Use of Food Attractant to Monitor and Forecast Population Dynamics of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), a Long-Distance Migratory Pest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Methods
2.2. Methods of Adult Dissection and Determination of the Age of Males
2.3. Determination of Adult Source Properties and Dynamic Prediction of Oviposition
2.4. Statistical Analysis
3. Results
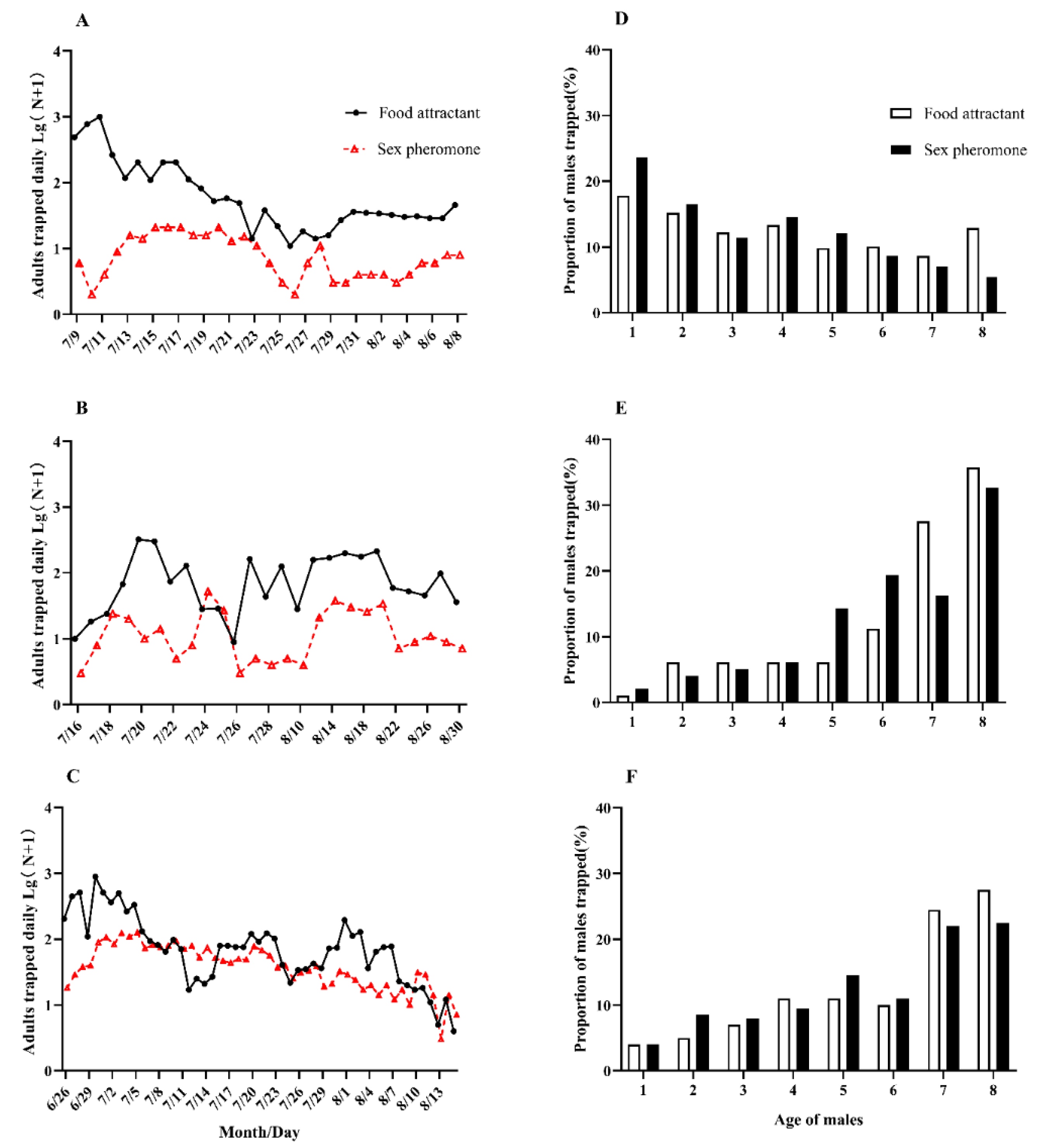
3.1. Comparison of Monitoring Effects of Food Attractants and Sex Pheromones
3.2. Monitoring Seasonal Population Migration Dynamics of C. medinalis Using Food Attractants
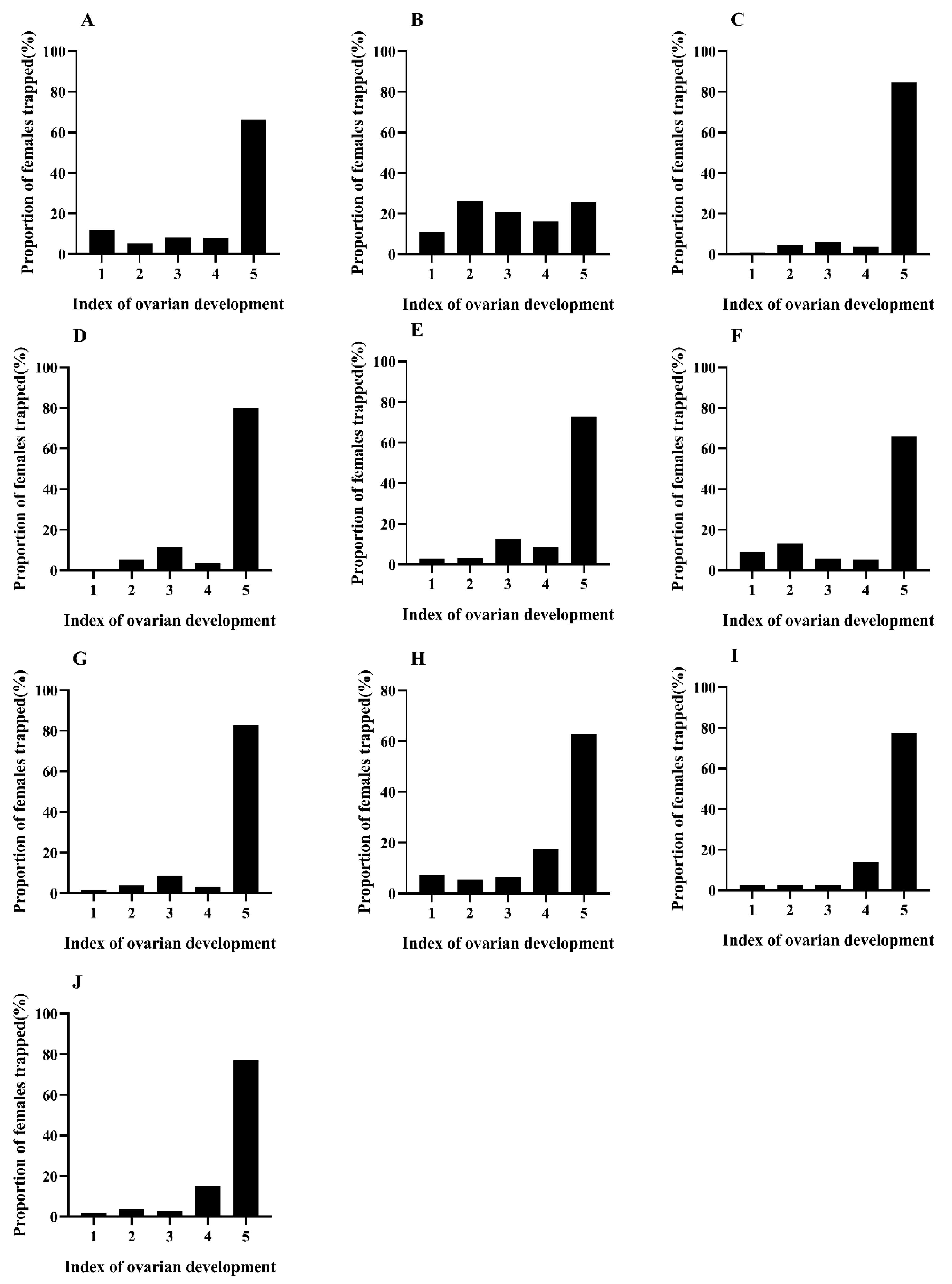
3.3. Monitoring the Reproductive Development Status of C. medinalis using Food Attractants
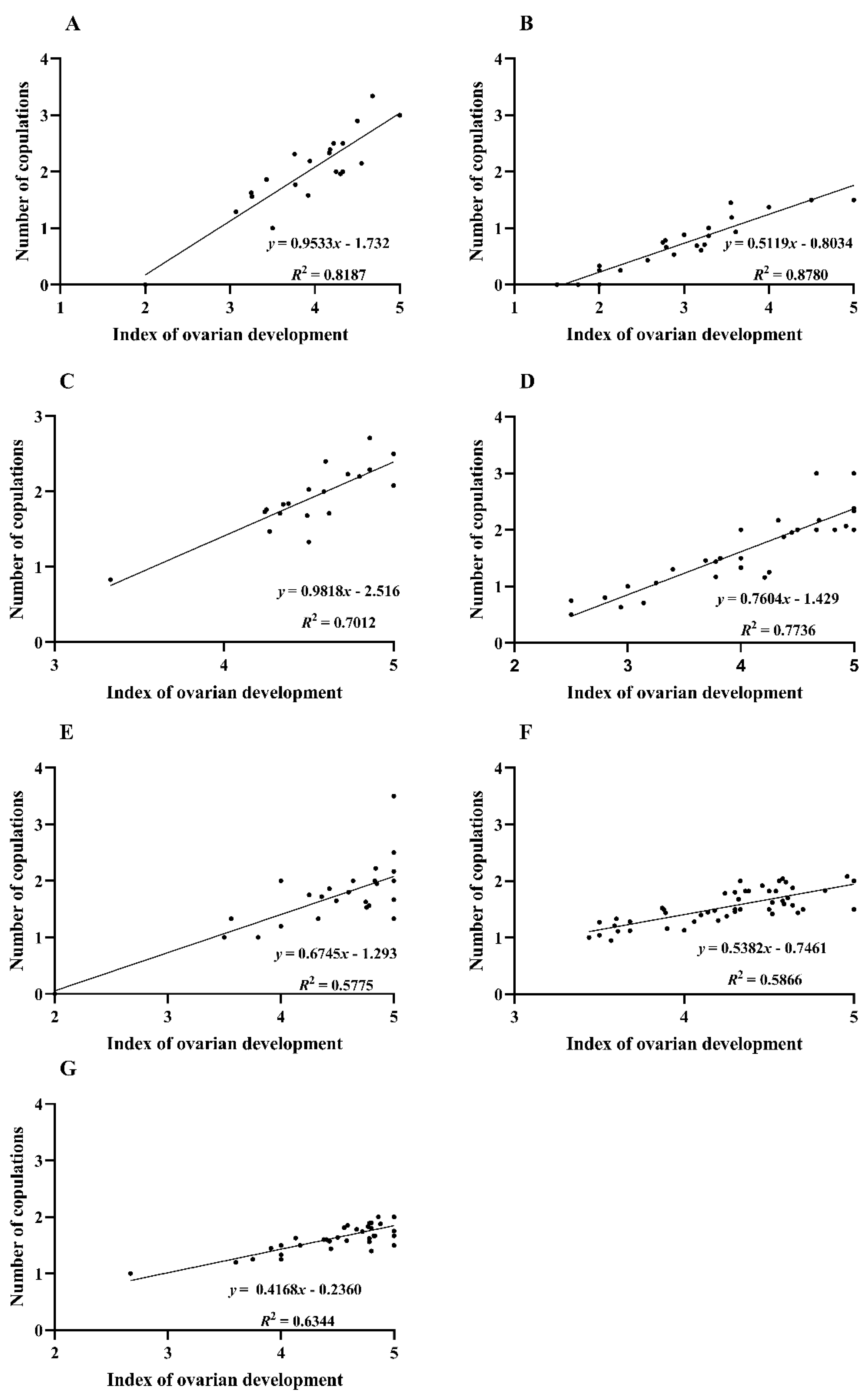
3.4. Prediction of Oviposition Dynamics of C. medinalis Based on Food Attractants Monitoring Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Padmavathi, C.; Katti, G.; Padmakumari, A.P.; Voleti, S.R.; Subba Rao, L.V. The effect of leaf folder Cnaphalocrocis medinalis (Guenee) [Lepidoptera: Pyralidae] injury on the plant physiology and yield loss in rice. J. Appl. Entomol. 2013, 137, 249–256. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Zhang, A.; Dong, H.B.; Zeng, F.F.; Pan, X.Y.; Wang, Y.; Wang, M.Q. Electrophysiological responses of the rice leaf folder, Cnaphalocrocis medinalis, to rice plant volatiles. J. Insect Sci. 2014, 14, 70. [Google Scholar] [CrossRef]
- Chintalapati, P.; Balakrishnan, D.; Venu Gopal Nammi, T.V.; Javvaji, S.; Muthusamy, S.K.; Lella Venkata, S.R.; Neelamraju, S.; Katti, G. Phenotyping and Genotype × Environment Interaction of Resistance to Leaf folder, Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) in Rice. Front. Plant Sci. 2019, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.R.; Barrion, A.T.; Litsinger, J.A.; Castilla, N.P.; Joshi, R.C. A Bibliography of Rice Leaf folders (Lepidoptera: Pyralidae). Int. J. Trop. Insect Sci. 1988, 9, 129–174. [Google Scholar] [CrossRef]
- Shanmugam, T.R.; Sendhil, R.; Thirumalvalavan, V. Quantification and prioritization of constraints causing yield loss in rice (Oryza sativa) in India. Agric. Trop. Et Subtrop. 2006, 39, 194–201. [Google Scholar]
