Phosphorus Mobility in Heavily Manured and Waterlogged Soil Cultivated with Ryegrass (Lolium multiflorum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Greenhouse Experiment
2.2.1. Collection of Soil Columns
2.2.2. Assembling the Lysimeters
2.2.3. Experimental Design and Treatments
2.3. Plant Sampling and Analysis
2.4. Soil Sampling and Analysis
2.5. Leachate Sampling and Analysis
2.6. Statistical Analyses
3. Results
3.1. Shoot Dry Matter Yield and P Offtake
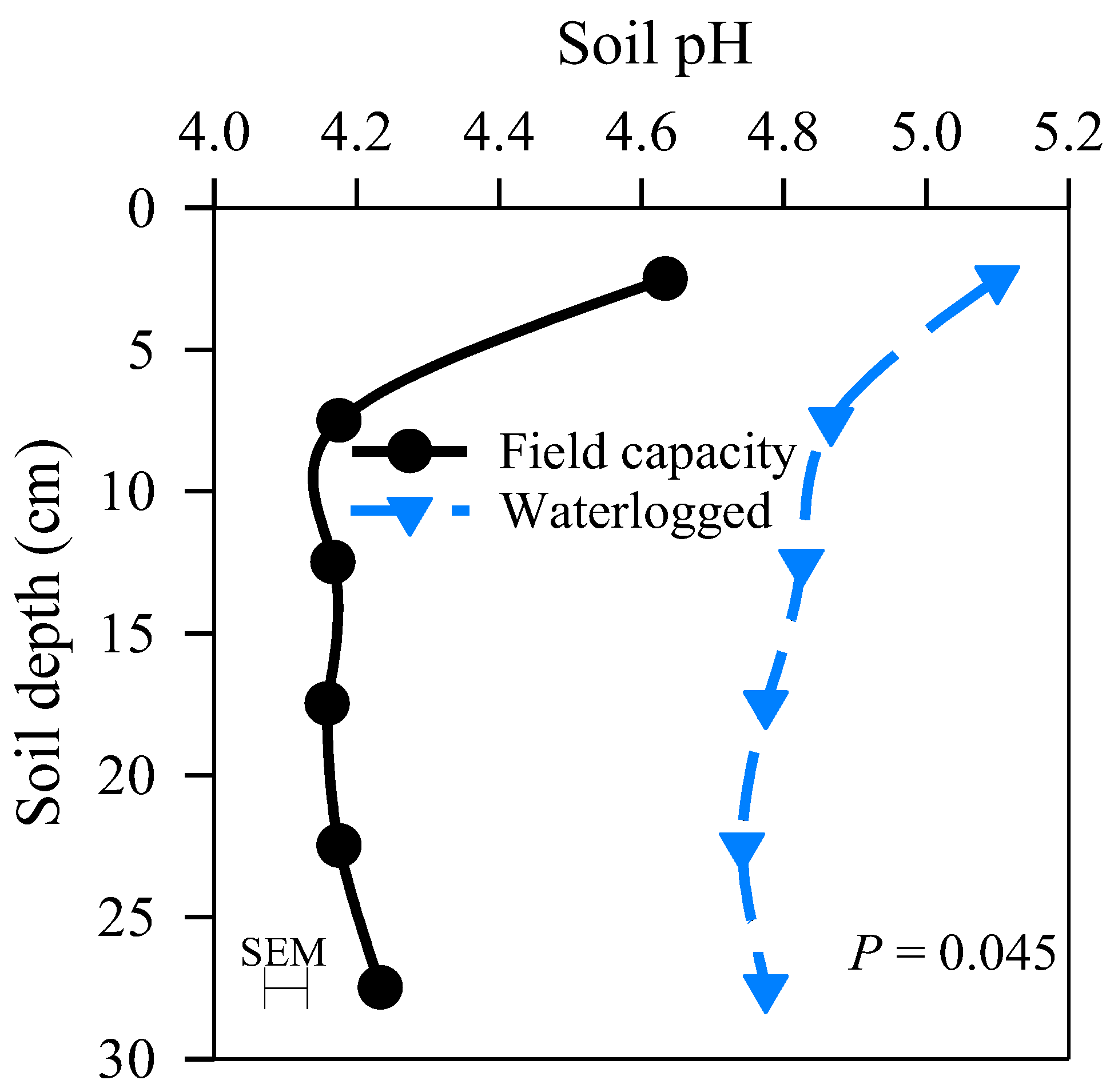
3.2. Change in Soil pH, ORP, and Fe Forms
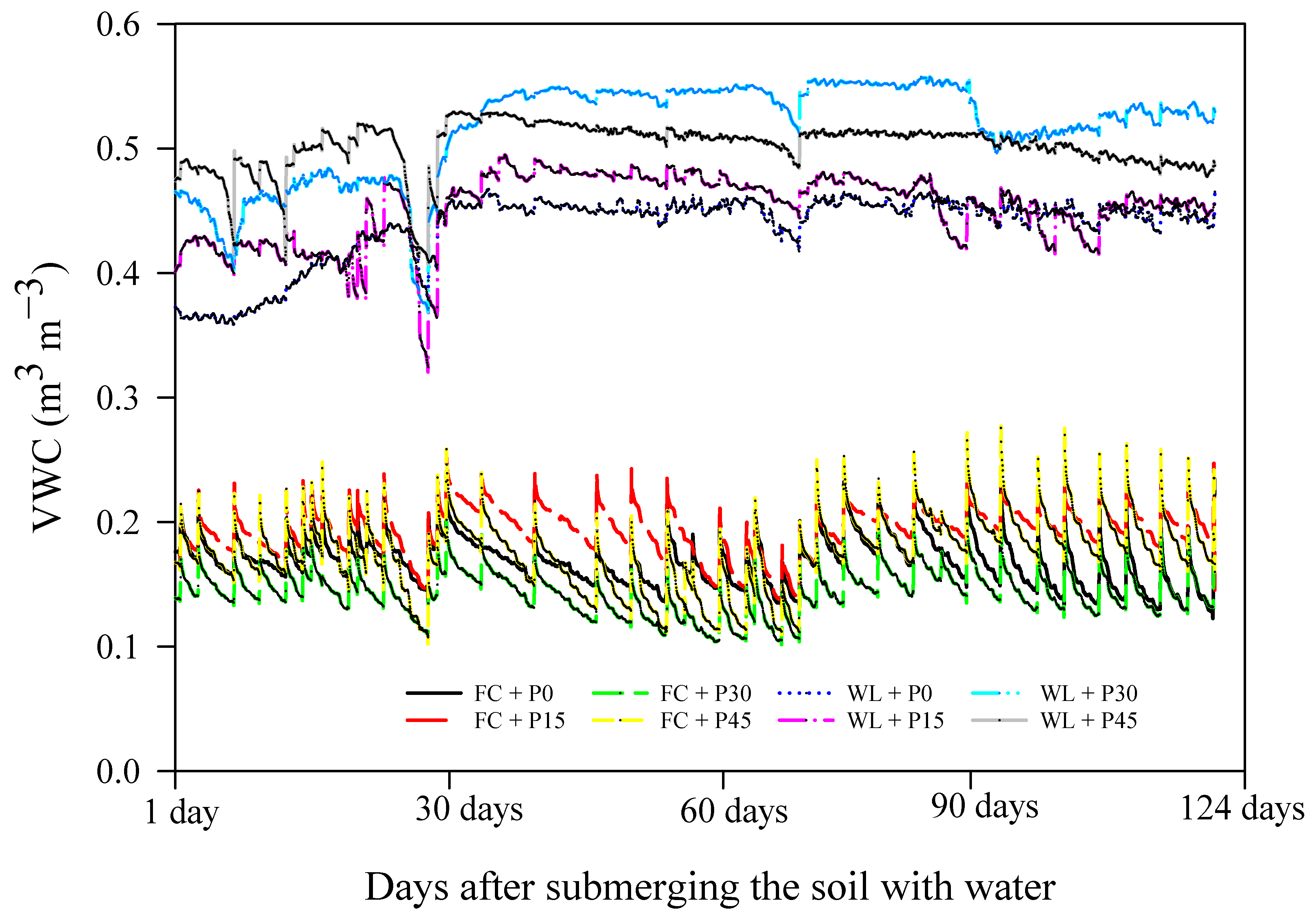
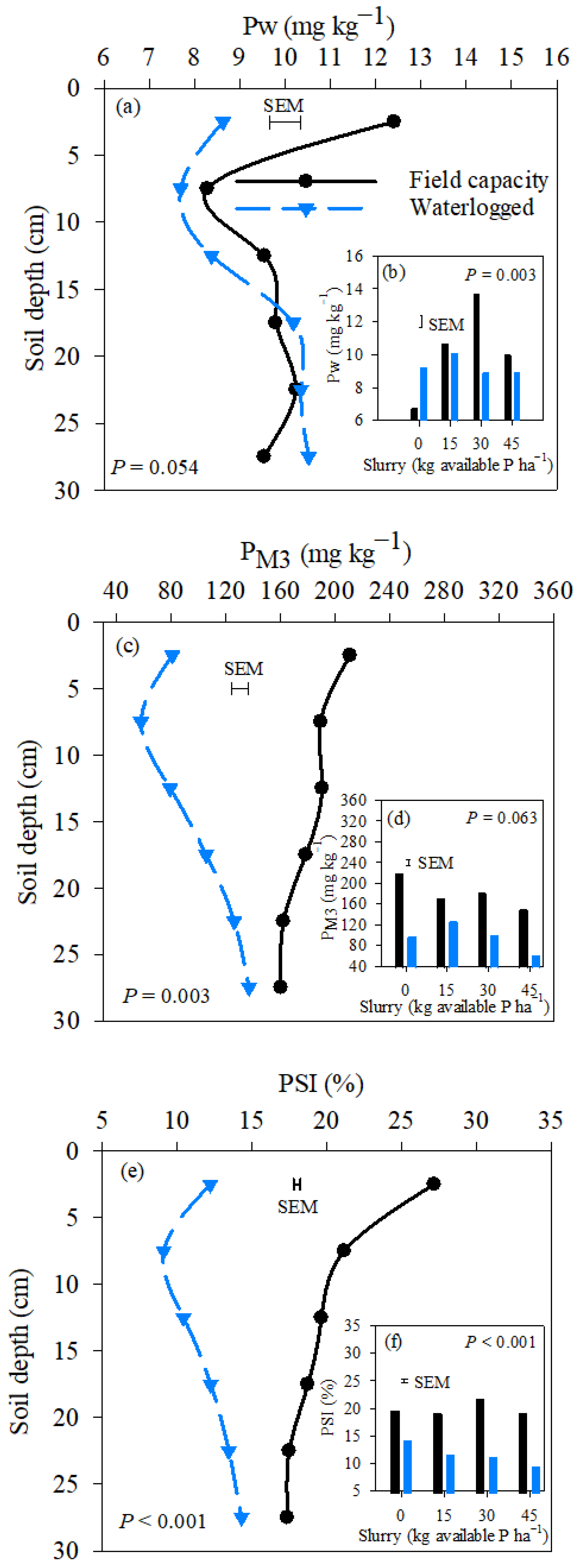
3.3. Changes in Pw, PM3, PSI, and TOP within the Soil Depth Profile
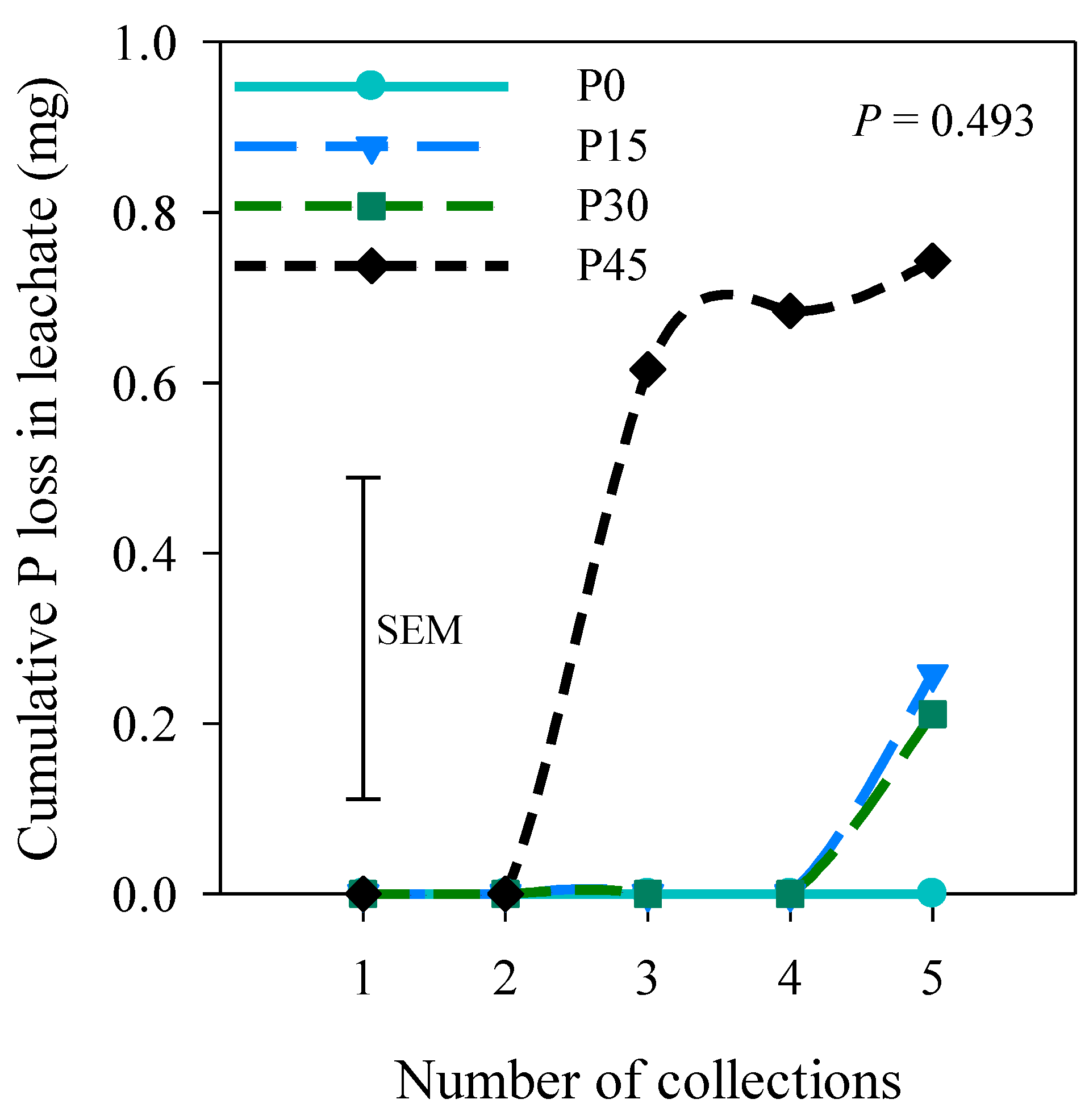
3.4. P Loss in Leachate under Field Capacity Regime
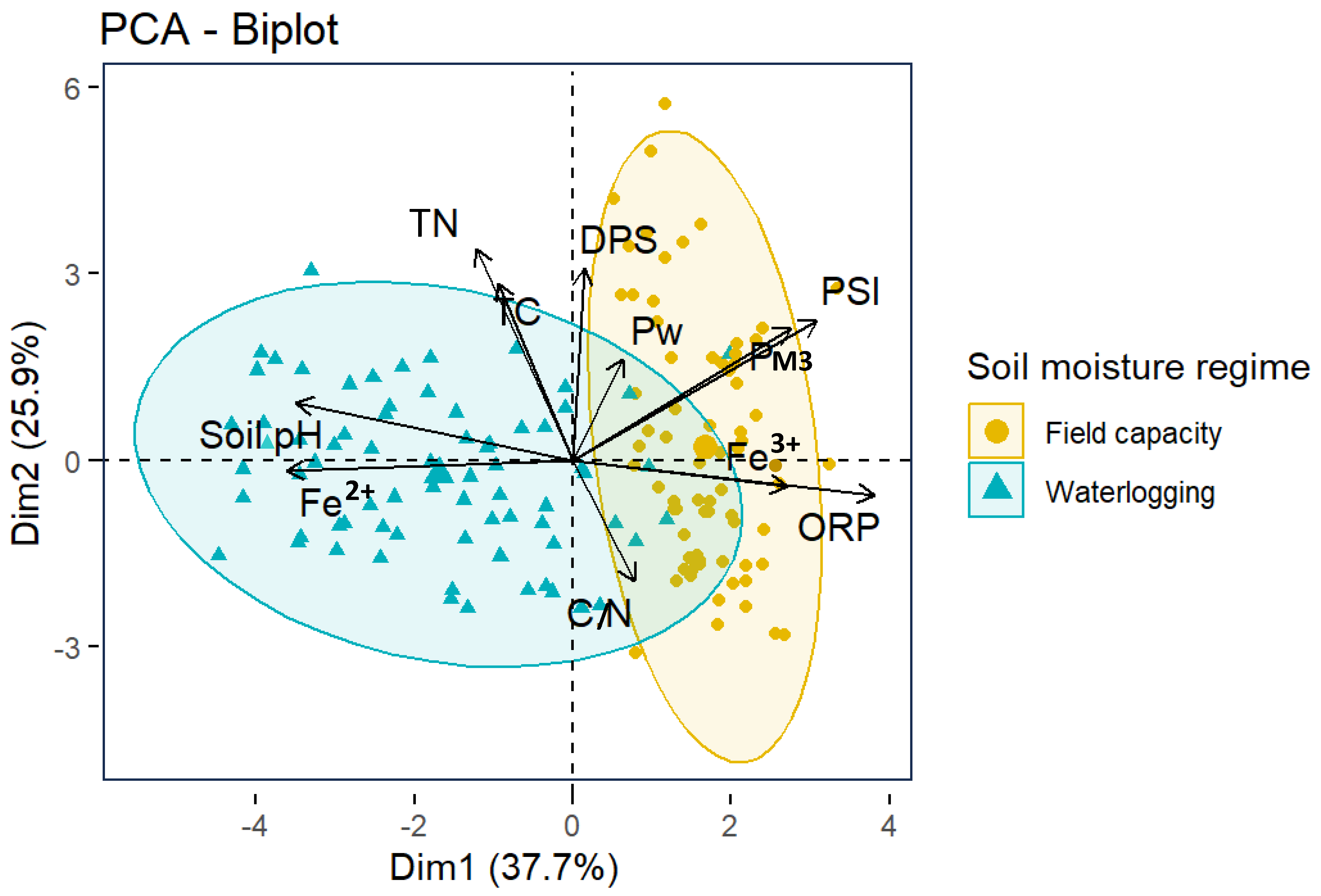
