Summer Rice–Winter Potato Rotation Suppresses Various Soil-Borne Plant Fungal Pathogens
Abstract
:1. Introduction
2. Materials and Methods
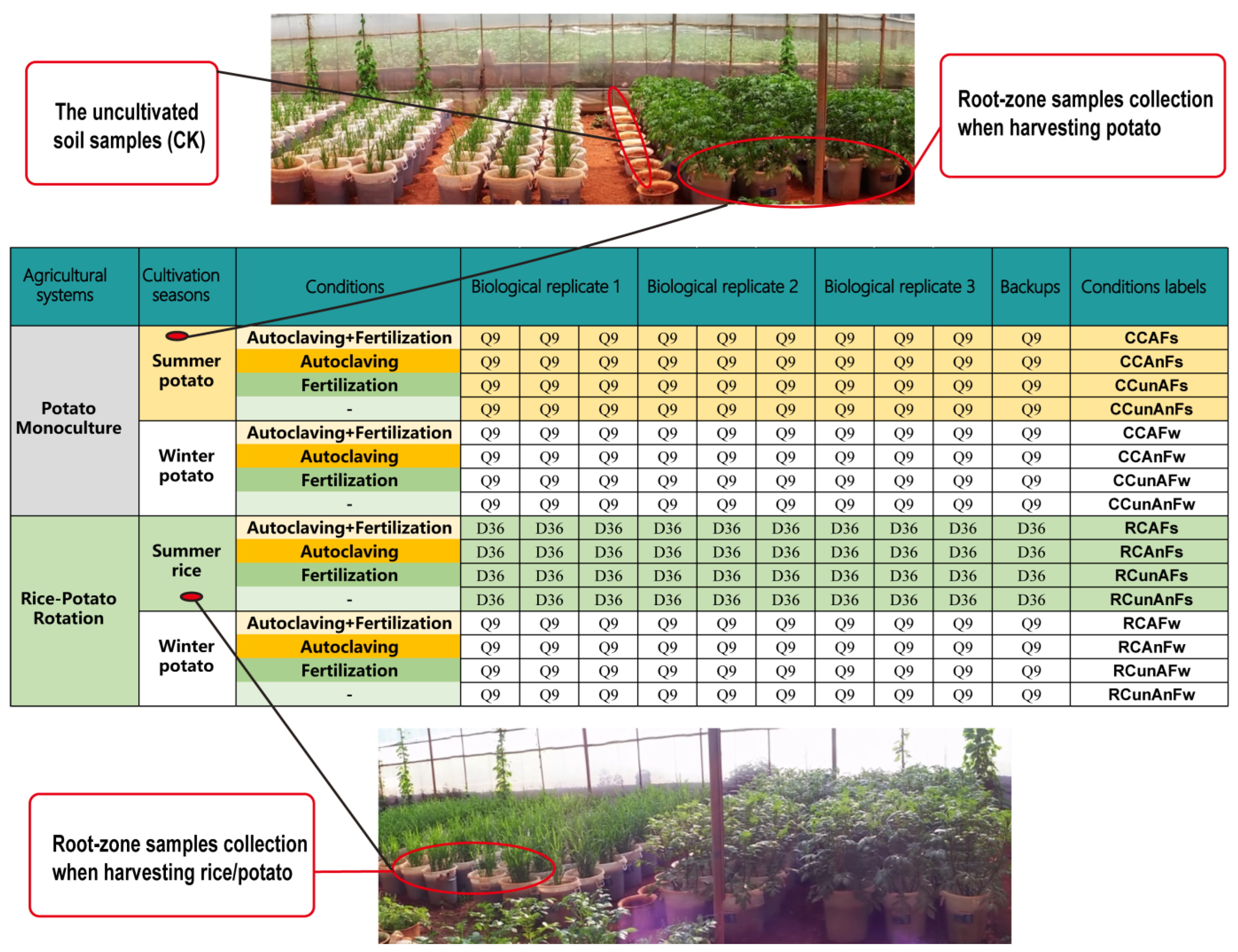
2.1. Experiment Description
2.1.1. Soil Pots Preparation
2.1.2. Experimental Arrangements
2.1.3. Soil Sampling and Preservation
2.2. Determination of Soil Physiochemical Properties and Enzymatic Activities
2.3. Fungal Community Analysis through Miseq Sequencing of Fungal 18S rRNA Genes
2.3.1. DNA Extraction and PCR Amplification
2.3.2. Illumina Miseq Sequencing
2.3.3. Processing of Sequencing Data
2.3.4. Fungal Guilds Annotation and Analysis
Comprehensive Evaluation of Fungal Pathogenic Suppressiveness of the Conditions
Differential Analysis of the Plant Pathogens under Factors
2.3.5. Bipartite Fungal Co-Occurrence Network Analysis between Plant Pathogens and the Remaining Non-Pathogenic Members
2.3.6. Redundancy Analysis (RDA) of the Plant Fungal Pathogens and the Soil Physiochemical Properties and Enzymatic Activities
2.4. Statistical Analysis
3. Results
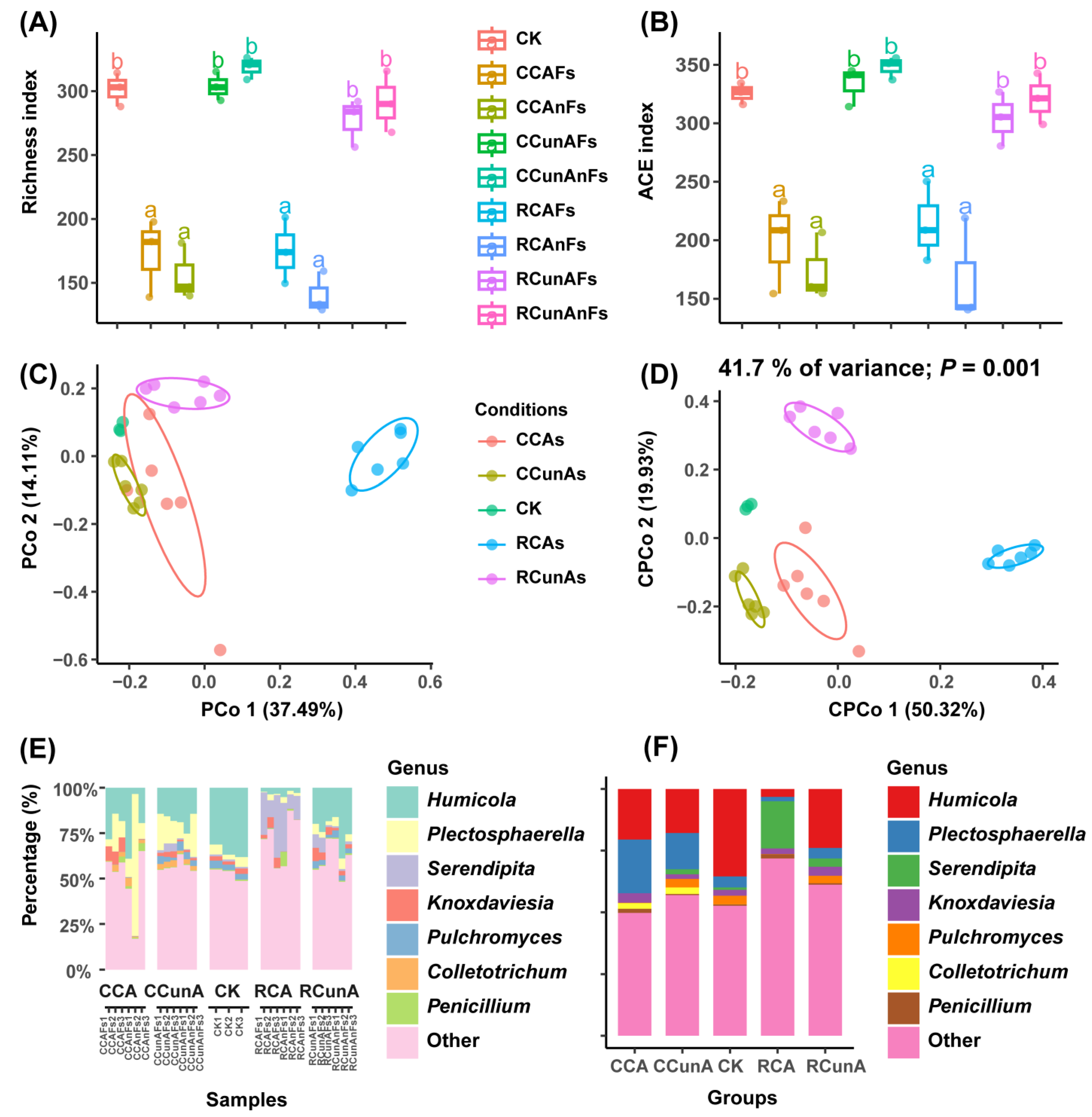
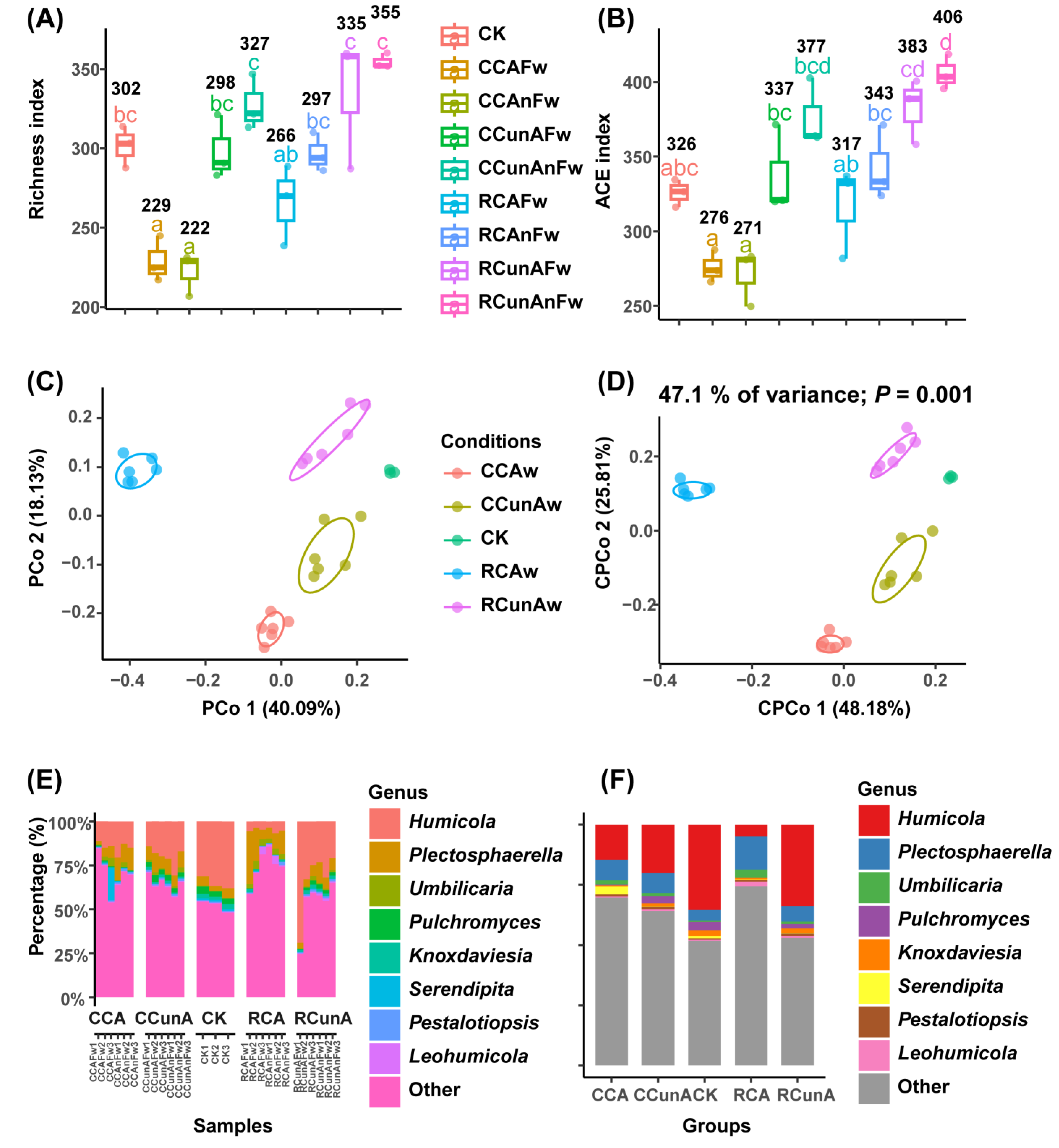
3.1. Taxon Compositional and Differential Analysis of Fungal Community under Different Taxonomic Levels among the Conditions of CK, CC, and RC
3.2. Fungal α- and β-Diversity Analysis
3.3. The Suppressiveness of the Summer Rice–Winter Potato Rotation System on Soilborne Plant Fungal Pathogens
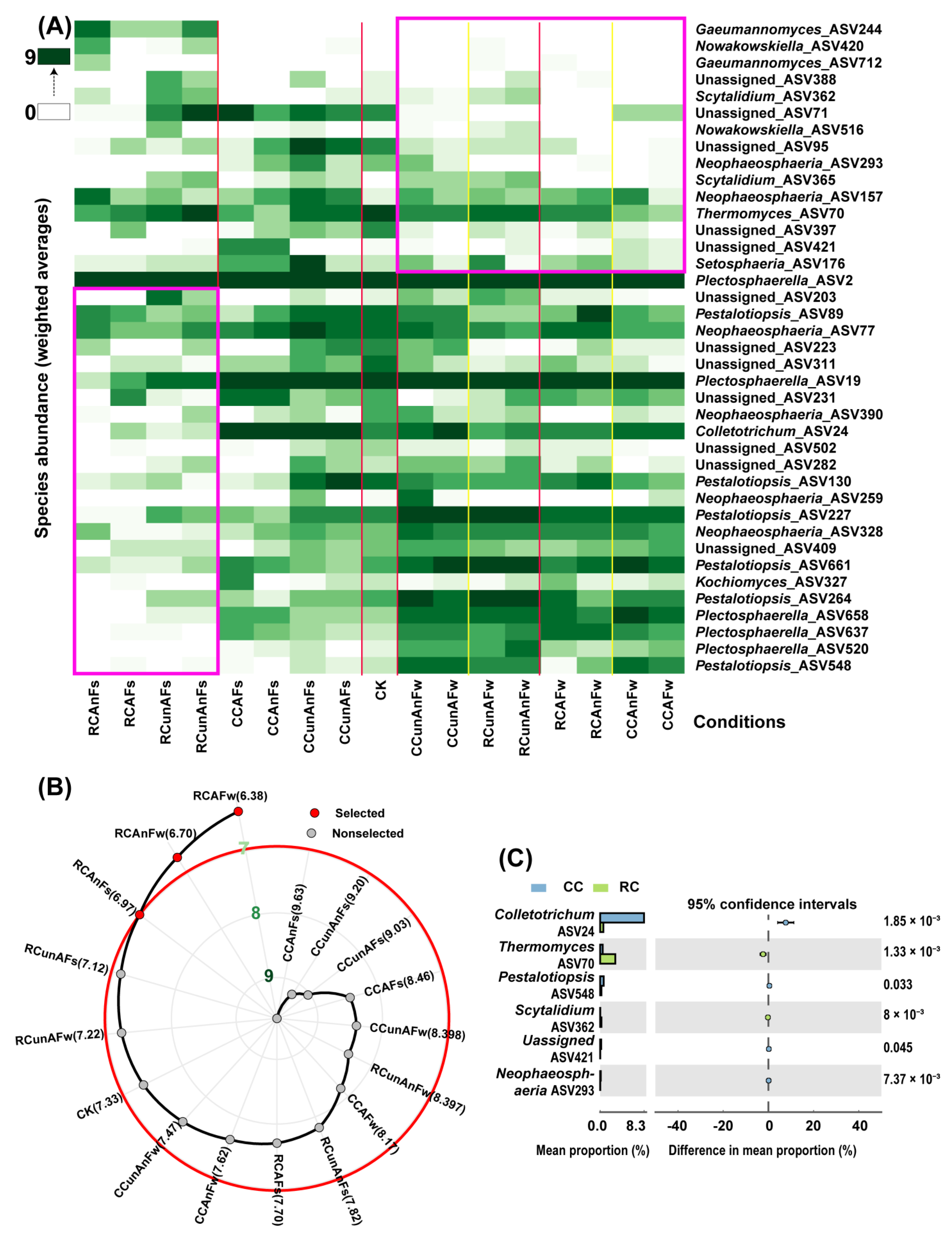
3.3.1. Comprehensive Evaluation of Suppressiveness of the Conditions
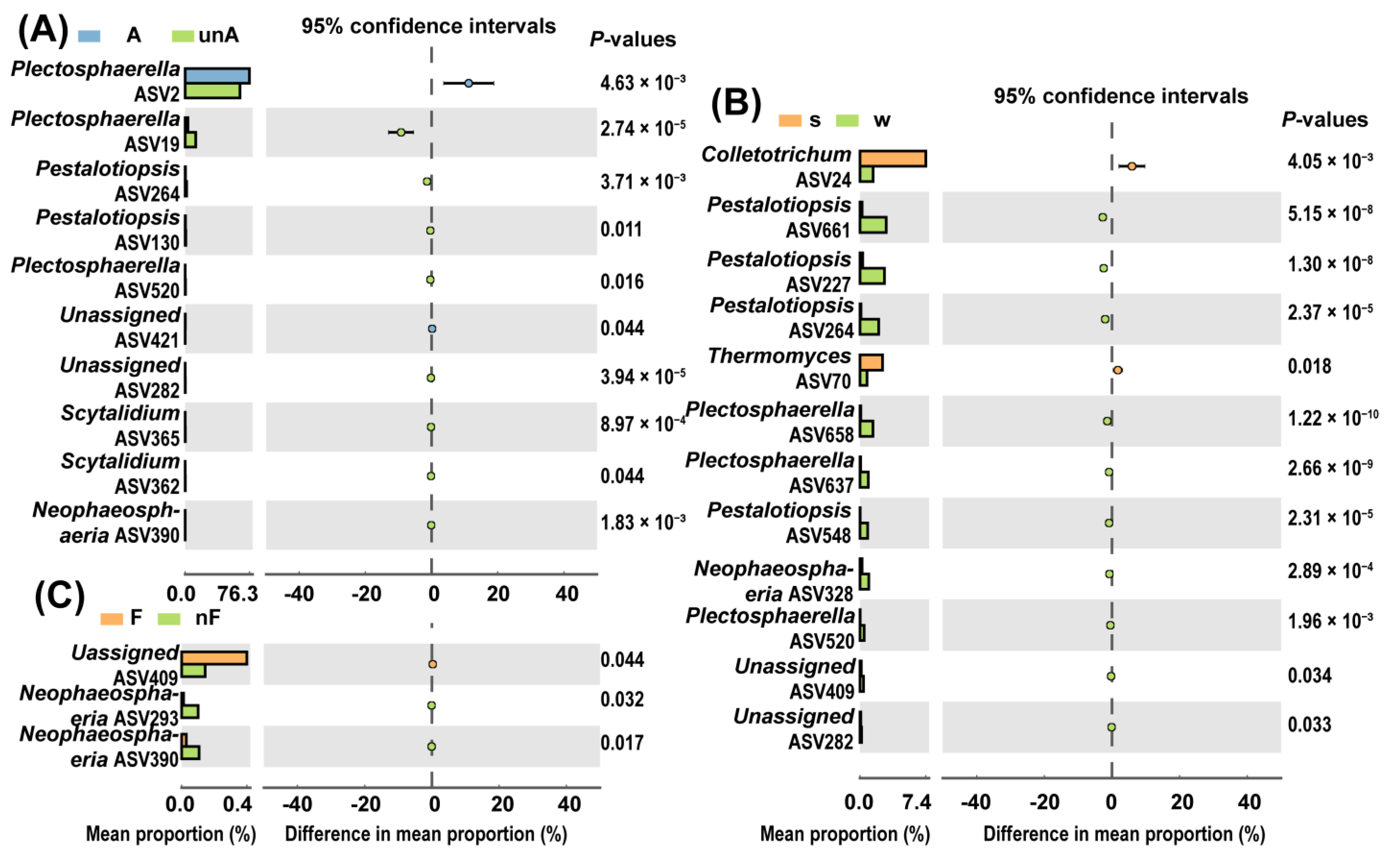
3.3.2. Suppressive Effect of Factors on Soilborne Plant Fungal Pathogens
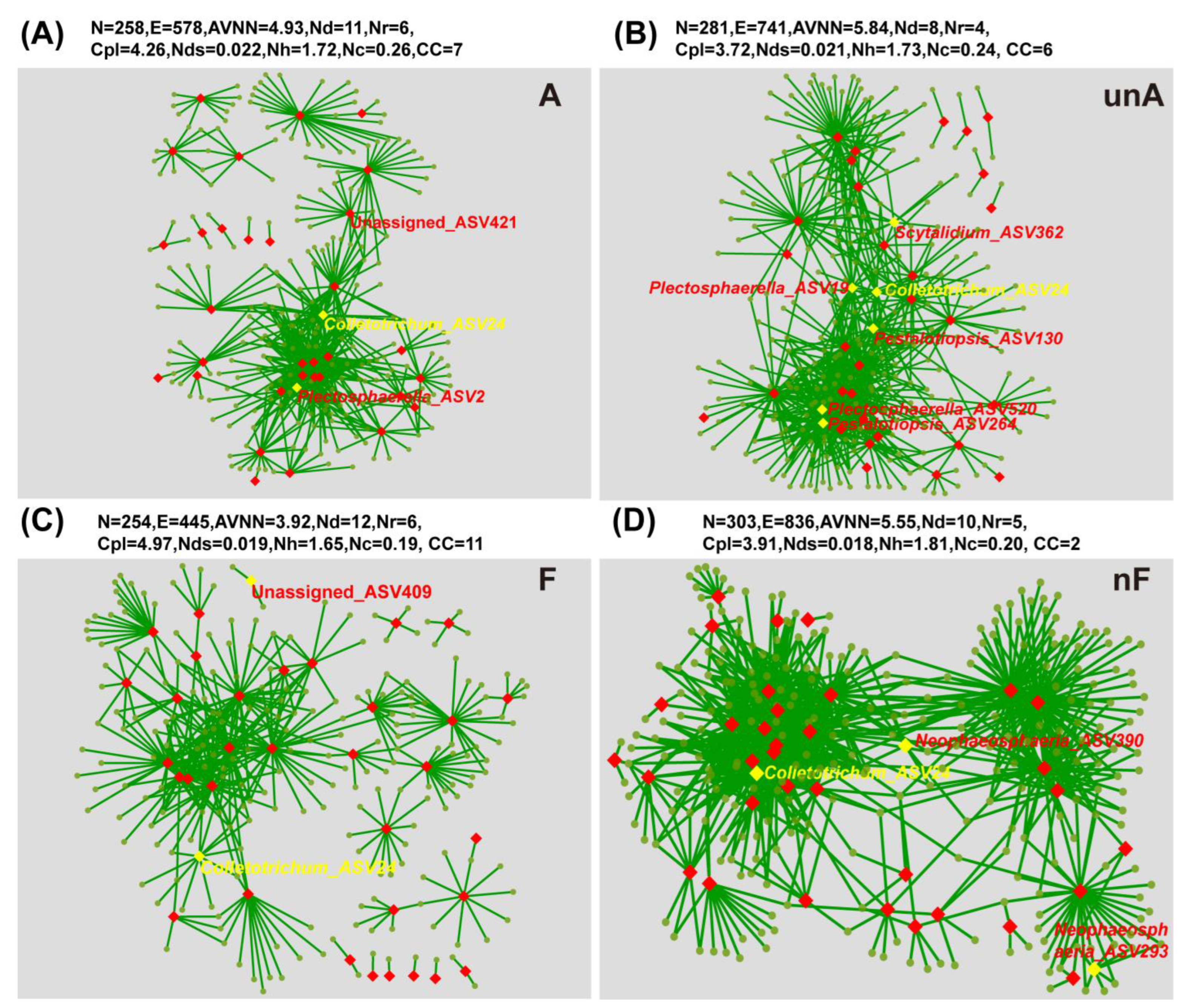
3.3.3. Bipartite Network between the Annotated Plant Pathogens and the Remaining Non-Pathogenic Fungal Species
3.3.4. The Potential Relationships between the Environmental Indicators (Enzymatic Activity and Physiochemical Properties) and the Soilborne Fungal Pathogens
4. Discussion
