Abstract
Juvenile hormones (JHs) play a crucial role in the development of the fall armyworm, Spodoptera frugiperda, with varying types and concentrations observed at different stages. However, the interplay between JHs and 20-hydroxyecdysone (20E) in co-ordinating the life cycle of S. frugiperda remains unknown. In this study, by using high-precision UPLC-MS/MS and qRT-PCR, we detected changes in JH and 20E levels and identified important 20E receptor and response genes. Our findings revealed that JH I antagonises JH II, whereas JH II promotes JH III synthesis. High JH I and JH II concentrations in the larval stage strongly affected moulting to the next instar. Furthermore, these hormones inhibit 20E synthesis and reduce its receptor expression, thereby affecting 20E signalling. During pupation, JH II plays a crucial role in stimulating 20E synthesis for larval–pupal transformation. JH I and JH II are essential for eclosion, precisely controlling emergence timing and subsequent reproductive organ maturation. These hormones likely regulate larval development, pupation, and adult reproduction in S. frugiperda. Further studies are warranted to explore the regulatory advantages of JH I and JH II over JH III.
1. Introduction
Spodoptera frugiperda is a well-known agricultural pest in its native range of North and South America and has become a major invasive pest around the globe in the past decade [1,2]. S. frugiperda invaded Yunnan, China, in December 2018 [3] and spread rapidly, and subsequently, outbreaks were detected in 26 provinces (autonomous regions, municipalities) [4]. Information on the potential economic loss of maize in China caused by S. frugiperda indicates a range from USD $5.4–47 billion per annum [5]. It is regarded as a super pest based on its host range (at least 353 host plants), its inherent ability to survive in a wide range of habitats, its strong migration ability, high fecundity, its rapid development of resistance to insecticides/viruses, and its gluttonous characteristics [6,7,8,9].
Juvenile hormone (JH) and 20-hydroxyecdysone (20E) collaboratively regulate various biological processes throughout the insect life cycle, such as embryonic development, moulting, metamorphosis, and reproduction [10,11]. These hormones can act either alone or in combination to modulate specific physiological events. An exemplary case demonstrating the co-ordination of JH and 20E is found in the tobacco hornworm Manduca sexta, in which 20E triggers each moult while JH governs the nature of the moulting, determining whether it leads to another larger larva or initiates the metamorphic programme. The anti-metamorphic function of JH is evident in early larval instars; however, its synthesis ceases once the larva reaches a critical weight, allowing 20E to instigate metamorphosis and transform the larva into a pupa [12].
Eight natural JHs have been identified, with JH III being predominant in most insects [13,14] and JH I and JH II being predominant in lepidopteran insects [15]. This suggests that JH I and JH II may serve as morphogenetic hormones, whereas JH III primarily functions as a gonadotropin [16], although this interpretation is questionable given the detection of both JH I and JH II in most morphogenetic insects. Furthermore, JH III has been identified as the primary hormone in both the larvae and adults of certain insect species [17]. In the fifth instar larvae of S. frugiperda, all three homologs, JH I, JH II, and JH III, have been detected [18], implying that JH III might have been the first JH to emerge during insect evolution, with JH I and JH II potentially considered special evolutionary products that are involved in regulating metamorphosis [19].
JH exerts its effects through methoprene-tolerant/germ-cell-expressed (Met/Gce) and Krüppel homolog 1 (Kr-h1) to inhibit ecdysone biosynthesis in Drosophila prothoracic glands [20]. A high JH titre hinders the production and release of prothoracicotropic hormone (PTTH) [21,22], leading to a decline in 20E synthesis and titre. By delaying metamorphosis until the appropriate developmental stage and size are achieved, the decline in JH secretion [23,24] enables PTTH release from the brain, stimulating the synthesis and release of ecdysone, thereby initiating the transition from larvae to pupae [25].
JHs and 20E are pivotal regulatory hormones that govern diverse biological processes in insects and exhibit differential responses across distinct developmental stages and tissues [26]. Insect moulting and metamorphosis are regulated by the dynamic balance between 20E and JH [26]. Overall, 20E co-ordinates the moulting process, while JH determines whether the larvae maintain their larval stage or pupate. Previous studies have suggested that 20E exerts its effects through the nuclear receptor complex of ecdysone receptor (EcR) and ultraspiracle protein (USP), whereas JH maintains larval characteristics and prevents metamorphosis by antagonising the action of 20E [22].