- Luo, S.J. Occurrence of rice leaf roller in China and its identification and prevention. Plant Dis. Pests 2010, 1, 13–18. [Google Scholar] [CrossRef]
- Li, S.W.; Yang, H.; Liu, Y.F.; Liao, Q.R.; Du, J.; Jin, D.C. Transcriptome and gene expression analysis of the rice leaf folder, Cnaphalocrosis medinalis. PLoS ONE 2012, 7, e47401. [Google Scholar] [CrossRef]
- Sun, B.B.; Zhang, L.; Jiang, X.F.; Luo, L.Z. Effects of temperature on reproduction in the rice leaf roller. Chin. J. Appl. Entomol. 2013, 50, 622–628. [Google Scholar] [CrossRef]
- Morshed, M.N.; Howlader, M.T.H.; Rafiqul, M.; Islam, N.S.; Hera, M.H.R. Effect of abiotic factors on the seasonal incidence of Rice yellow stem borer, Scirpophaga incertulas (Walker) and rice leaf folder, Cnaphalocrocis medinalis (Guenee) population at the south-east coastal region of. J. Entomol. Zool. Stud. 2020, 8, 1321–1326. [Google Scholar]
- Lu, M.H.; Liu, W.C.; Hu, G.; Zhai, B.P.; Hoang, A.T.; Do, H.K. Analysis of the relationships of rice planthopper and rice leaf folder occurrence between China and Vietnam. Plant Prot. 2018, 44, 31–36. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Zeng, J.; Huang, C.; Zhang, T. Occurrence of, and damage caused by, major migratory pests and techniques for monitoring and forecasting these in China. Chin. J. Appl. Entomol. 2021, 58, 542–551. [Google Scholar]
- Fu, X.W.; Li, C.; Feng, H.Q.; Liu, Z.F.; Chapman, J.W.; Reynolds, D.R.; Wu, K.M. Seasonal migration of Cnaphalocrocis medinalis (Lepidoptera: Crambidae) over the Bohai Sea in northern China. Bull. Entomol. Res. 2014, 104, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.X.; Cao, Y.; Xie, X.J.; Lu, M.; Li, X.; Wang, C.Z.; Liu, W.C. Migration pattern of rice leaf roller and impact of atmospheric conditions on a heavy migration event in China. Acta Ecol. Sin. 2015, 35, 3519–3533. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Luo, M.; Fu, X.G.; Zheng, L.X.; Wei, H.Y. Mating disruption of Chilo suppressalis from sex pheromone of another pyralid rice pest Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Insect Sci. 2020, 20, 19. [Google Scholar] [CrossRef]