4.1. Rice–Potato Rotation (RC) Could Significantly Change Soil Fungal Community Composition Compared with the Uncultivated (CK) and the Potato Monoculture (CC) Soil Samples
4.2. The Rice–Potato Rotation System Could Promote the Fungal Species Diversity Compared with the Potato Monoculture System
4.3. The Rice–Potato Rotation System Could Suppress the Soil Fungal Pathogens in Comparision with the Potato Monoculture System
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Conditions | SOM | pH | EC | TN | AN | TP | AP | TK | AK |
|---|---|---|---|---|---|---|---|---|---|
| CK | 4.61 ± 0.32 | 6.64 ± 0.17 | 408.00 ± 10.00 | 0.20 ± 0.01 | 42.12 ± 1.33 | 0.70 ± 0.02 | 82.79 ± 7.25 | 1.87 ± 0.07 | 489.89 ± 33.89 |
| CCAFs | 7.19 ± 0.16 | 6.61 ± 0.18 | 497.00 ± 15.00 | 0.26 ± 0.01 | 73.97 ± 0.73 | 0.84 ± 0.00 | 137.83 ± 10.77 | 2.44 ± 0.12 | 416.34 ± 16.82 |
| CCAFw | 8.45 ± 0.43 | 6.82 ± 0.08 | 640.00 ± 14.00 | 0.27 ± 0.01 | 74.08 ± 1.23 | 0.83 ± 0.03 | 138.40 ± 6.42 | 2.36 ± 0.12 | 494.26 ± 66.77 |
| CCAnFs | 5.99 ± 0.54 | 6.81 ± 0.04 | 236.00 ± 8.00 | 0.23 ± 0.02 | 73.03 ± 1.41 | 0.77 ± 0.06 | 97.34 ± 2.55 | 1.94 ± 0.09 | 365.01 ± 51.07 |
| CCAnFw | 8.13 ± 0.30 | 6.85 ± 0.08 | 609.00 ± 22.00 | 0.27 ± 0.00 | 73.73 ± 2.05 | 0.81 ± 0.02 | 127.11 ± 11.44 | 2.39 ± 0.12 | 439.17 ± 30.12 |
| CCunAFs | 7.19 ± 0.37 | 6.81 ± 0.05 | 516.00 ± 12.00 | 0.26 ± 0.02 | 76.07 ± 1.76 | 0.77 ± 0.04 | 121.71 ± 3.93 | 2.62 ± 0.19 | 524.09 ± 31.93 |
| CCunAFw | 8.01 ± 0.57 | 6.80 ± 0.11 | 575.00 ± 7.00 | 0.27 ± 0.02 | 80.27 ± 3.18 | 0.81 ± 0.03 | 139.83 ± 8.95 | 2.37 ± 0.21 | 545.20 ± 48.03 |
| CCunAnFs | 4.57 ± 0.42 | 6.85 ± 0.18 | 209.00 ± 10.00 | 0.19 ± 0.00 | 65.22 ± 2.02 | 0.67 ± 0.01 | 81.45 ± 3.13 | 1.77 ± 0.06 | 350.87 ± 36.55 |
| CCunAnFw | 5.02 ± 0.32 | 6.95 ± 0.03 | 288.00 ± 14.00 | 0.21 ± 0.01 | 63.70 ± 1.85 | 0.73 ± 0.03 | 82.40 ± 2.30 | 1.60 ± 0.08 | 394.05 ± 26.45 |
| RCAFs | 7.50 ± 0.61 | 6.81 ± 0.04 | 545.00 ± 10.00 | 0.26 ± 0.03 | 77.82 ± 1.41 | 0.82 ± 0.06 | 89.46 ± 5.49 | 2.40 ± 0.08 | 468.67 ± 62.38 |