Starvation during the sensitive period of S. frugiperda adults results in an increased JH titre [27]. A study on the effects of food stress on gene expression in S. frugiperda identified four differentially expressed genes (E75, EcR, BrC, and USP) related to 20E biosynthesis, suggesting that starvation stress also affects 20E synthesis [28].
In the present study, we assessed the trends in JHs and 20E across the various developmental stages of S. frugiperda. Our findings revealed three distinct JH types present during the S. frugiperda larval stage: JH I, JH II, and JH III. Different JHs reached their peak levels at different times during the various developmental stages, suggesting a unique role for each hormone at each stage. However, the precise functions of these JHs and their roles during specific periods remain unclear. In order to elucidate the effects and temporal dynamics of distinct JHs, we augmented their titres in S. frugiperda through in vivo injection and investigated the interplay between these hormones. The in vivo injection of different JHs during the larval stage allowed us to explore their sensitivity at different stages and their impact on development. By using molecular detection, we identified upregulated and downregulated ecdysone-related genes during critical periods of specific JHs, thereby providing insights into the underlying mechanisms driving regulatory processes.
2. Materials and Methods
2.1. Insects
S. frugiperda were collected from cornfields located on the outskirts of Nanning City, Guangxi, China, in July 2022. The insects were reared in bacterial culture dishes at a temperature range of 24–26 °C, a relative humidity of 60–70%, and a photoperiod of 14:10 (L:D) hours. The larvae were reared on maize leaves and kernels to simulate their natural food source in the field. The adult moths were provided with a 10% honey water solution and laid eggs on nylon webs, which were then collected for subsequent subculturing and the propagation of the next generation. Throughout this process, S. frugiperda was maintained under controlled environmental conditions without exposure to any insecticides.
2.2. Chemical Drugs
JH I (≥98%), JH II (≥96%), JH III (≥95%), and 20-hydroxyecdysone (≥98%) were procured from Toronto Research Chemicals, Canada. High-performance liquid chromatography-grade acetonitrile was sourced from Merck, Germany; analytical-grade methanol, acetonitrile, and hexane were obtained from Chengdu Kelong Chemical Reagent Factory. Analytical-grade methanol, acetonitrile, and hexane were procured from Chengdu Kelong Chemical Reagent Factory. A 200 μL glass cannula with a polymer stent was obtained from Shanghai Anpu Company. Ultrapure water was purified using the Millipore treatment system (Millipore, Billerica, MA, USA) and was utilized throughout the experiment.
2.3. Solvent Configuration
Three JH standards (2.5 mg JH I, 2.5 mg JH II, and 100 mg JH III) were accurately weighed, and the standards were dissolved in 10 mL of acetonitrile to obtain stock solutions. Thus, standard solutions of three JHs were prepared at a concentration of 50 mg/L by acetonitrile dilution. The 20-hydroxyecdysone standard was accurately weighed at 20 mg, and the standard was dissolved in 10 mL of ethanol to obtain the stock solution. Thus, standard solutions of 20E were prepared at a concentration of 50 mg/L by ethanol dilution.
Take 1 mL of JH I, JH II, JH III, and 20E standard solutions, dried under nitrogen flow, and dissolve them with 1 mL of 0.5% dimethyl sulfoxide and 10% ethanol in water, respectively, for subsequent injection experiments.
2.4. Experimental Treatment
Newly moulting and soon-moulting larvae of each instar were collected. New pupae were collected from 8 a.m. to 11 a.m. every day and divided into 24 h pupae with 96 h pupae and 168 h pupae according to the days after pupation. Newly emerged female adults were collected from 8 to 11 a.m. each day, after which they were fed individually to maintain virgin status, mated freely 48 h later, and female adults were collected after laying eggs.
The fourth-instar larvae of the same size were selected and divided into two groups, with 50 larvae in each group. The starvation group was fed with corn kernels after 24 h of starvation, and the control group was fed with corn kernels all the time. The levels of the three JHs and 20E in the body were determined, and the pupation time was recorded.
On the fourth day, the fourth-instar larvae and the sixth instar larvae (prepupa) were injected with 1 μL of 50 mg/L of different types of juvenile hormone, and this was injected into the blood vessel of the dorsal midline. Immediately after the injection, the injection was sealed with instant sol to prevent leakage of the injection. After half an hour of treatment, the larvae were frozen in the refrigerator at −80 °C for subsequent experiments.