- Wu, J.; Wu, X.; Chen, H.; Xu, L.; Liu, G.; Mao, B.; Quo, R.; Du, Y. Optimization of the sex pheromone of the rice leaf folder moth Cnaphalocrocis medinalis as a monitoring tool in China. J. Appl. Entomol. 2013, 137, 509–518. [Google Scholar] [CrossRef]
- Sun, G.J.; Liu, S.H.; Luo, H.L.; Feng, Z.L.; Yang, B.J.; Luo, J.; Tang, J.; Yao, Q.; Xu, J.J. Intelligent Monitoring System of Migratory Pests Based on Searchlight Trap and Machine Vision. Front. Plant Sci. 2022, 13, 897739. [Google Scholar] [CrossRef]
- Zhu, F.; Zeng, J.; Tian, Z.H. Monitoring of population dynamics and development status of rice leaf folder moth by sex pheromone trapping. China Plant Prot. 2018, 38, 43–47. [Google Scholar] [CrossRef]
- Kawazu, K.; Kamimuro, T.; Kamiwada, H.; Nagata, K.; Matsunaga, T.; Sugie, H.; Fukumoto, T.; Adati, T.; Tatsuki, S. Effective pheromone lures for monitoring the rice leaffolder moth, Cnaphalocrocis medinalis (Lepidoptera: Crambidae). Crop. Prot. 2004, 23, 589–593. [Google Scholar] [CrossRef]
- Cho, J.R.; San Choi, K.; Park, H.H.; Lee, S.; Yum, K.H.; Jung, J.K.; Seo, B.Y.; Lee, M. Electroantennogram and field responses of Korean population of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Crambidae), to sex attractant candidates. J. Asia-Pac. Entomol. 2013, 16, 61–66. [Google Scholar] [CrossRef]
- Zeng, J.; Du, Y.J.; Jiang, Y.Y.; Liu, W.C. Development and application of sex pheromone in population monitoring of crop insect pests in China. Plant Prot. 2015, 41, 9–15. [Google Scholar]
- Zeng, F.F.; Liu, H.; Zhang, A.; Lu, Z.X.; Leal, W.S.; Abdelnabby, H.; Wang, M.Q. Three chemosensory proteins from the rice leaf folder Cnaphalocrocis medinalis involved in host volatile and sex pheromone reception. Insect Mol. Biol. 2018, 27, 710–723. [Google Scholar] [CrossRef]
- Zhu, P.J.; Shen, H.R.; Chen, Z.G.; Li, H.M.; Jiang, Y.P. Screening test on lure core of rice leaf folder detection. China Plant Prot. 2010, 30, 41–42. [Google Scholar] [CrossRef]