| RCAFw | 8.02 ± 0.42 | 6.92 ± 0.12 | 743.00 ± 25.00 | 0.28 ± 0.02 | 73.73 ± 3.59 | 0.84 ± 0.04 | 95.61 ± 8.28 | 2.42 ± 0.25 | 529.84 ± 68.73 |
| RCAnFs | 5.64 ± 0.25 | 7.03 ± 0.03 | 269.00 ± 17.58 | 0.21 ± 0.00 | 71.05 ± 2.45 | 0.73 ± 0.03 | 50.36 ± 0.59 | 1.51 ± 0.06 | 183.91 ± 12.49 |
| RCAnFw | 7.17 ± 0.65 | 6.97 ± 0.04 | 828.00 ± 17.00 | 0.25 ± 0.03 | 70.23 ± 2.46 | 0.80 ± 0.07 | 62.46 ± 5.96 | 1.84 ± 0.19 | 373.33 ± 51.26 |
| RCunAFs | 7.46 ± 0.69 | 6.85 ± 0.06 | 598.67 ± 11.37 | 0.26 ± 0.02 | 74.20 ± 4.26 | 0.84 ± 0.02 | 94.03 ± 1.03 | 2.83 ± 0.23 | 620.99 ± 21.98 |
| RCunAFw | 9.12 ± 0.30 | 6.84 ± 0.08 | 1122.33 ± 55.90 | 0.30 ± 0.00 | 101.73 ± 1.33 | 0.92 ± 0.02 | 156.07 ± 4.30 | 3.33 ± 0.07 | 685.39 ± 88.05 |
| RCunAnFs | 5.08 ± 0.33 | 7.01 ± 0.11 | 256.00 ± 9.00 | 0.21 ± 0.00 | 69.65 ± 4.04 | 0.78 ± 0.02 | 66.43 ± 1.78 | 1.75 ± 0.09 | 305.58 ± 26.43 |
| RCunAnFw | 5.78 ± 0.52 | 7.02 ± 0.03 | 304.00 ± 18.00 | 0.22 ± 0.01 | 72.57 ± 4.23 | 0.78 ± 0.05 | 73.97 ± 5.90 | 1.77 ± 0.06 | 328.13 ± 38.52 |
| Conditions | S-PP0 | S-CAT | S-SC | S-CL | S-UE | S-ACP |
|---|---|---|---|---|---|---|
| CK | 25.98 ± 0.64 | 13.89 ± 0.34 | 36.09 ± 5.28 | 33.95 ± 0.81 | 1217.51 ± 44.16 | 12,300.43 ± 669.61 |
| CCAFs | 32.84 ± 3.46 | 36.31 ± 0.10 | 37.81 ± 7.35 | 39.20 ± 7.45 | 712.34 ± 58.56 | 16,116.82 ± 935.68 |
| CCAFw | 16.16 ± 2.15 | 22.07 ± 0.68 | 33.53 ± 9.49 | 40.54 ± 0.80 | 941.03 ± 29.47 | 11,163.51 ± 1542.02 |
| CCAnFs | 40.56 ± 0.76 | 32.65 ± 3.56 | 33.02 ± 2.09 | 40.48 ± 3.11 | 575.41 ± 10.50 | 12,414.70 ± 1187.62 |
| CCAnFw | 19.96 ± 1.90 | 23.06 ± 6.44 | 32.87 ± 0.73 | 41.42 ± 1.00 | 955.02 ± 12.33 | 9746.65 ± 2062.82 |
| CCunAFs | 35.46 ± 2.47 | 37.67 ± 2.02 | 45.24 ± 0.25 | 36.59 ± 3.07 | 988.82 ± 80.12 | 20,144.60 ± 565.69 |
| CCunAFw | 21.57 ± 1.96 | 27.51 ± 1.86 | 40.52 ± 1.56 | 41.27 ± 1.31 | 1007.46 ± 38.20 | 16,676.72 ± 541.19 |
| CCunAnFs | 23.71 ± 1.59 | 31.94 ± 2.57 | 21.50 ± 4.27 | 23.84 ± 6.74 | 1002.51 ± 71.01 | 11,517.73 ± 2792.63 |
| CCunAnFw | 19.91 ± 1.04 | 33.55 ± 2.38 | 35.27 ± 2.64 | 37.27 ± 0.45 | 1204.11 ± 60.12 | 16,928.09 ± 389.46 |