The 24 h pupae were divided into four groups, and 50 pupae in each group were treated with 2 μL of 50 mg/L JH I, JH II, JH III, and the control, respectively. Emergence time and emergence rate were observed, and the freshly emerging adults were dissected. All treatments were repeated three times.
2.5. Quantification of JHs and 20E by UPLS-MS/MS
For the extraction of JH, add 1 mL 0.7% NaCl aqueous solution and acetonitrile (1:1) and 10 small steel balls per gram of S. frugiperda larvae. Grind at a frequency of 70 Hz for two minutes, then add 2 mL ethyl acetate and centrifuge at 4000 RPM. The upper supernatant was collected and transferred to a new centrifuge tube; this process was repeated three times. The extract was dried under nitrogen flow and then dissolved in 200 μL methanol/acetonitrile (1:1). Finally, the sample was filtered through double organic membranes with pore sizes of 0.35 μM.
For the extraction of 20E, add 1 mL saturated NaCl solution and 10 small steel balls per gram of S. frugiperda larvae. Grind at a frequency of 70 Hz for two minutes, then add 2 mL ethyl acetate and centrifuge at 4000 RPM. The upper supernatant was collected and transferred to a new centrifuge tube; this process was repeated three times. The extract was dried under nitrogen flow and then dissolved in 200 μL methanol/acetonitrile (1:1). Finally, the sample was filtered through double organic membranes with pore sizes of 0.35 μM.
The samples were frozen in the refrigerator at −20 °C. Finally, UPLS-MS/MS was used to detect the juvenile hormone and 20E titres of S. frugiperda at different ages. For the detection of juvenile hormone, we referred to the detection method of Yi Guoqiang [29]. The chromatographic detection of 20E was performed with a Waters XBridge HPLC C18 column (2.1 × 150 mM, 3.5 μM) at room temperature (25 °C) with a 5 μL injection volume and flow rate of 0.3 mL/min. The mass spectrometry conditions were as follows: electrospray ion source and positive ion scanning mode (ESI+); capillary voltage, 4.0 kV; air temperature, 330 °C; and atomizing gas pressure, 103.425 kPa. The retention time was 3.53 min; precursor ion, 481.3; fragmentor, 170 V; quantifying ion, 445.2; collision energy, 10 V; qualitative ion, 371.1; collision energy, 5 V.
2.6. qRT-PCR
Total RNA was extracted from S. frugiperda larvae using a Trizol (MRC, Cincinnati, OH, USA) reagent kit according to the manufacturer’s instructions. cDNA was synthesized using the Hifair® III reverse Transcription kit (Yease Biotechnology (Shanghai, China) Co., Ltd., Shanghai, China) for subsequent qPCR (real-time fluorescent quantitative PCR) experiments, qPCR was performed using a QuantStudio 6 Flex instrument (Thermo Fisher Scientific, Waltham, MA, USA) with a total reaction volume of 10 mL. Primers can be found in the attachment (Table S1).
The t-test was used to determine the significance of the difference in gene expression between the control and treatment groups (RT-qPCR). Two-tailed probability was used in the test. Multi-level parametric tests were used to determine the significance of changes in JH and 20E titres between the control and treatment groups. All analyses were performed using SPSS Statistics 25.0 software.
3. Results
3.1. JH and 20E Dynamics during S. frugiperda Development
The larval stage of S. frugiperda is characterised by the presence of three types of JHs: JH I, JH II, and JH III. In order to gain insight into the changes in the JHs and 20E in S. frugiperda, the levels of JH I, JH II, JH III, and 20E were measured during the larval, pupal, and adult stages. This investigation aimed to understand the roles of different JHs and 20E during these stages.
