Effects of Paclobutrazol and Mepiquat Chloride on the Physiological, Nutritional, and Morphological Behavior of Potted Icterina Sage under Greenhouse Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Parameters
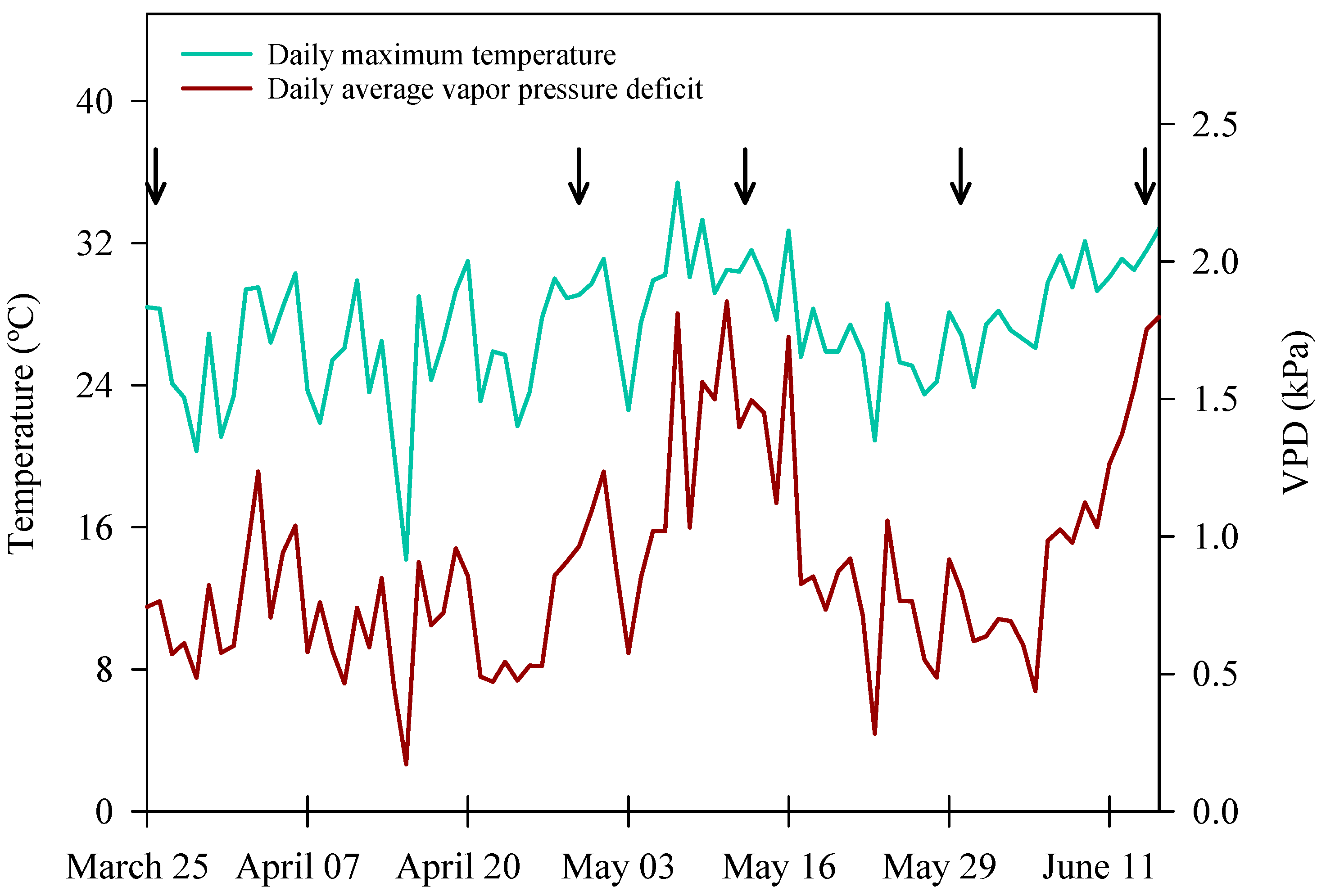
2.2. Automated Irrigation System and Soil Moisture Sensors
2.3. Water Usage and Water Use Efficiency
2.4. Plant Growth Regulator Treatments
2.5. Plant Growth and Chlorophyll Content
2.6. Mineral Ion Content in Plant Tissues
2.7. Stomatal Conductance and Photosynthesis
2.8. Chlorophyll Fluorescence
2.9. Statistical Analysis
3. Results and Discussion
3.1. Growth, Water Consumption, and Leaf Chlorophyll
3.2. Water Usage, Water Use Efficiency, and Relative Water Content
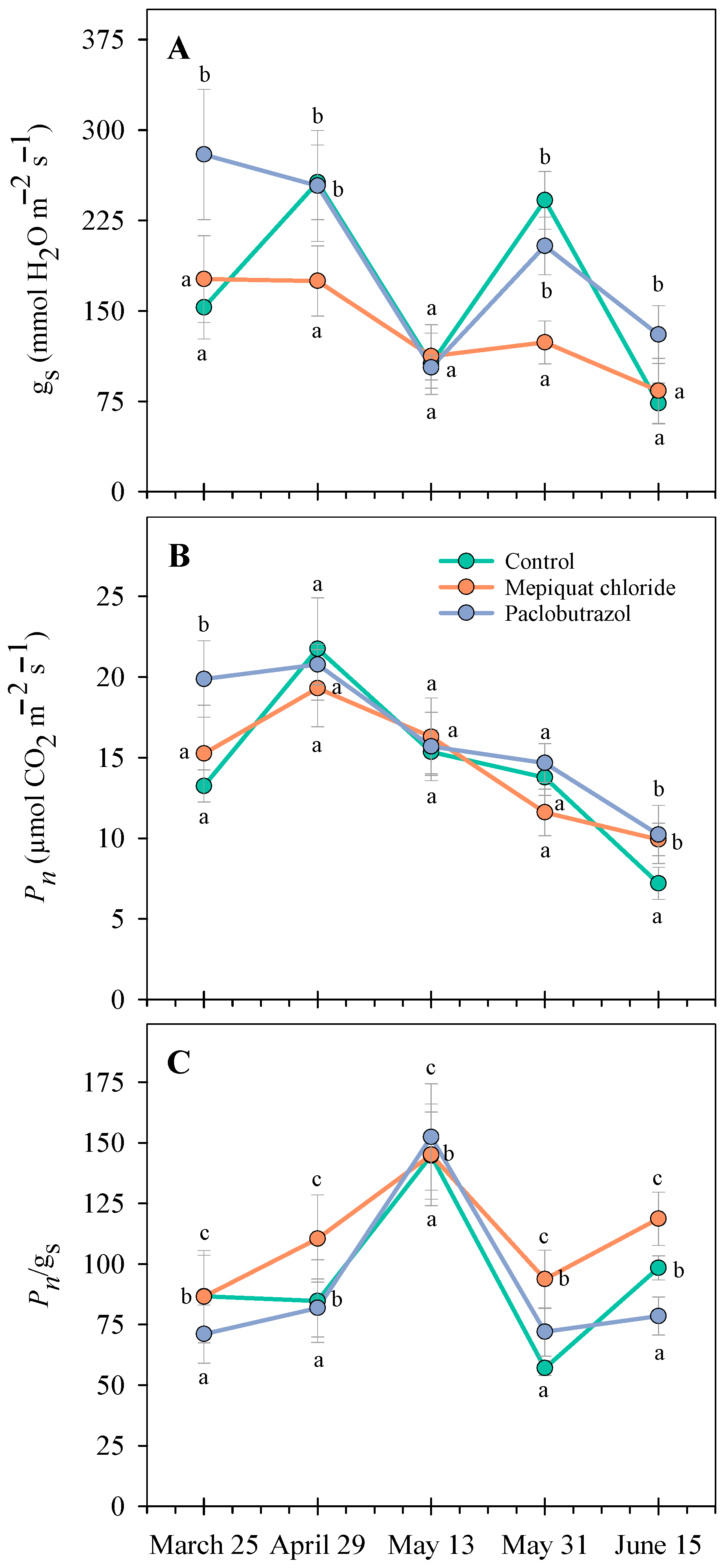
3.3. Photosynthesis and Stomatal Conductance
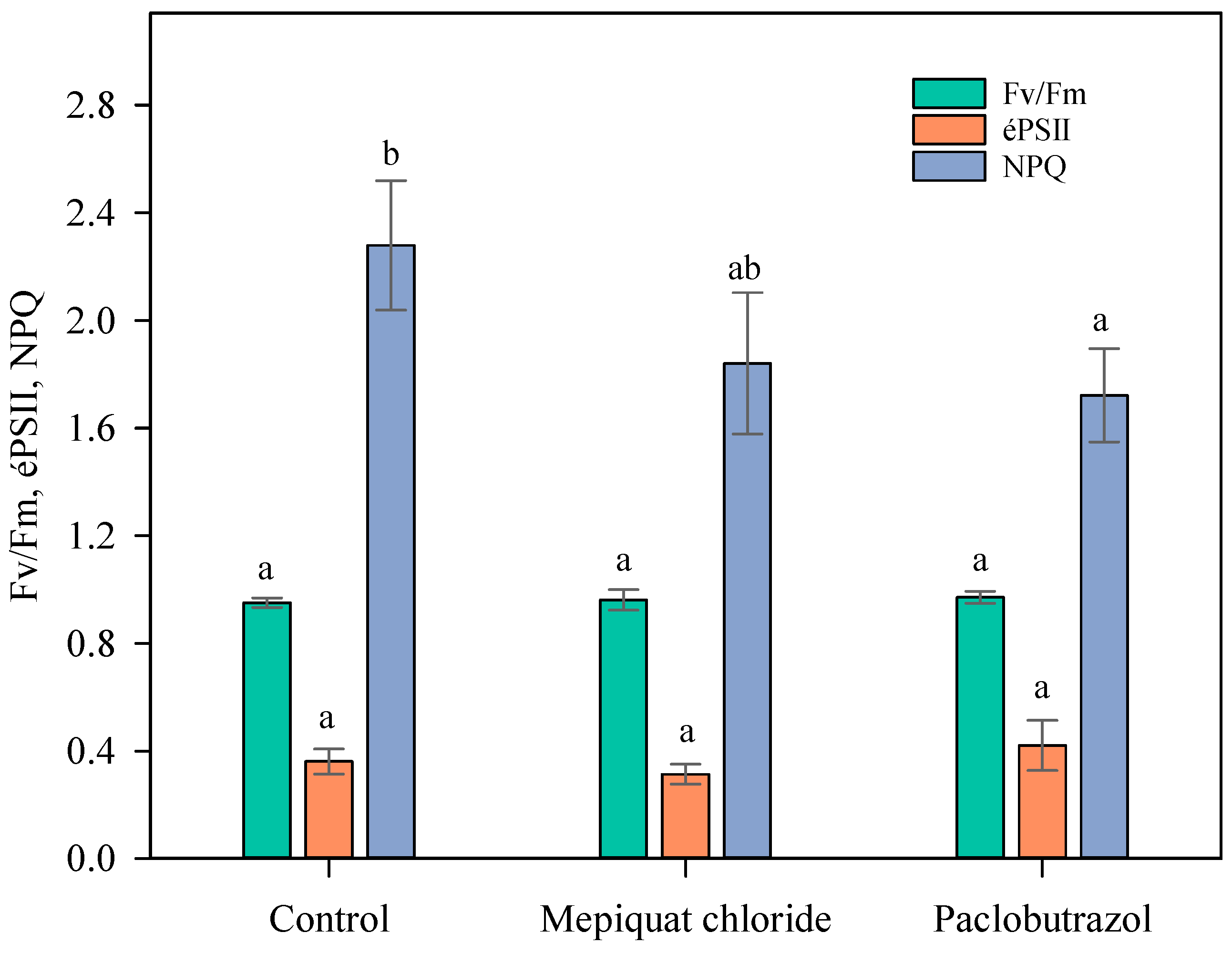
3.4. Chlorophyll Fluorescence
3.5. Plant Mineral Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Desta, B.; Amare, G. Paclobutrazol as a Plant Growth Regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Bañón, S.; Fernández, J.A.; Ochoa, J.; Sánchez-Blanco, M.J. Paclobutrazol as an Aid to Reduce Some Effects of Salt Stress in Oleander Seedlings. Eur. J. Hortic. Sci. 2005, 70, 43–49. [Google Scholar]
- Navarro, A.; Sanchez-Blanco, M.J.; Morte, A.; Bañón, S. The Influence of Mycorrhizal Inoculation and Paclobutrazol on Water and Nutritional Status of Arbutus unedo L. Environ. Exp. Bot. 2009, 66, 362–371. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Sajjad, Y.; Jaskani, M.J.; Asif, M.; Qasim, M. Application of Plant Growth Regulators in Ornamental Plants: A Review. Pak. J. Agric. Sci. 2017, 54, 327–333. [Google Scholar]
- Guimarães, R.F.B.; Maia Júnior, S.d.O.; Lima, R.F.d.; Souza, A.R.d.; Andrade, J.R.d.; Nascimento, R.d. Growth and Physiology of Ornamental Sunflower under Salinity in Function of Paclobutrazol Application Methods. Rev. Bras. Eng. Agrícola E Ambient. 2021, 25, 853–861. [Google Scholar] [CrossRef]