| RCAFs | 25.24 ± 5.37 | 30.52 ± 2.41 | 12.45 ± 3.78 | 30.69 ± 4.83 | 259.32 ± 84.99 | 6850.08 ± 1191.45 |
| RCAFw | 17.60 ± 1.87 | 25.34 ± 2.52 | 22.65 ± 6.58 | 42.35 ± 0.78 | 510.74 ± 111.28 | 11,592.00 ± 4642.37 |
| RCAnFs | 20.70 ± 1.98 | 26.98 ± 1.03 | 7.80 ± 1.50 | 20.44 ± 1.89 | 233.97 ± 34.06 | 5564.62 ± 1372.05 |
| RCAnFw | 19.30 ± 1.73 | 19.20 ± 4.80 | 25.68 ± 10.05 | 40.46 ± 1.36 | 483.06 ± 64.46 | 10,083.73 ± 252.68 |
| RCunAFs | 46.15 ± 9.11 | 33.20 ± 2.01 | 43.32 ± 0.88 | 36.31 ± 0.96 | 943.08 ± 13.99 | 16,008.27 ± 1671.69 |
| RCunAFw | 28.34 ± 0.87 | 23.80 ± 1.86 | 32.22 ± 2.78 | 41.67 ± 0.24 | 973.96 ± 28.08 | 10,917.85 ± 312.77 |
| RCunAnFs | 28.16 ± 2.95 | 26.73 ± 4.62 | 19.14 ± 2.89 | 23.87 ± 6.27 | 904.03 ± 16.61 | 13,865.84 ± 238.11 |
| RCunAnFw | 20.00 ± 2.23 | 23.76 ± 3.89 | 26.39 ± 4.13 | 40.05 ± 1.17 | 994.93 ± 337.50 | 12,906.03 ± 2850.36 |
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing Essential Grains Yield for Sustainable Food Security and Bio-Safe Agriculture through Latest Innovative Approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Lu, Y.; Kear, P.; Lu, X.; Gatto, M. The Status and Challenges of Sustainable Intensification of Rice-Potato Systems in Southern China. Am. J. Potato Res. 2021, 98, 361–373. [Google Scholar] [CrossRef]
- Gatto, M.; Petsakos, A.; Hareau, G. Sustainable Intensification of Rice-Based Systems with Potato in Eastern Indo-Gangetic Plains. Am. J. Potato Res. 2020, 97, 162–174. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Zhong, L.; Li, L.; Zheng, Y.; Zhou, Y.; Zeng, Y.; Zhu, W.; Chen, F. First Report of Black Dot Caused by Colletotrichum coccodes on Potato in the Tibet Autonomous Region of China. Plant Dis. 2022, 106, 2746. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Bian, C.; Duan, S.; Wang, W.; Li, G.; Jin, L. Effects of different rotation cropping systems on potato yield, rhizosphere microbial community and soil biochemical properties. Front. Plant Sci. 2022, 13, 999730. [Google Scholar] [CrossRef]