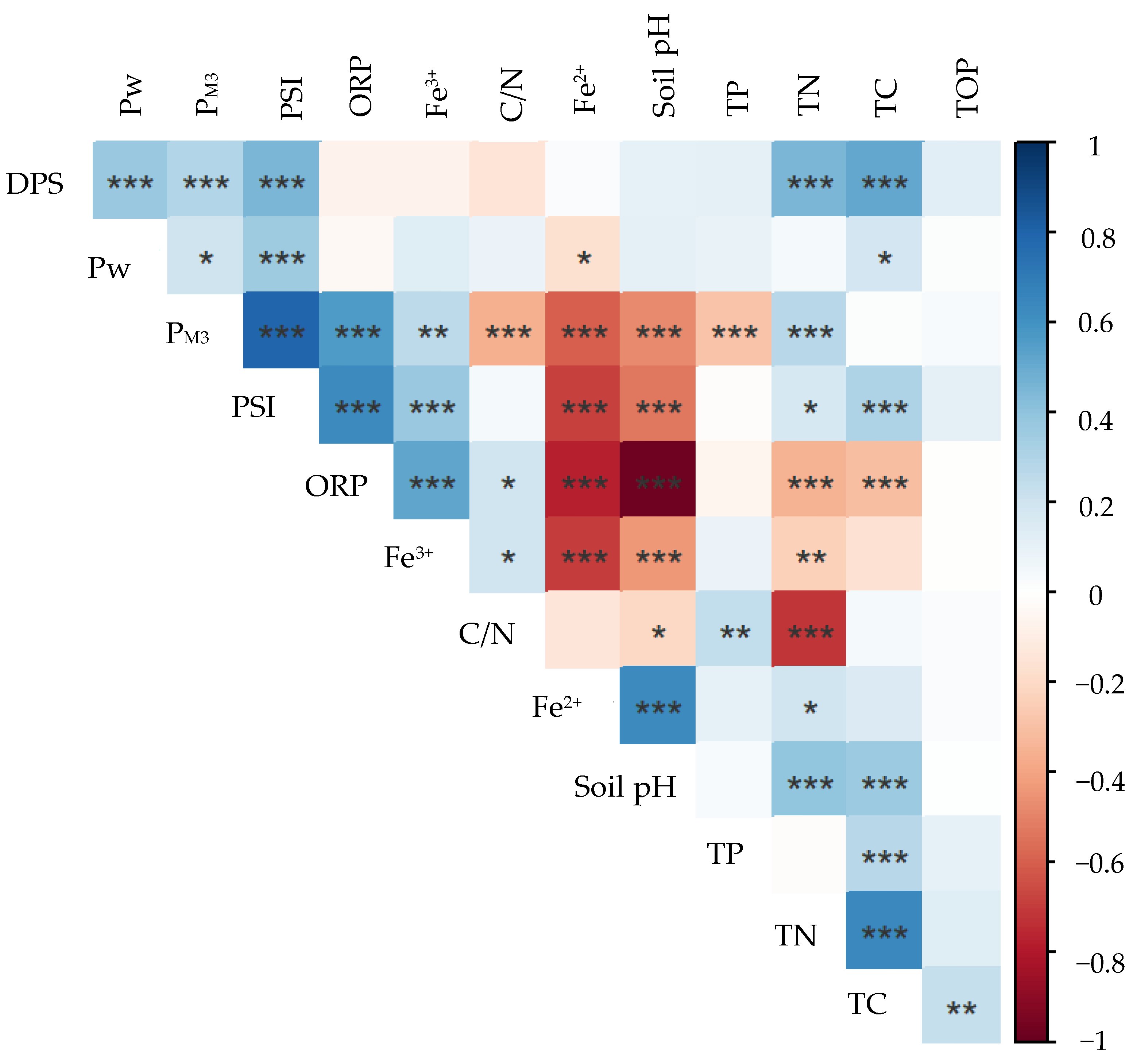
3.5. Relationship between the Soil Parameters
4. Discussion
4.1. Effects of Soil Moisture Regimes, Slurry Rate, and Duration of Plant Growth on Shoot Dry Matter Yield and P Offtake
4.2. Effects of Soil Moisture Regimes, Slurry Rate, and Soil Depth on Reduction of Fe3+ and Soil pH
4.3. Effects of Soil Moisture Regimes, Slurry Rate, and Soil Depth on Pw, PM3, and PSI
4.4. Effects of Soil Moisture Regimes, Slurry Rate, and P Offtake on P Loss
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Insurance Bureau of Canada. British Columbia Floods Cause $450 Million in Insured Damage. 2021. Available online: http://www.ibc.ca/bc/resources/media-centre/media-releases/british-columbia-floods-cause-450-million-in-insured-damage (accessed on 17 May 2022).