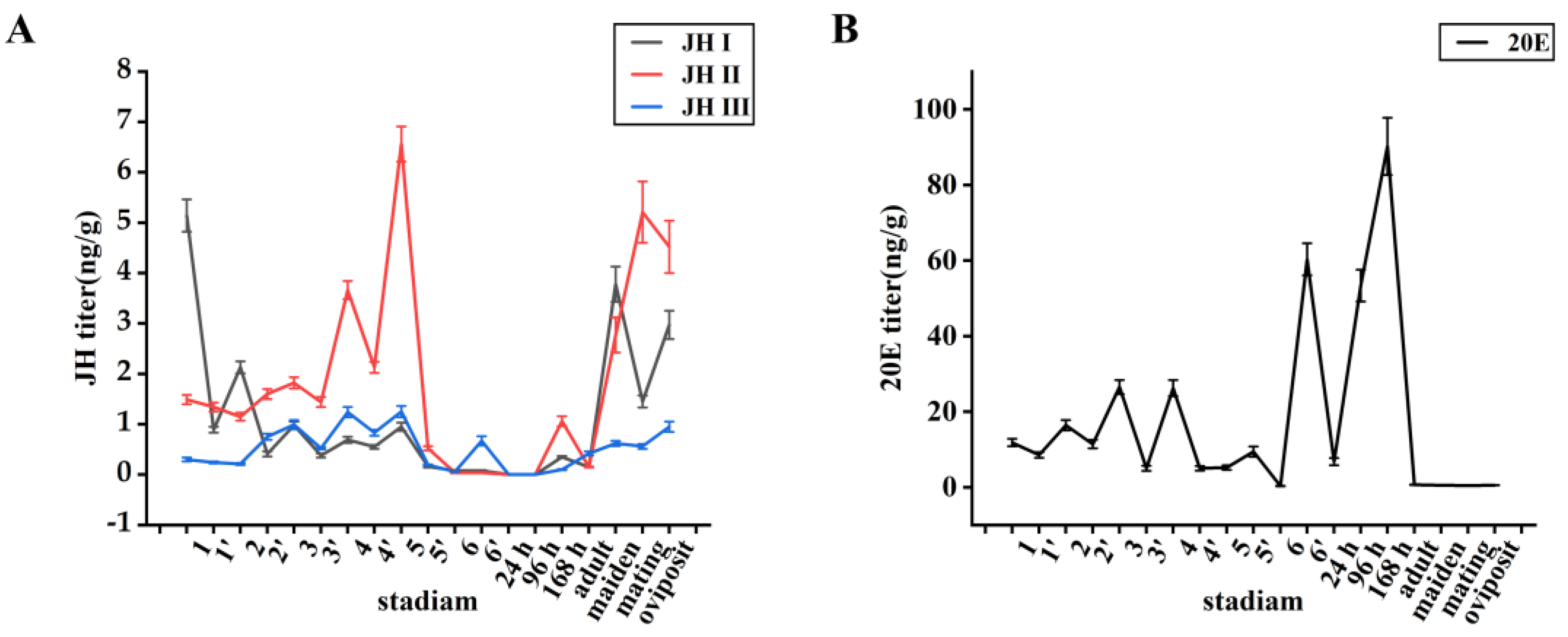
In the larval stage, the JH levels increased initially and then decreased over time, with each hormone declining at the end of each instar and subsequently increasing again in the next instar. Conversely, the 20E levels peaking at the end of each instar decreased and then peaked again at the end of the next instar. Thus, the 20E levels exhibited an initial increase, followed by a decrease, and then another increase (Figure 1A). The titre of 20E was low in the first-instar larvae but then continuously increased, reaching its maximum level in fourth-instar larvae, except at the prepupal stage, and subsequently decreased to an undetectable level in the sixth instar. The titre of 20E rose again during the prepupal stage and reached its highest peak during the larval stage (Figure 1B). In the pupae, JH was not synthesised until the end of the pupal stage, whereas the 20E levels increased throughout this stage. In the adult stage, the JH levels continuously increased, reaching a peak after mating and decreasing after egg laying, whereas 20E was not synthesised.
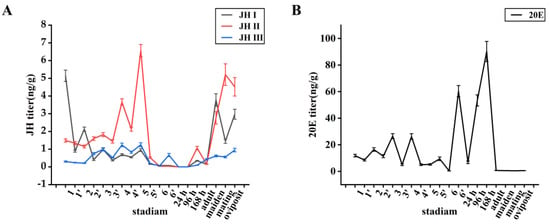
Figure 1.
(A): Trends of juvenile hormone changes in different periods.(B): Trends of 20E in different periods. Data shown in the figures are means ± standard errors. 1 represents the first instar, 1’ represents the end of the first instar, and so on. 24 h, 96 h, and 168 h represent the pupal stage at 24 h, 96 h, and 168 h; adult represents the newly emerged adult; maiden represents the unmated adult; mating represents the mated unlaid adult; oviposit represents the post-laid adult.
The JH I titre in first-instar larvae was the highest among all JHs, reaching approximately 5.14 ng/g, which was 3.5- and 17.0-fold higher than the JH II and JH III titres, respectively. The JH I titre decreased as the larvae aged and remained between 0.5 and 1.0 ng/g during the third, fourth, and fifth instars, respectively, declining to a very low level at the end of the pupal stage (<0.03 ng/g) [29]. During the adult stage, the JH I titre was initially only 0.25 ng/g but increased to 3.78 ng/g 48 h after emergence before significantly decreasing to 1.45 ng/g 24 h after mating and rising to 2.97 ng/g after egg laying (Figure 1A).