- Dong, F.; Chen, X.; Lei, X.; Wu, D.; Zhang, Y.; Lee, Y.-W.; Mokoena, M.P.; Olaniran, A.O.; Li, Y.; Shen, G.; et al. Effect of Crop Rotation on Fusarium Mycotoxins and Fusarium Species in Cereals in Sichuan Province (China). Plant Dis. 2022, 107, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Woodhall, J.W.; Brown, L.; Harrington, M.; Murdock, M.; Pizolotto, C.A.; Wharton, P.S.; Duellman, K. Anastomosis Groups of Rhizoctonia solani and Binucleate Rhizoctonia Associated with Potatoes in Idaho. Plant Dis. 2022, 106, 3127–3132. [Google Scholar] [CrossRef]
- Acharya, U.; Das, T.; Ghosh, Z.; Ghosh, A. Defense Surveillance System at the Interface: Response of Rice Towards Rhizoctonia solani During Sheath Blight Infection. Mol. Plant-Microbe Interact. 2022, 35, 1081–1095. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, L.; Wang, Y.; Chen, Y.; Hu, K.; Yang, W.; Zuo, S.; Xu, J.; Kang, Z.; Xiao, X.; et al. A High-Quality Genome of Rhizoctonia solani, a Devastating Fungal Pathogen with a Wide Host Range. Mol. Plant-Microbe Interact. 2022, 35, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Manzar, N.; Meshram, S.; Sharma, P.K. Screening microbial inoculants and their interventions for cross-kingdom management of wilt disease of solanaceous crops- a step toward sustainable agriculture. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Guo, S.; Tao, C.; Jousset, A.; Xiong, W.; Wang, Z.; Shen, Z.; Wang, B.; Xu, Z.; Gao, Z.; Liu, S.; et al. Trophic interactions between predatory protists and pathogen-suppressive bacteria impact plant health. ISME J. 2022, 16, 1932–1943. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.; Qian, W.; Liu, H.; Jiang, X. Rotations improve the diversity of rhizosphere soil bacterial communities, enzyme activities and tomato yield. PLoS ONE 2023, 18, e0270944. [Google Scholar] [CrossRef]