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Sasidharan, R. Ethylene–and oxygen signaling–drive plant survival during flooding. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Rupngam, T.; Messiga, A.; Karam, A. Solubility of Soil phosphorus in extended waterlogged conditions: An incubation study. Heliyon 2023, 9, e13502. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dong, G.; Karthikeyan, R.; Li, L.; Harmel, R.D. Phosphorus dynamics in long-term flooded, drained, and reflooded soils. Water 2017, 9, 531. [Google Scholar] [CrossRef]
- Makowski, V.; Julich, S.; Feger, K.-H.; Julich, D. Soil phosphorus translocation via preferential flow pathways: A comparison of two sites with different phosphorus stocks. Front. For. Glob. Chang. 2020, 3, 48. [Google Scholar] [CrossRef]
- Gu, S.; Gruau, G.; Dupas, R.; Petitjean, P.; Li, Q.; Pinay, G. Respective roles of Fe-oxyhydroxide dissolution, pH changes and sediment inputs in dissolved phosphorus release from wetland soils under anoxic conditions. Geoderma 2019, 338, 365–374. [Google Scholar] [CrossRef]
- Reid, K.; Schneider, K.D. Phosphorus accumulation in Canadian agricultural soils over 30 yr. Can. J. Soil Sci. 2019, 99, 520–532. [Google Scholar] [CrossRef]
- Messiga, A.J.; Lam, C.; Li, Y. Phosphorus saturation index and water-extractable phosphorus in high-legacy phosphorus soils in southern British Columbia, Canada. Can. J. Soil Sci. 2021, 101, 365–377. [Google Scholar] [CrossRef]
- Amarawansha, E.A.G.S.; Kumaragamage, D.; Flaten, D.; Zvomuya, F.; Tenuta, M. Phosphorus mobilization from manure-amended and unamended alkaline soils to overlying water during simulated flooding. J. Environ. Qual. 2015, 44, 1252–1262. [Google Scholar] [CrossRef]
- Canadian Agricultural Services Coordinating Committee; Soil Classification Working Group; National Research Council Canada; Canada Agriculture & Agri-Food Canada Research Branch. The Canadian System of Soil Classification; NRC Research Press: Ottawa, ON, Canada, 1998.
- Soil Survey Staff. Soil Survey Manual. Soil Conservation Service; USDA: Washington, DC, USA, 2014.
- Boll, J.; Steenhuis, T.S.; Selker, J.S. Fiberglass wicks for sampling of water and solutes in the vadose zone. Soil Sci. Soc. Am. J. 1992, 56, 701–707. [Google Scholar] [CrossRef]
- BC Ministry of Agriculture [BCMA]. Nutrient Management Reference Guide; Poon, D., Schmidt, O., Eds.; BC Agricultural Research and Development Corporation: Abbotsford, BC, Canada, 2010. Available online: http://ardcorp.ca/wp-content/uploads/2017/11/nutrientmgmt_refguide.pdf (accessed on 31 October 2022).