- Feng, B.; Guo, Q.S.; Zhu, F.; Wang, X.; Liu, W.C.; Jiang, Y.Y.; Zhong, L.; Du, Y.J. Ovarian development and synthetic sex pheromone lure trapping of adults of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Acta Entomol. Sin. 2017, 60, 211–221. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.D.; Henderson, G.S. Development of a synthetic plant volatile-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 21–30. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Birch, A.N.E.; Lindzey, S.; Meadow, R.; Snyder, W.E. A mechanistic framework to improve understanding and applications of push-pull systems in pest management. J. Appl. Ecol. 2016, 53, 202–212. [Google Scholar] [CrossRef]
- Cheng, J.J.; Zhu, J.; Liu, F. EAG response of Cnaphalocrocis medinalis to 43 graminaceous plant volatiles. Chin. J. Appl. Entomol. 2016, 53, 472–481. [Google Scholar] [CrossRef]
- Wei, B.; Gao, H.Y.; Zheng, X.S.; Xu, H.X.; Ruan, Y.M.; Lv, Z.X.; Zhu, P.Y. EAG responses of adult Cnaphalocrocis medinalis to plant volatiles. Chin. J. Appl. Entomol. 2022, 59, 988–996. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, T.; Wang, L.Y.; Wu, Q.L.; Lu, M.H.; Wu, K.M. Preliminary application of food attractant trapping in monitoring population dynamics of leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) in China. Plant Prot. 2021, 47, 203–214. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Xia, H.X.; Zhang, C.; Chang, X.Q.; Zhang, S.; Lv, L.; Chang, W. Preliminary study on monitoring the occurrence of rice leaf folder by biological food attractants in field. Hubei Plant Prot. 2022, 3, 31–33. [Google Scholar] [CrossRef]
- He, W.; Zhao, X.C.; Ali, A.; Ge, S.S.; Zhang, H.W.; He, L.M.; Wu, K.M. Population dynamics and reproductive developmental analysis of Helicoverpa armigera (Lepidoptera: Noctuidae) trapped using food attractants in the field. J. Econ. Entomol. 2021, 114, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, L.Y.; Lv, C.Y.; Ge, S.S.; Zhang, H.W.; Jiang, S.; Chu, B.; Yang, X.M.; Wyckhuys, K.A.; Wu, K.M. Use of food attractants to monitor and forecast Spodoptera frugiperda (JE Smith) seasonal abundance in southern China. J. Pest Sci. 2023, 96, 1–13. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zeng, J.; Zeng, W.; Li, Q.; Chen, X.J.; Zou, Y. Application of the morphological indicators of the male internal reproductive system in forecasting the population dynamics of the rice leaf roller, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) by sex pheromone trapping. Acta Entomol. Sin. 2017, 60, 927–935. [Google Scholar] [CrossRef]