The JH II titre was 1.49 ng/g in the first instar and increased with each subsequent instar until the sixth instar stage. It reached a peak of 6.56 ng/g during the fifth instar and then declined rapidly, decreasing to a level considered undetectable (<0.02 ng/g) [29] during the sixth instar. After a slight peak at 0.67 ng/g during the prepupal stage, the JH II levels rapidly declined and remained very low. During the larval stage, JH II exhibited a significantly higher titre than JH I and JH III (approximately 3–5-fold higher). Although JH II was not detected throughout the early pupal stage, it was present at the end of this stage, reaching 1.06 ng/g. In the adult stage, the JH II titre was initially only 0.15 ng/g but significantly increased to 2.77 ng/g 48 h after emergence, further increasing to 5.21 ng/g 24 h after mating and then slightly decreased to 4.52 ng/g after egg laying (Figure 1A).
JH III exhibited the lowest content among the three JHs, with a titre of 0.3 ng/g in the first-instar larvae, which increased over time until the fifth instar, peaking at 1.25 ng/g. In the sixth instar, the JH III titre rapidly decreased to an undetectable level (<0.05 ng/g) [29] and, subsequently, remained low. From the end of the pupal stage to the adult stage, the JH III titre was <0.92 ng/g (Figure 1A).
Similar to JH II, the 20E levels increased with each instar, reached a peak of 26.3 ng/g during the third and fourth instars, decreased during the fifth instar, and declined to undetectable levels during the sixth instar. During the prepupal stage, the peak 20E content was 60.3 ng/g, which rapidly declined to an undetectable level (<0.4 ng/g) over time. Throughout the pupal stage, the 20E levels continued to increase, reaching 90 ng/g. Unlike JH, which only appeared at the end of the pupal stage, the 20E levels continued to increase and became significantly higher than those during the larval stage. However, the 20E levels significantly decreased during the adult stage, remaining low (<0.4 ng/g) (Figure 1B).