- Peters, J.; Combs, S.M.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.E.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis (A3769); University of Wisconsin: Madison, WI, USA, 2003. [Google Scholar]
- CEM Corporation. Microwave Digestion of Cannabis (Plant). Available online: https://cemcontenttype.s3.amazonaws.com/content/media-library/attachments/MetNote_SPD80_Cannabis_Plant_.pdf (accessed on 5 May 2022).
- Hendershot, W.W.H.; Lalande, H.; Duquette, M. Soil Reaction and Exchangeable Acidity. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 173–174. [Google Scholar]
- Vizier, J.F. Étude de l’état d’oxydo-réduction du sol et de ses conséquences sur la dynamique du fer dans les sols hydromorphes. Bull. Liaison Thème C-Orstom 1971, 9, 19–64. [Google Scholar]
- LECO Corporation. Trumac CNS/NS Determinators; LECO Corporation: St. Joseph, MI, USA, 2012. [Google Scholar]
- Self-Davis, M.L.; Moore, P.A., Jr.; Joern, B.C. Determination of water-and/or dilute salt extractable phosphorus. Methods Phosphorus Anal. Soils Sediments Residuals Waters 2000, 396, 24–26. [Google Scholar]
- Ziadi, N.; Tran, T.S. Mehlich 3-extractable elements. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 81–88. [Google Scholar]
- Schoumans, O.F. Determination of the degree of phosphate saturation in non-calcareous soils. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, 2nd ed.; North Carolina State University: Raleigh, NC, USA, 2000; pp. 29–32. [Google Scholar]
- Kowalenko, C.G.; Babuin, D. Use of lithium metaborate to determine total phosphorus and other element concentrations in soil, plant, and related materials. Commun. Soil Sci. Plant Anal. 2014, 45, 15–28. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; O’Halloran, I.P. Total and organic phosphorus. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press; Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 265–292. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Van der Zee, S.E.A.T.M.; Fokkink, V.D.L.; Van Riemsdijk, W.H. A new technique for assessment of reversibly adsorbed phosphate. Soil Sci. Soc. Am. J. 1987, 51, 599–604. [Google Scholar] [CrossRef]
- Khiari, L.; Parent, L.E.; Pellerin, A.; Alimi, A.R.A.; Tremblay, C.; Simard, R.R.; Fortin, J. An agri-environmental phosphorus saturation index for acid coarse-textured soils. J. Environ. Qual. 2000, 29, 1561–1567. [Google Scholar] [CrossRef]
- Benjannet, R.; Khiari, L.; Nyiraneza, J.; Thompson, B.; He, J.; Geng, X.; Stiles, K.; Jiang, Y.; Fillmore, S. Identifying environmental phosphorus risk classes at the scale of Prince Edward Island, Canada. Can. J. Soil Sci. 2018, 98, 317–329. [Google Scholar] [CrossRef]
- Yu, W.; Lawrence, N.C.; Sooksa-Nguan, T.; Smith, S.D.; Tenesaca, C.; Howe, A.C.; Hall, S.J. Microbial linkages to soil biogeochemical processes in a poorly drained agricultural ecosystem. Soil Biol. Biochem. 2021, 156, 108228. [Google Scholar] [CrossRef]
- Huang, W.; Hall, S.J. Optimized high-throughput methods for quantifying iron biogeochemical dynamics in soil. Geoderma 2017, 306, 67–72. [Google Scholar] [CrossRef]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Mui, N.T.; Zhou, M.; Parsons, D.; Smith, R.W. Aerenchyma Formation in Adventitious Roots of Tall Fescue and Cocksfoot under Waterlogged Conditions. Agronomy 2021, 11, 2487. [Google Scholar] [CrossRef]
- Lkhagvasuren, B.; Schoenau, J.J.; Anderson, D.W.; Malhi, S.S. Plant and soil responses to nitrogen and phosphorus fertilization of bromegrassdominated haylands in Saskatchewan, Canada. Grass Forage Sci. 2011, 66, 351–360. [Google Scholar] [CrossRef]
- Tan, M.; Hou, Y.; Zhang, L.; Shi, S.; Long, W.; Ma, Y.; Zhang, T.; Oenema, O. Nutrient use efficiency of intensive dairy farms in China—Current situation and analyses of options for improvement. Agric. Syst. 2022, 203, 103495. [Google Scholar] [CrossRef]
- Popovic, O.; Hjorth, M.; Stoumann Jensen, L. Phosphorus, copper and zinc in solid and liquid fractions from full-scale and laboratory-separated pig slurry. Environ. Technol. 2012, 33, 2119–2131. [Google Scholar] [CrossRef]
- Gao, R.; Duan, Y.; Zhang, J.; Ren, Y.; Li, H.; Liu, X.; Zhao, P.; Jing, Y. Effects of long-term application of organic manure and chemical fertilizer on soil properties and microbial communities in the agro-pastoral ecotone of North China. Front. Environ. Sci. 2022, 10, 993973. [Google Scholar] [CrossRef]
- Hall, S.J.; Liptzin, D.; Buss, H.L.; DeAngelis, K.; Silver, W.L. Drivers and patterns of iron redox cycling from surface to bedrock in a deep tropical forest soil: A new conceptual model. Biogeochemistry 2016, 130, 177–190. [Google Scholar] [CrossRef]
- Messiga, A.J.; Ziadi, N.; Jouany, C.; Virkajärvi, P.; Suomela, R.; Sinaj, S.; Belanger, G.; Stroia, C.; Morel, C. Soil test phosphorus and cumulative phosphorus budgets in fertilized grassland. AMBIO 2015, 44, 252–262. [Google Scholar] [CrossRef]
- Watson, C.J.; Matthews, D.I. A 10-year study of phosphorus balances and the impact of grazed grassland on total P redistribution within the soil profile. Eur. J. Soil Sci. 2008, 59, 1171–1176. [Google Scholar] [CrossRef]
- Hejcman, M.; Klaudisová, M.; Štursa, J.; Pavlů, V.; Schellberg, J.; Hejcmanová, P.; Hakl, J.; Rauch, O.; Vacek, S. Revisiting a 37 years abandoned fertilizer experiment on Nardus grassland in the Czech Republic. Agric. Ecosyst. Environ. 2007, 118, 231–236. [Google Scholar] [CrossRef]
- Pradhan, S.N.; Ghosh, A.K.; Ram, S.; Pal, Y.; Pradhan, C. Changes in degree of phosphorus saturation and risk of P loss upon twelve years of manuring and reduced tillage. Geoderma 2021, 404, 115277. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Environmental Indicators of Water Quality in the United States; US Government Printing Office: Washington, DC, USA, 1996.