- Zhang, X.X.; Lu, Z.Q.; Geng, J.G. Application of anatomy of female rice leaf folder in measuring and reporting. Chin. Bulletion Entomol. 1979, 3, 97–99. [Google Scholar]
- Wu, K.M.; Guo, Y.Y. The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef]
- Chouinard, G.; Pelletier, F.; Vincent, C. Pest Activity and Protection Practices: Four Decades of Transformation in Quebec Apple Orchards. Insects 2021, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhao, X.C.; Ge, S.S.; Wu, K.M. Food attractants for field population monitoring of Spodoptera exigua (Hübner). Crop. Prot. 2021, 145, 105616. [Google Scholar] [CrossRef]
- Wu, K.M.; Guo, Y.Y.; Wu, Y. Ovarian development of adult females of cotton bollworm and its relation to migratory behavior around Bohai bay of China. Acta Ecol. Sin. 2002, 22, 1075–1078. [Google Scholar] [CrossRef]
- Zhang, X.X.; Lu, Z.Q.; Geng, J.G.; Li, G.Z.; Chen, X.L.; Wu, X.W. Study on migration route of rice leaf folder. Acta Entomol. Sin. 1980, 3, 130–140. [Google Scholar]
- Liu, W.; Cai, X.W.; Liu, Y.; Guo, A.H.; Wang, C.Z.; Lu, M.H.; Bao, Y.X. Study on immigration path of rice leaf roller based on FLEXPART and atmospheric background. Meteorol. Mon. 2017, 43, 460–467. [Google Scholar] [CrossRef]
- Chakraborty, K.; Deb, D.C. Incidence of adult leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) on paddy crop in the agro climatic conditions of the northern parts of West Bengal, India. World J. Agric. Sci. 2011, 7, 738–742. [Google Scholar]
- Toledo-Hernández, R.A.; Lasa, R.; Montoya, P.; Liedo, P.; Rodríguez, D.; Sánchez, A.; Toledo, J. Efficacy of food-based attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in berry crops. Crop. Prot. 2021, 150, 105797. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Cha, D.H.; Loeb, G.M. Evaluating a push–pull strategy for management of Drosophila suzukii Matsumura in red raspberry. Pest Manag. Sci. 2018, 74, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lamy, F.; Dugravot, S.; Cortesero, A.M.; Chaminade, V.; Faloya, V.; Poinsot, D. One more step toward a push-pull strategy combining both a trap crop and plant volatile organic compounds against the cabbage root fly Delia radicum. Environ. Sci. Pollut. Res. 2017, 25, 29868–29879. [Google Scholar] [CrossRef] [PubMed]
- Thwaite, W.G.; Mooney, A.M.; Eslick, M.A.; Nicol, H.I. Evaluating pear-derived kairomone lures for monitoring Cydia pomonella’(L.) (Lepidoptera: Tortricidae) in Granny Smith apples under mating disruption. Gen. Appl. Entomol. 2004, 33, 55–60. [Google Scholar]
- Knight, A.L.; Light, D.M. Seasonal flight patterns of codling moth (Lepidoptera: Tortricidae) monitored with pear ester and codlemone-baited traps in sex pheromone-treated apple orchards. Environ. Entomol. 2005, 34, 1028–1035. [Google Scholar] [CrossRef]
- Loeb, G.M.; Cha, D.H.; Hesler, S.P.; Linn, J.C.E.; Zhang, A.J.; Teal, P.E.; Roelofs, W.L. Monitoring grape berry moth (Paralobesia viteana: Lepidoptera) in commercial vineyards using a host plant based synthetic lure. Environ. Entomol. 2011, 40, 1511–1522. [Google Scholar] [CrossRef]
- Broughton, S.; Harrison, J. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop. Prot. 2012, 42, 156–163. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, M.J.; Wang, K.F.; Lu, Y. Preliminary study on the monitoring effect of the rice leaf folder’s food attractions. China Plant Prot. 2021, 41, 43–44. [Google Scholar] [CrossRef]




| Experimental Year | Experimental Site/Province | Experimental Time (Month/Day) | Longitude/Latitude |
|---|---|---|---|
| 2020 | Guigang/Guangxi | 28 May–20 June | 109°43′48″/22°46′12″ |
| 2020 | Liling/Hunan | 8 July–8 Aug. | 113°31′12″/27°36′36″ |
| 2020 | Shaodong/Zhejiang | 3 July–21 July | 111°43′11″/27°18′36″ |
| 2020 | Zhangjiagang/Jiangsu | 16 July–12 Aug. | 120°31′48″/31°48′36″ |
| 2020 | Changzhou/Jiangsu | 17 July–18 Aug. | 119°29′24″/31°40′12″ |
| 2020 | Quzhou/Zhejiang | 23 July–25 Aug. | 119°11′24″/29°7′12″ |
| 2020 | Xiangshan/Zhejiang | 16 July–26 Aug. | 121°54′36″/29°24′36″ |
| 2021 | Shaodong/Zhejiang | 26 June–15 Aug. | 111°43′12″/27°18′36″ |