The high titres of JH II during the fourth and fifth instars suggest that JH may play a more important role in maintaining the larval state than JH I and JH III. The sudden increase in JH II and 20E levels during the prepupal stage indicates that the successful pupation of S. frugiperda larvae requires the participation of JH II and 20E. The significant increase in JH I and JH II during the adult period, with these JHs reaching their highest peak after mating and, subsequently, decreasing after egg laying, suggests that JH I and JH II are necessary for adult reproduction. Notably, JH III did not appear to be involved in regulating larval metamorphosis, pupation, and reproduction.
3.2. Effects of Starvation Stress on JH and 20E Levels
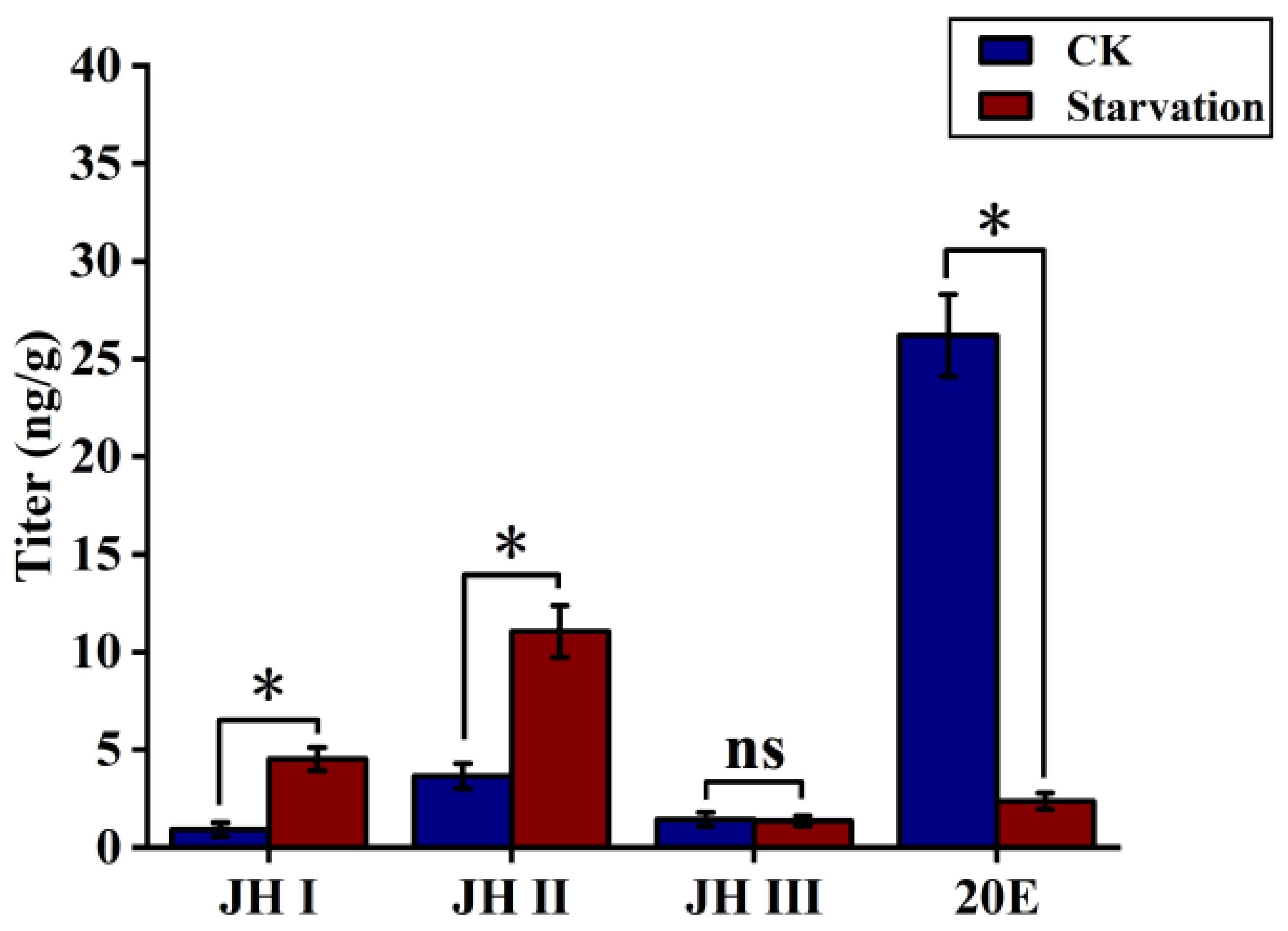
In the natural environment, S. frugiperda growth is often influenced by starvation. In Section 3.1, we examined the trends of JH and 20E levels in S. frugiperda under sufficient food conditions. Previous studies have indicated that stress, such as starvation, can alter insect JH titres and impact developmental processes. In order to clarify the effect of starvation stress on JHs and moulting hormones in S. frugiperda, we subjected fourth-instar larvae to 24 h of starvation, after which normal feeding conditions were resumed.
The results showed that the JH I and JH II levels significantly increased in fourth-instar larvae subjected to 24 h of starvation when compared with fourth-instar larvae fed a normal diet. The JH I level increased five-fold, from 0.93 to 4.53 ng/g, and the JH II level increased three-fold, from 3.66 to 11.06 ng/g. In contrast, the JH III titre remained at a low level of 1.37 ng/g and did not change significantly. However, the 20E titre decreased significantly, exhibiting an 11-fold decrease, from 26.2 to 2.37 ng/g, under starvation stress (Figure 2).
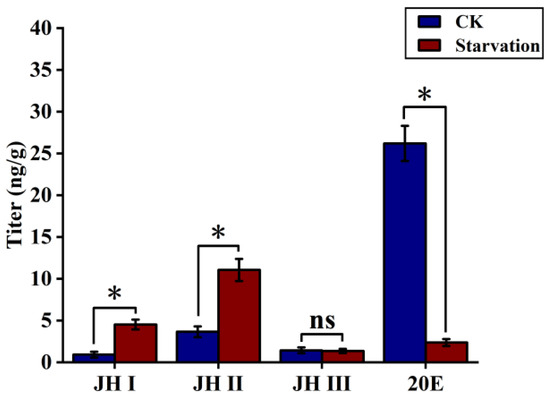
Figure 2.
The JH and 20E titres of fourth-instar larvae after 24 h of starvation and under adequate food conditions (CK). Data shown in the figures are means ± standard errors. With significantly expression differences between different treatment groups and control groups are indicated using asterisks: * p < 0.05; ns represents no significant difference (independent sample t-test).