- Messiga, A.J.; Ziadi, N.; Plénet, D.; Parent, L.E.; Morel, C. Long-term changes in soil phosphorus status related to P budgets under maize monoculture and mineral P fertilization. Soil Use Manag. 2010, 26, 354–364. [Google Scholar] [CrossRef]




| 0–15 cm | 15–30 cm | |
|---|---|---|
| Particle size distribution (g kg−1) | ||
| Sand | 585 (7) a | 610 (21) |
| Silt | 310 (0) | 290 (14) |
| Soil textural class | Sandy Loam | Sandy Loam |
| Soil pH | 4.2 (0.1) | 4.2 (0.0) |
| Organic matter (g kg−1) | 43 (1.0) | 43 (1.0) |
| CEC (cmol(+) kg−1) | 6.7 (3.9) | 10.2 (4.5) |
| Total carbon (g kg−1) | 27(2.6) | 24 (1.4) |
| Total nitrogen (mg kg−1) | 2088 (33) | 2046 (161) |
| Carbon to nitrogen ratio (%) | 13 (1.0) | 12 (1.3) |
| Total phosphorus (mg kg−1) | 6635 (130) | 6402 (25) |
| Total organic phosphorus (mg kg−1) | 4282 (193) | 4745 (459) |
| Total iron (g kg−1) | 190 (1.7) | 184 (2.5) |
| Water extractable phosphorus (mg kg−1) | 9.4 (0.9) | 9.5 (1.2) |
| Mehlich-3 phosphorus (mg kg−1) | 214 (6) | 244 (2) |
| Mehlich-3 aluminum (mg kg−1) | 895 (10) | 822 (19) |
| Mehlich-3 iron (mg kg−1) | 64 (2.3) | 64 (1.0) |
| Phosphorus saturation index (%) | 18.9 (0.6) | 21.9 (0.9) |
| Oxalate phosphorus (mmol kg−1) | 29 (0.3) | 30 (0.4) |
| Oxalate aluminum (mmol kg−1) | 276 (1.1) | 279 (0.2) |
| Oxalate iron (mmol kg−1) | 138 (0.6) | 137 (0.8) |
| Degree of phosphorus saturation (%) | 14 (0.1) | 14 (0.2) |
| Values | ||
|---|---|---|
| pH | 7.0 (0.07) a | |
| Dry matter (%) | 2.05 (0.18) | |
| Values (wet dairy slurry basis) | Values (dry dairy slurry basis) | |
| Total nitrogen (mg kg−1) | 1190.50 (0.01) | 58,897.73 (6083.51) |
| NH4-N (mg kg−1) | 598.45 (0.00) | 29,746.13 (3959.92) |
| Total phosphorus (mg kg−1) | 206.01 (13.71) | 10,105.41 (517.21) |
| Phosphate (mg kg−1 P as P2O5) | 471.76 (31.39) | 23,141.39 (1184.41) |
| Total potassium (mg kg−1) | 890.81 (11.65) | 44,478.13 (6441.3) |
| Soil pH | Calculated ORP | Fe3+ | Fe2+ | Pw | PM3 | PSI | TC | TN | C-to-N | TP | TOP | FeM3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture content (MC) | |||||||||||||
| Field capacity | 4.3 b | 297.9 a | 1990.3 a | 242.2 b | 10.3 a | 181.8 a | 20.2 a | 25,686.2 a | 2182.6 a | 12.1 a | 6385.6 a | 4887.7 a | 69 b |
| Waterlogged | 4.8 a | 139.9 b | 1535.4 b | 2356.6 a | 9.3 a | 97.6 b | 11.9 b | 25,386.4 a | 2254.3 a | 11.5 a | 6334.0 a | 4709.3 b | 196 a |
| SEM (MC) | 0.02 | 3.91 | 39.15 | 74.70 | 0.34 | 4.96 | 0.31 | 17.7 | 4.22 | 0.98 | 7.89 | 96.09 | 0.25 |
| Slurry (S) | |||||||||||||
| 0 | 4.4 b | 253.8 a | 1862.2 a | 890.9 b | 7.9 b | 159.4 a | 17.2 a | 24,941.1 b | 2117.4 b | 11.9 a | 6410.9 a | 4594.3 a | 124 b |
| 15 | 4.6 a | 212.1 b | 1732.9 a | 1272.7 ab | 10.4 ab | 150.7 a | 15.7 b | 25,988.2 a | 2403.8 a | 11.2 a | 6393.9 a | 4873.4 a | 130 ab |
| 30 | 4.6 a | 204.6 b | 1776.2 a | 1459.2 ab | 11.3 a | 142.1 a | 16.8 ab | 25,813.1 a | 2162.1 ab | 12.2 a | 6364.9 a | 4647.7 a | 135 a |