- Ju, S.; Xu, D.; Zhan, C.; Ji, L.; Yin, T.; Li, Z.; Lu, Z. Influence of Paclobutrazol on the Growth and Photosynthesis of Seedlings. J. Hortic. Res. 2019, 27, 21–30. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Schubert, S. Water-Use Efficiency of Maize May Be Increased by the Plant Growth Regulator Paclobutrazol. J. Agron. Crop Sci. 2021, 207, 521–534. [Google Scholar] [CrossRef]
- Soumya, P.R.; Kumar, P.; Pal, M. Paclobutrazol: A Novel Plant Growth Regulator and Multi-Stress Ameliorant. Indian J. Plant Physiol. 2017, 22, 267–278. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Ali, S.; Hafeez, A.; Shah, A.N.; Song, X.; Ma, X.; Luo, D.; Yang, G. Mepiquat Chloride Application Does Not Favor Leaf Photosynthesis and Carbohydrate Metabolism as Well as Lint Yield in Late-Planted Cotton at High Plant Density. Field Crop. Res. 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Tashmatova, M.; Azizov, B.; Aberkulov, M.; Baboev, S.; Ikromov, O. Use of Retardants Against Lodging of Medium-Sized Soft Wheat Varieties. In Proceedings of the XV International Scientific Conference “INTERAGROMASH 2022”, Rostov-on-Don, Russia, 25 May 2022; Beskopylny, A., Shamtsyan, M., Artiukh, V., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 2179–2186. [Google Scholar]
- Wang, X.; Zhou, Q.; Wang, X.; Song, S.; Liu, J.; Dong, S. Mepiquat Chloride Inhibits Soybean Growth but Improves Drought Resistance. Front. Plant Sci. 2022, 13, 982415. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, E.; Uysal, İ.; Doğan, M. Cultivated Salvia Species in Turkey. Biyolojik Çeşitlilik Koruma 2009, 2, 71–77. [Google Scholar]
- Singh, V.; Sood, R.; Ramesh, K.; Singh, B. Effects of Growth Regulator Application on Growth, Flower, Oil Yield, and Quality of Clary Sage (Salvia sclarea L.). J. Herbs Spices Med. Plants 2008, 14, 29–36. [Google Scholar] [CrossRef]
- Snyder, R.; Shaw, R.H.; Dawld, K.T. Vapour Pressure Deficit and Other Psychrometric Properties of Air from Temperature and Relative Humidity. Agric. Res. 1986, 2, 183–192. [Google Scholar]
