069.4282 | 971.9625 | 379.2664 | 439.77 | 796.974 |
| A-4 | 488.6195 | 1069.4282 | 971.9625 | 379.2664 | 439.77 | 796.974 |
| B-1 | 427.4854 | 1074.9431 | 983.6514 | 326.7462 | 382.2906 | 734.6369 |
| B-2 | 405.7781 | 1066.8813 | 975.9632 | 339.8691 | 361.6792 | 712.099 |
| B-3 | −3725.6512 | 1069.4358 | 973.1946 | 311.9996 | 439.7769 | 796.9766 |
| B-4 | 401.9971 | 1067.4377 | 971.1955 | 384.1081 | 437.7815 | 794.9778 |
| B-5 | 488.6201 | 1069.4288 | 971.7995 | 335.4233 | 439.7705 | 796.9744 |
| B-6 | 486.6202 | 1067.4289 | 969.7973 | 373.051 | 437.7705 | 794.9744 |
| Trait | Model | U12 | U22 | U32 | nW2 | Dn |
|---|---|---|---|---|---|---|
| Abnormal (crown) bud | B-3 | 2.0744 (1) | 6.2797 (0.0122) | 4.8239 (0.0281) | 0.8465 | 1.4232 |
| B-4 | 1.9728 (0.951) | 0.016 (0.8992) | 0.0577 (0.8101) | 0 | 1.0072 | |
| Plant height | A-0 | 0.2835 (0.5944) | 0.3555 (0.551) | 0.1043 (0.7467) | 0.1165 | 0.0072 |
| A-1 | 0.0468 (0.8286) | 0.086 (0.7693) | 0.112 (0.7379) | 0.0469 | 0.0071 | |
| Plant crown width | A-0 | 0.0535 (0.817) | 0.035 (0.8515) | (0.8828) | 0.0968 | 0.0076 |
| A-6 | 31.8982 (0.4246) | 0.0001 (0.9934) | 0.0029 (0.9573) | 0.0332 | 0.0863 | |
| Daylength at budding (VBD) date | B-1 | 0.001 (0.0095) | 0.000 (0.9929) | 0.0067 (0.9348) | 0.0939 | 0.0126 |
| B-3 | 0.0969 (0.7556) | 0.0867 (0.7684) | 0.0008 (0.9781) | 0.14 | 0.0179 | |
| Daylength at full flowering (anthesis) date | A-1 | 0.2575 (0.6118) | 0.1432 (0.7051) | 0.204 (0.6515) | 0.467 | 0.0044 |
| B-2 | 1.303 (0.902) | 0.0713 (0.7894) | 0.0428 (0.8361) | 0.0427 | 0.4051 | |
| No. of petal layers | B-1 | 0.4936 (0.4823) | 0.7712 (0.3799) | 0.6268 (0.4285) | 0.3077 | 0.0074 |
| B-2 | 0 (0.9994) | 0.0142 (0.9051) | 0.23 (0.6315) | 0.1592 | 0.0074 |
| Genetic Parameters | Abnormal (Crown) Bud | Daylength at Budding Date (h) | Daylength at Full Flowering Date (h) | No. of Petal Layers |
|---|---|---|---|---|
| M | 14.3109 | 14.3109 | 13.4165 | 9.6336 |
| da | 0.3470 | 0.3470 | 1.3595 | 4.9622 |
| db | 1.3349 | 1.3349 | / | 4.9527 |
| ha | / | / | −1.3586 | −7.4372 |
| hb | / | / | / | −4.9179 |
| i | / | / | / | 4.9516 |
| jab | / | / | / | −4.9178 |
| jba | / | / | / | −2.4682 |
| l | / | / | / | 7.3981 |
| σ2 p | 2.0744 | 1.1168 | 1.0459 | 11.4283 |
| σ2 mg | 2.0744 | 1.1314 | 1.4446 | 20.3640 |
| h2 mg | 1 | 0.9871 | 0.7240 | 0.5612 |
| Traits | Abnormal (Crown) Bud | Plant Height | Plant Crown Width | Daylength at Budding (VBD) Date | Daylength at Full Flowering (Anthesis) Date | No. of Petal Layers |
|---|---|---|---|---|---|---|
| Abnormal (crown) bud | 1 | |||||
| Plant height | −0.425 ** | 1 | ||||
| Plant crown width | 0.335 ** | −0.226 ** | 1 | |||
| Daylength at budding (VBD) date | 0.620 ** | −0.468 ** | 0.460 ** | 1 | ||
| Daylength at full flowering (anthesis) date | 0.181 * | −0.310 ** | 0.287 ** | 0.461 ** | 1 | |
| No. of petal layers | −0.025 | 0.074 | −0.010 | 0.024 | −0.006 | 1 |
| Genotype No. | Abnormal (Crown) Bud | Plant Height (cm) | Plant Crown Width (cm) | Budding (VBD) Date | Full Flowering (Anthesis) Date | No. of Petal Layers | Comprehensive Evaluation Scores |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 23 | 36 | 5.10 | 6.20 | 5 | 84 |
| 2 | 1 | 23 | 36 | 5.10 | 6.20 | 4 | 84 |
| 3 | 2 | 31 | 31 | 5.29 | 8.25 | 6 | 71 |
| 4 | 1 | 33 | 30 | 5.25 | 8.10 | 4 | 80 |
| 5 | 1 | 30 | 34 | 6.01 | 8.05 | 6 | 74 |
| 6 | 1 | 36 | 40 | 6.20 | 8.20 | 6 | 80 |
| 7 | 1 | 38 | 42 | 7.15 | 10.1 | 12 | 72 |
| 8 | 2 | 32 | 30 | 9.03 | 9.20 | 7 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhao, X.; Gao, K.; Tian, Y.; Zhang, M.; Anderson, N.O.; Dai, S. Heterosis and Mixed Genetic Analysis of Flowering Traits in Cross Breeding of Day-Neutral Chrysanthemum (Asteraceae). Agronomy 2023, 13, 2107. https://doi.org/10.3390/agronomy13082107
Wu X, Zhao X, Gao K, Tian Y, Zhang M, Anderson NO, Dai S. Heterosis and Mixed Genetic Analysis of Flowering Traits in Cross Breeding of Day-Neutral Chrysanthemum (Asteraceae). Agronomy. 2023; 13(8):2107. https://doi.org/10.3390/agronomy13082107
Chicago/Turabian StyleWu, Xiaoyun, Xiaogang Zhao, Kang Gao, Yuankai Tian, Mengmeng Zhang, Neil O. Anderson, and Silan Dai. 2023. "Heterosis and Mixed Genetic Analysis of Flowering Traits in Cross Breeding of Day-Neutral Chrysanthemum (Asteraceae)" Agronomy 13, no. 8: 2107. https://doi.org/10.3390/agronomy13082107
APA StyleWu, X., Zhao, X., Gao, K., Tian, Y., Zhang, M., Anderson, N. O., & Dai, S. (2023). Heterosis and Mixed Genetic Analysis of Flowering Traits in Cross Breeding of Day-Neutral Chrysanthemum (Asteraceae). Agronomy, 13(8), 2107. https://doi.org/10.3390/agronomy13082107