| 45 | 4.6 a | 205.2 b | 1680.3 a | 1574.8 a | 9.5 ab | 106.8 b | 14.6 bc | 25,402.8 ab | 2190.5 ab | 11.8 a | 6269.5 a | 5075.4 a | 141 a |
| SEM (S) | 0.03 | 7.56 | 45.31 | 134.67 | 0.33 | 5.85 | 0.46 | 38.9 | 10.59 | 0.98 | 7.99 | 95.54 | 0.62 |
| Soil depth (SD) | |||||||||||||
| 0–5 cm | 4.9 a | 154.9 c | 1588.9 b | 1573.5 ab | 11.5 a | 145.7 a | 19.7 a | 32,846.8 a | 2805.9 a | 11.9 a | 6629.8 a | 5210.5 a | 144 ab |
| 5–10 cm | 4.5 b | 214.7 b | 1663.8 ab | 2075.9 a | 7.9 a | 123.5 a | 15.1 b | 27,348.3 b | 2395.7 b | 11.6 a | 6502.8 a | 5056.8 ab | 160 a |
| 10–15 cm | 4.5 b | 221.4 ab | 1528.1 b | 1848.1 a | 8.9 a | 134.7 a | 15.0 b | 24,589.6 c | 2193.4 bc | 11.5 a | 6415.5 a | 4813.4 ab | 145 ab |
| 15–20 cm | 4.5 b | 238.9 a | 1861.3 ab | 946.1 bc | 9.9 a | 141.9 a | 15.5 b | 23,308.2 d | 2076.0 c | 11.6 a | 6064.7 a | 4721.7 ab | 128 b |
| 20–25 cm | 4.5 b | 243.2 a | 1891.0 ab | 889.7 bc | 10.3 a | 144.1 a | 15.5 b | 22,682.3 d | 1921.4 c | 12.2 a | 6306.8 a | 4537.7 ab | 113 c |
| 25–30 cm | 4.5 b | 240.3 a | 2044.2 a | 463.1 c | 10.0 a | 148.5 a | 15.8 b | 22,442.6 d | 1918.2 c | 12.0 a | 6239.3 a | 4451.4 b | 105 c |
| SEM (SD) | 0.03 | 7.32 | 42.19 | 118.65 | 0.33 | 6.12 | 0.44 | 333.96 | 28.28 | 0.16 | 7.74 | 74.75 | 0.59 |
| p-values | |||||||||||||
| MC | <0.001 | <0.001 | <0.001 | <0.001 | ns | <0.001 | <0.001 | ns | ns | ns | ns | 0.039 | <0.001 |
| S | <0.001 | <0.001 | ns | 0.024 | 0.005 | ns | <0.001 | 0.003 | 0.021 | ns | ns | ns | 0.004 |
| MC × S | ns | 0.032 | ns | 0.023 | 0.003 | 0.264 | <0.001 | ns | 0.039 | ns | ns | ns | ns |
| SD | <0.001 | <0.001 | 0.002 | <0.001 | ns | <0.001 | <0.001 | <0.001 | <0.001 | ns | ns | 0.005 | <0.001 |
| MC × SD | 0.045 | <0.001 | 0.027 | <0.001 | 0.054 | <0.001 | <0.001 | <0.001 | ns | ns | ns | ns | 0.002 |
| S × SD | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| MC × S × SD | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| N | 144 | 144 | 144 | 144 | 144 | 144 | 144 | 144 | 144 | 144 | 144 | 143 | 144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupngam, T.; Messiga, A.J.; Karam, A. Phosphorus Mobility in Heavily Manured and Waterlogged Soil Cultivated with Ryegrass (Lolium multiflorum). Agronomy 2023, 13, 2168. https://doi.org/10.3390/agronomy13082168
Rupngam T, Messiga AJ, Karam A. Phosphorus Mobility in Heavily Manured and Waterlogged Soil Cultivated with Ryegrass (Lolium multiflorum). Agronomy. 2023; 13(8):2168. https://doi.org/10.3390/agronomy13082168
Chicago/Turabian StyleRupngam, Thidarat, Aimé J. Messiga, and Antoine Karam. 2023. "Phosphorus Mobility in Heavily Manured and Waterlogged Soil Cultivated with Ryegrass (Lolium multiflorum)" Agronomy 13, no. 8: 2168. https://doi.org/10.3390/agronomy13082168
APA StyleRupngam, T., Messiga, A. J., & Karam, A. (2023). Phosphorus Mobility in Heavily Manured and Waterlogged Soil Cultivated with Ryegrass (Lolium multiflorum). Agronomy, 13(8), 2168. https://doi.org/10.3390/agronomy13082168