| 2021 | Zhijiang/Hunan | 19 June–14 Aug. | 109°40′48″/27°26′24″ |
| 2021 | Taihe/Jiangxi | 1 June–27 June | 114°52′48″/26°47′24″ |
| Adult Age (Day) | Range of the Semi-Major Axis Length of Testes (μm) |
|---|---|
| 1 | >421.1 |
| 2 | 387.6–421.1 |
| 3 | 367.3–387.6 |
| 4 | 348.0–367.3 |
| 5 | 327.9–348.0 |
| 6 | 307.4–327.9 |
| 7 | 277.8–307.4 |
| 8 | <277.8 |
| Experimental Year | Experimental Site/Province | Number of Females | Number of Males | χ2 | p |
|---|---|---|---|---|---|
| 2020 | Guigang/Guangxi | 800 | 1503 | 214.59 | <0.001 |
| 2020 | Liling/Hunan | 1729 | 2413 | 112.95 | <0.001 |
| 2020 | Shaodong/Hunan | 1272 | 2075 | 192.65 | <0.001 |
| 2020 | Changzhou/Jiangsu | 538 | 1591 | 520.81 | <0.001 |
| 2020 | Zhangjiagang/Jiangsu | 1593 | 976 | 148.19 | <0.001 |
| 2020 | Quzhou/Zhejiang | 419 | 677 | 60.73 | <0.001 |
| 2020 | Xiangshan/Zhejiang | 493 | 988 | 165.45 | <0.001 |
| 2021 | Shaodong/Hunan | 2442 | 4173 | 452.97 | <0.001 |
| 2021 | Taihe/Jiangxi | 185 | 207 | 1.24 | 0.270 |
| 2021 | Zhijiang/Hunan | 464 | 648 | 30.45 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Yan, R.; He, W.; Wu, K. Use of Food Attractant to Monitor and Forecast Population Dynamics of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), a Long-Distance Migratory Pest. Agronomy 2023, 13, 2141. https://doi.org/10.3390/agronomy13082141
Gao L, Yan R, He W, Wu K. Use of Food Attractant to Monitor and Forecast Population Dynamics of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), a Long-Distance Migratory Pest. Agronomy. 2023; 13(8):2141. https://doi.org/10.3390/agronomy13082141
Chicago/Turabian StyleGao, Lingyun, Ran Yan, Wei He, and Kongming Wu. 2023. "Use of Food Attractant to Monitor and Forecast Population Dynamics of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), a Long-Distance Migratory Pest" Agronomy 13, no. 8: 2141. https://doi.org/10.3390/agronomy13082141
APA StyleGao, L., Yan, R., He, W., & Wu, K. (2023). Use of Food Attractant to Monitor and Forecast Population Dynamics of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), a Long-Distance Migratory Pest. Agronomy, 13(8), 2141. https://doi.org/10.3390/agronomy13082141