- in’t Zandt, D.; Kolaříková, Z.; Cajthaml, T.; Münzbergová, Z. Plant community stability is associated with a decoupling of prokaryote and fungal soil networks. Nat. Commun. 2023, 14, 3736. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, X.; Zhao, D.; Wei, K.; Yuan, Y.; Li, Y.; Liu, M.; Zhang, C.S. Exploring Biocontrol Agents From Microbial Keystone Taxa Associated to Suppressive Soil: A New Attempt for a Biocontrol Strategy. Front. Plant Sci. 2021, 12, 655673. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, T.; Friman, V.-P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glockner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2021, 105, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.-s.; Gao, G.-l.; Ren, Y.; Ding, G.-d.; Zhang, Y. Growing season stage determines the stability of root symbiotic and pathogenic fungi associated with Pinus sylvestris var. mongolica in a semi-arid desert. Appl. Soil Ecol. 2023, 190, 104993. [Google Scholar] [CrossRef]
- Liu, S.; García-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.A.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K.; et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022, 6, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-L.; Mgelwa, A.S.; Singh, A.N.; Zeng, D.-H. Differential responses of the soil nutrient status, biomass production, and nutrient uptake for three plant species to organic amendments of placer gold mine-tailing soils. Land Degrad. Dev. 2018, 9, 2836–2845. [Google Scholar] [CrossRef]
- Skujiņš, J.; Burns, R.G. Extracellular Enzymes in Soil. CRC Crit. Rev. Microbiol. 1976, 4, 383–421. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.-B.; Xi, J.; Lu, H.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, e83. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.-X.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. iMeta 2022, 1, e32. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, W.; Yang, Y.; Hu, J.; Bian, C.; Jin, L.; Li, G.; Xiong, X. Rhizosphere microbial diversity and community dynamics during potato cultivation. Eur. J. Soil Biol. 2020, 98, 103176. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Li, D.; Xia, J.; Zhang, X. Morphological and Phylogenetic Analyses Reveal Three New Species of Pestalotiopsis (Sporocadaceae, Amphisphaeriales) from Hainan, China. Microorganisms 2023, 11, 1627. [Google Scholar] [CrossRef]