- Valdés, R.; Miralles, J.; Ochoa, J.; Sánchez-Blanco, M.J.; Bañón, S. Saline Reclaimed Wastewater Can Be Used to Produce Potted Weeping Fig (Ficus benjamina L.) with Minimal Effects on Plant Quality. Span. J. Agric. Res. 2012, 10, 1167–1175. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction Coefficients of Chlorophyll a and B in n,n-Dimethylformamide and 80% Acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, G.C.; Liu, X.; Xia, J.B. The Responses of Photosynthetic Rate and Stomatal Conductance of Fraxinus Rhynchophylla to Differences in CO2 Concentration and Soil Moisture. Photosynthetica 2013, 51, 359–369. [Google Scholar] [CrossRef]
- Miralles, J.; Martínez-Sánchez, J.J.; Franco, J.A.; Bañón, S. Rhamnus Alaternus Growth under Four Simulated Shade Environments: Morphological, Anatomical and Physiological Responses. Sci. Hortic. 2011, 127, 562–570. [Google Scholar] [CrossRef]
- Matsoukis, A.; Gasparatos, D.; Chronopoulou-Sereli, A. Mepiquat Chloride and Shading Effects on Specific Leaf Area and K, P, Ca, Fe and Mn Content of Lantana camara L. Emir. J. Food Agric. 2015, 27, 121–125. [Google Scholar] [CrossRef]
- Ahmade, E. Effect of Pinching and Paclobutrazol on Growth and Flowering of Garland Chrysanthemum (Chrysanthemum coronarium L.). Syr. J. Agric. Res. 2019, 6, 409–419. [Google Scholar]
- Chaney, W.R. Growth Retardants: A Promising Tool for Managing Urban Trees; Purdue University: West Lafayette, IN, USA, 2005. [Google Scholar]
- Maghsoudi, E.; Abbaspour, H.; Pirbalouti, A.G.; Saeidi-Sar, S. Effects of Paclobutrazol and 24-Epibrassinolide on Some Morphological and Biochemical Characteristics of Salvia Officinalis under Different Irrigation Regimes. Iran. J. Plant Physiol. 2020, 11, 3523–3532. [Google Scholar]
- Symons, P.; Wolstenholme, B.N. Field Trial Using Paclobutrazol Foliar Sprays on Hass Avocado Trees. S. Afr. Avocado Grow. Assoc. Yearb. 1990, 13, 35–36. [Google Scholar]
- Wu, Q.; Du, M.; Wu, J.; Wang, N.; Wang, B.; Li, F.; Tian, X.; Li, Z. Mepiquat Chloride Promotes Cotton Lateral Root Formation by Modulating Plant Hormone Homeostasis. BMC Plant Biol. 2019, 19, 573. [Google Scholar] [CrossRef]