When the fourth-instar larvae were starved for 24 h, they exhibited prolonged development and appeared as over-aged larvae. Subsequently, when the larvae reached the sixth instar, instead of pupating, they shed their head capsule and moulted into over-aged larvae, referred to as the seventh instar larvae. The seventh instar larvae were the same length as the sixth instar larvae but appeared yellowish and nearly transparent. Under normal feeding conditions, the S. frugiperda larvae took only 16.21 days to pupate, whereas larvae subjected to starvation treatment took 18.51 days to pupate, representing a delay of 2.3 days (Figure 3).

Figure 3.
(A): The end of the penultimate stage; (B): The beginning of the last stage; (C): The last stage; starvation treatment on the left and normal feeding on the right.
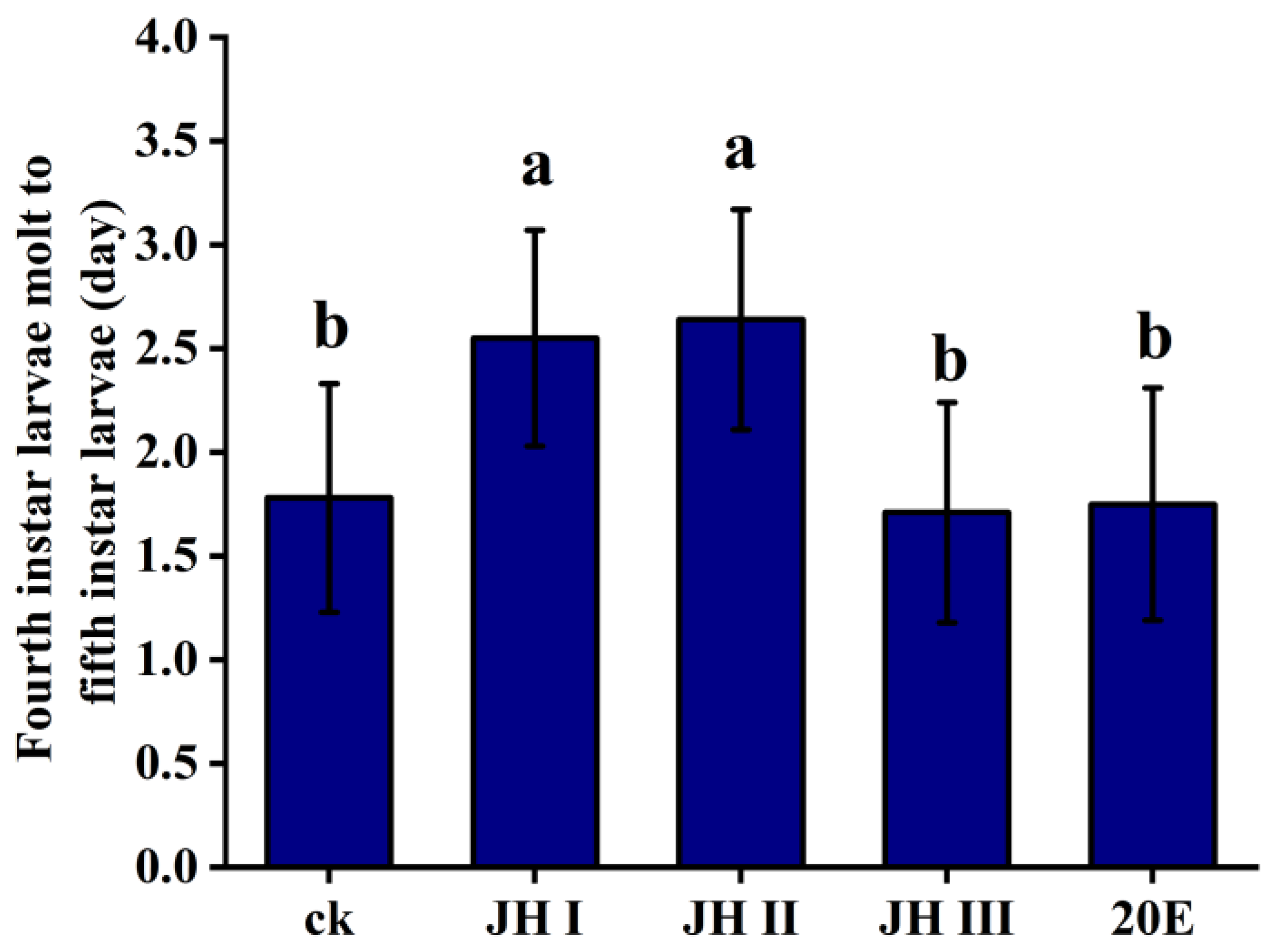
In order to further investigate the role of different JHs and 20E in development, the hormones were injected into the fourth-instar larvae of S. frugiperda, and the time taken for the next instar to occur was recorded. The injection of JH I and JH II significantly extended the time to the next instar, from 1.78 days to 2.55 days and 2.62 days, respectively. However, the injection of JH III and 20E did not affect the time taken for the next instar to occur (1.71 and 1.75 days, respectively) (Figure 4).