- Johnson, D.A.; Cummings, T.F. Effect of Extended Crop Rotations on Incidence of Black Dot, Silver Scurf, and Verticillium Wilt of Potato. Plant Dis. 2015, 99, 257–262. [Google Scholar] [CrossRef]
- Palm, M.E.; Gams, W.; Nirenberg, H.I. Plectosporium, a new genus for Fusarium, tabacinum, the anamorph of Plectosphaerella cucumerina. Mycologia 1995, 87, 397–406. [Google Scholar] [CrossRef]
- Rossman, A.Y. Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 1996, 88, 1–19. [Google Scholar] [CrossRef]
- Pétriacq, P.; Stassen, J.H.M.; Ton, J. Spore Density Determines Infection Strategy by the Plant Pathogenic Fungus Plectosphaerella cucumerina. Plant Physiol. 2016, 170, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lv, F.; Lv, R.; Lin, H.; Zhang, Z.; Wei, L. Sampling period and disease severity of bacterial wilt significantly affected the bacterial community structure and functional prediction in the sesame rhizosphere soil. Rhizosphere 2023, 26, 100704. [Google Scholar] [CrossRef]
- Woo, S.L.; De Filippis, F.; Zotti, M.; Vandenberg, A.; Hucl, P.; Bonanomi, G. Pea-Wheat Rotation Affects Soil Microbiota Diversity, Community Structure, and Soilborne Pathogens. Microorganisms 2022, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; Bruijn, I.; Dekkers, E.; Voort, M.; Schneider, J.; Piceno, Y.; DeSantis, T.; Andersen, G.; Bakker, P.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Klironomos, J.N.; HilleRisLambers, J.; Kinkel, L.L.; Reich, P.B.; Xiao, K.; Rillig, M.C.; Sikes, B.A.; Callaway, R.M.; Mangan, S.A.; et al. Soil microbes drive the classic plant diversity–productivity pattern. Ecology 2011, 92, 296–303. [Google Scholar] [CrossRef]