- Almeida, A.Q.d.; Rosolem, C.A. Cotton Root and Shoot Growth as Affected by Application of Mepiquat Chloride to Cotton Seeds. Acta Sci. Agron. 2012, 34, 61–65. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Responses of Antioxidant Defense System of Catharanthus Roseus (L.) G. Don. to Paclobutrazol Treatment under Salinity. Acta Physiol. Plant 2007, 29, 205–209. [Google Scholar] [CrossRef]
- Sheena, A.; Sheela, V.L. Effects of the Growth Retardant Triadimefon on the Ex Vitro Establishment of Gladiolus (Gladiolus grandiflorus L.) Cv. Vinks Glory. Plant Tissue Cult. Biotechnol. 2010, 20, 171–178. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Hafeez, A.; Ali, S.; Liu, A.; Chattha, M.S.; Ahmad, S.; Yang, G. Morpho-Physiological Effects and Molecular Mode of Action of Mepiquat Chloride Application in Cotton: A Review. J. Soil. Sci. Plant Nutr. 2020, 20, 2073–2086. [Google Scholar] [CrossRef]
- Matsoukis, A.S.; Tsiros, I.; Kamoutsis, A. Leaf Area Response of Lantana camara L. subsp. camara to Plant Growth Regulators under Different Photosynthetic Flux Conditions. HortScience 2004, 39, 1042–1044. [Google Scholar] [CrossRef]
- Berova, M.; Zlatev, Z. Physiological Response and Yield of Paclobutrazol Treated Tomato Plants (Lycopersicon Esculentum Mill.). Plant Growth Regul. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Marshall, J.; Beardmore, T.; Whittle, C.A.; Wang, B.; Rutledge, R.G.; Blumwald, E. The Effects of Paclobutrazol, Abscisic Acid, and Gibberellin on Germination and Early Growth in Silver, Red, and Hybrid Maple. Can. J. For. Res. 2000, 30, 557–565. [Google Scholar] [CrossRef]
- Navarro, A.; Sánchez-Blanco, M.J.; Bañón, S. Influence of Paclobutrazol on Water Consumption and Plant Performance of Arbutus Unedo Seedlings. Sci. Hortic. 2007, 111, 133–139. [Google Scholar] [CrossRef]
- Mohamed, G.F.; Agamy, R.A.; Rady, M.M. Ameliorative Effects of Some Antioxidants on Water-Stressed Tomato (Lycopersicon Esculentum Mill.) Plants. J. Appl. Sci. Res. 2011, 7, 2470–2478. [Google Scholar]
- Maghsoudi, E.; Abbaspour, H.; Ghasemi Pirbalouti, A.; Saeidi-Sar, S. Influence of the Foliar Applications of Paclobutrazol and 24-Epibrassinolide on the Quantitative and Qualitative Traits of Sage (Salvia officinalis L.) Volatile Oil Under Different Soil Moisture Conditions. J. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Kishorekumar, A. Differential Effects of Hexaconazole and Paclobutrazol on the Foliage Characteristics of Chinese Potato (Solenostemon Rotundifolius Poir., JK Morton). Acta Biol. Szeged. 2006, 50, 127–129. [Google Scholar]
- Zhu, L.-H.; van de Peppel, A.; Li, X.-Y.; Welander, M. Changes of Leaf Water Potential and Endogenous Cytokinins in Young Apple Trees Treated with or without Paclobutrazol under Drought Conditions. Sci. Hortic. 2004, 99, 133–141. [Google Scholar] [CrossRef]
- Ahmad Nazarudin, M.R. Plant Growth Retardants Effect on Growth and Flowering of Potted Hibiscus rosa-sinensis L. J. Trop. Plant Physiol. 2012, 4, 29–40. [Google Scholar]
- Fletcher, R.A.; Gilley, A.; Sankhla, N.; Davis, T.D. Triazoles as Plant Growth Regulators and Stress Protectants. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999; pp. 55–138. ISBN 978-0-470-65077-6. [Google Scholar]
- Reddy, A.R.; Reddy, K.R.; Hodges, H.F. Mepiquat Chloride (PIX)-Induced Changes in Photosynthesis and Growth of Cotton. Plant Growth Regul. 1996, 20, 179–183. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Oosterhuis, D.M.; Souza, F.S. de Cotton Response to Mepiquat Chloride and Temperature. Sci. Agric. 2013, 70, 82–87. [Google Scholar] [CrossRef]
- Liu, B.; Long, S.; Liu, K.; Zhu, T.; Gong, J.; Gao, S.; Wang, R.; Zhang, L.; Liu, T.; Xu, Y. Paclobutrazol Ameliorates Low-Light-Induced Damage by Improving Photosynthesis, Antioxidant Defense System, and Regulating Hormone Levels in Tall Fescue. Int. J. Mol. Sci. 2022, 23, 9966. [Google Scholar] [CrossRef]
- Cregg, B.; Ellison-Smith, D. Application of Paclobutrazol to Mitigate Environmental Stress of Urban Street Trees. Forests 2020, 11, 355. [Google Scholar] [CrossRef]
- Landis, T.D.; Nisley, R.G. The Container Tree Nursery Manual: Seedling Processing, Storage, and Outplanting; US Department of Agriculture, Forest Service: Washington, DC, USA, 2010; Volume 674, ISBN 9781782662419. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kishore, K.; Singh, H.S.; Kurian, R.M. Paclobutrazol Use in Perennial Fruit Crops and Its Residual Effects: A Review. Indian J. Agric. Sci. 2015, 85, 863–872. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium Influences on Yield and Quality Production for Maize, Wheat, Soybean and Cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Du, M.; Tian, X.; Eneji, A.E.; Duan, L.; Li, Z. Plant Growth Regulation Enhanced Potassium Uptake and Use Efficiency in Cotton. Field Crop. Res. 2014, 163, 109–118. [Google Scholar] [CrossRef]
- Symons, P.R.R.; Hofman, P.J.; Wolstenholme, B.N. Responses to Paclobutrazol of Potted “Hass” Avocado Trees. In Proceedings of the Acta Horticulturae (Netherlands), Nelspruit, South Africa, 6 November 1989; ISHS: Leuven, Belgium, 1990. [Google Scholar]
- Rieger, M.; Scalabrelli, G. Paclobutrazol, Root Growth, Hydraulic Conductivity, and Nutrient Uptake of Nemaguard’Peach. HortScience 1990, 25, 95–98. [Google Scholar] [CrossRef]
- Swietlik, D.; Miller, S.S. The Effect of Paclobutrazol on Mineral Nutrition of Apple Seedlings. J. Plant Nutr. 1985, 8, 369–382. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Ahmed, M.; Shahin, S. Effect of Magnetite and Paclobutrazol on Growth and Chemical Composition of Schefflera Arboricola Endl. Cv. Gold Capella Plant under Salt Stress Conditions. Egypt. J. Chem. 2023. [Google Scholar] [CrossRef]
- Kitayama, M.; Tisarum, R.; Samphumphuang, T.; Cha-um, K.; Takagaki, M.; Himanshu, S.K.; Cha-um, S. Promotion of Mineral Contents and Antioxidant Compounds in Water Spinach Using Foliar Paclobutrazol and Salt Elicitors. J. Soil Sci. Plant Nutr. 2023, 23, 275–289. [Google Scholar] [CrossRef]
| Parameters | Treatments | ||
|---|---|---|---|
| Control | Mepiquat | Paclobutrazol | |
| Plant dry weight (g) | 21.98 c | 17.8 b | 9.7 a |
| Aerial dry weight (g) | 15.7 c | 11.79 b | 4.89 a |
| Root dry weight (g) | 6.28 b | 6.01 b | 4.81 a |
| Shoot-to-root ratio | 2.5 c | 1.96 b | 1.02 a |
| Size index (cm) | 23.17 c | 19.43 b | 15.42 a |
| Leaf area (dm2) | 13.88 c | 6.29 b | 3.34 a |
| Number of leaves per plant | 242 c | 160 b | 141 a |
| Blade area (cm2) | 5.74 c | 3.93 b | 2.37 a |
| Leaf chlorophyll (mg gFW−1) | 0.88 a | 1.02 b | 1.18 c |