- Resendiz-Nava, C.N.; Alonso-Onofre, F.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Stasiewicz, M.J.; Nava, G.M.; Mercado-Silva, E.M. Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System. Microorganisms 2023, 11, 1633. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, H.; Chang, G.; Liang, S.; Ma, K.; Cai, Y.; Tian, B.; Shi, X. Effects of Rhizosphere Microbial Communities on Cucumber Fusarium wilt Disease Suppression. Microorganisms 2023, 11, 1576. [Google Scholar] [CrossRef]
- Hunjan, M.S.; Sabhikhi, H.S. Designing a crop rotation strategy to manage Streptomyces scabies causing potato scab in north India. J. Phytopathol. 2020, 168, 469–477. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-g.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Moreno, A.; Stefanato, F.L.; Ford, J.J.; Trippel, C.; Uszkoreit, S.; Ferrafiat, L.; Grenga, L.; Dickens, R.; Kelly, N.; Kingdon, A.D.H.; et al. Pan-genome analysis identifies intersecting roles for specialized metabolites in potato pathogen inhibition. eLife 2021, 10, e71900. [Google Scholar] [CrossRef]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Gárriz, A.; Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Liu, Y.; Nie, X.; Zheng, H.; Zhang, G.; Wang, S.; Ma, Y. Soil nutrients shape the composition and function of fungal communities in abandoned ancient rice terraces. J. Environ. Manag. 2023, 329, 117064. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Luo, W.; Li, M.; Wang, Q.; Liu, Y.; Guo, H. Summer Rice–Winter Potato Rotation Suppresses Various Soil-Borne Plant Fungal Pathogens. Agronomy 2023, 13, 2143. https://doi.org/10.3390/agronomy13082143
Zhou Y, Luo W, Li M, Wang Q, Liu Y, Guo H. Summer Rice–Winter Potato Rotation Suppresses Various Soil-Borne Plant Fungal Pathogens. Agronomy. 2023; 13(8):2143. https://doi.org/10.3390/agronomy13082143
Chicago/Turabian StyleZhou, Yuanping, Wenjiao Luo, Maoxing Li, Qiong Wang, Yongxin Liu, and Huachun Guo. 2023. "Summer Rice–Winter Potato Rotation Suppresses Various Soil-Borne Plant Fungal Pathogens" Agronomy 13, no. 8: 2143. https://doi.org/10.3390/agronomy13082143