| Water applied (L pot−1) | 16.1 c | 12.8 b | 8.05 a |
| Relative water content (%) | 79.02 a | 80.09 a | 82.87 b |
| Water use efficiency (gDW L−1) | 1.37 b | 1.39 b | 1.21 a |
| Element (mg g−1) | Plant Organ | Control | Mepiquat | Paclobutrazol |
|---|---|---|---|---|
| NO3− | Leaves | 7.42 a | 9.94 b | 6.62 a |
| Shoots | 6.08 a | 11.48 b | 6.45 a | |
| Roots | 6.9 a | 11.05 b | 6.42 a | |
| P | Leaves | 1.19 a | 2.64 b | 1.2 a |
| Shoots | 1.42 a | 1.39 a | 1.31 a | |
| Roots | 1.46 a | 1.73 b | 1.28 a | |
| K+ | Leaves | 1.48 b | 2.34 c | 1.21 a |
| Shoots | 1.64 b | 1.68 b | 1.39 a | |
| Roots | 1.72 b | 1.86 b | 1.29 a | |
| Ca2+ | Leaves | 6.17 a | 10.45 c | 8.58 b |
| Shoots | 8.4 a | 9.59 ab | 10.51 b | |
| Roots | 9.08 a | 9.59 a | 9.47 a | |
| Mg2+ | Leaves | 4.82 a | 5.88 b | 5.93 b |
| Shoots | 6.02 ab | 5.2 a | 6.27 b | |
| Roots | 5.42 a | 5.05 a | 5.3 a | |
| Cl− | Leaves | 7.42 a | 23.57 b | 33.1 c |
| Shoots | 6.08 a | 28.67 b | 37.2 c | |
| Roots | 6.9 a | 24.51 b | 29.05 c | |
| Na+ | Leaves | 7.35 a | 6.75 a | 10.1 b |
| Shoots | 7.84 a | 6.98 a | 11.06 b | |
| Roots | 6.74 a | 6.63 a | 9.26 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañón, D.; Ortuño, M.F.; Sánchez-Blanco, M.J.; Pagán, B.L.; Bañón, S. Effects of Paclobutrazol and Mepiquat Chloride on the Physiological, Nutritional, and Morphological Behavior of Potted Icterina Sage under Greenhouse Conditions. Agronomy 2023, 13, 2161. https://doi.org/10.3390/agronomy13082161
Bañón D, Ortuño MF, Sánchez-Blanco MJ, Pagán BL, Bañón S. Effects of Paclobutrazol and Mepiquat Chloride on the Physiological, Nutritional, and Morphological Behavior of Potted Icterina Sage under Greenhouse Conditions. Agronomy. 2023; 13(8):2161. https://doi.org/10.3390/agronomy13082161
Chicago/Turabian StyleBañón, Daniel, María Fernanda Ortuño, María Jesús Sánchez-Blanco, Beatriz Lorente Pagán, and Sebastián Bañón. 2023. "Effects of Paclobutrazol and Mepiquat Chloride on the Physiological, Nutritional, and Morphological Behavior of Potted Icterina Sage under Greenhouse Conditions" Agronomy 13, no. 8: 2161. https://doi.org/10.3390/agronomy13082161