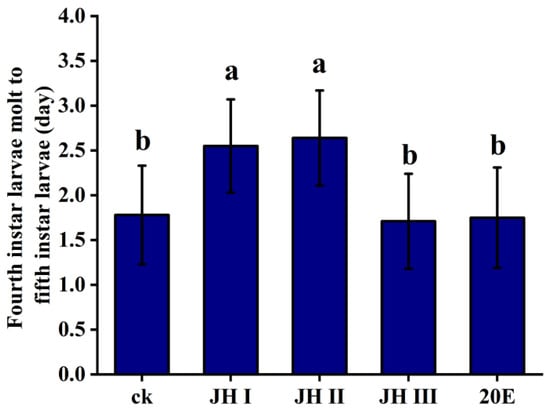
Figure 4.
Time from moulting to the next instar of the fourth-instar larvae after the injection of JH and 20E. The data shown in the figures are means ± standard errors. The averages within the columns followed by the same letter do not significantly differ according to Tukey’s multiple range test (α = 0.05).
Based on these findings, we hypothesise that JH I and JH II play critical roles in maintaining the larval state. High levels of these JHs, along with low levels of 20E in the haemolymph, result in excess larval instars. During the larval stage, JH I and JH II may be the most important hormones controlling metamorphosis and development, as they affect moulting hormone synthesis and subsequently influence the moulting process.
3.3. Interactions among Three JHs
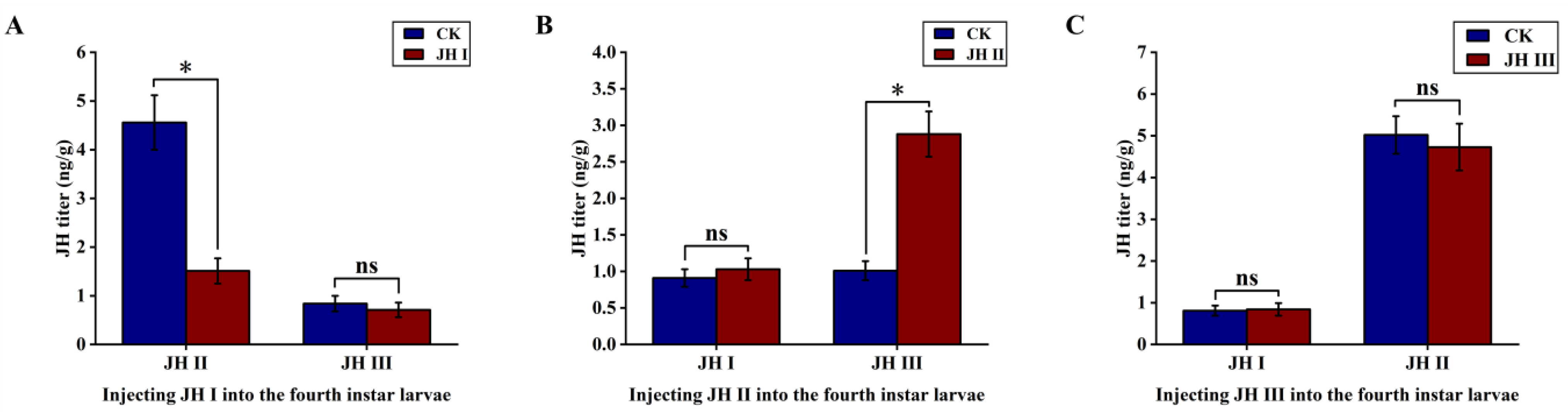
In order to understand the interactions among different JHs, we observed changes in JH I, JH II, and JH III levels in the fourth-instar larvae of S. frugiperda. We selected fourth-instar larvae with high JH levels and injected them with different JHs to investigate whether the hormones promoted or antagonised the effects on each other.
The results showed that JH I injection significantly reduced the content of JH II in the larvae, causing an approximate three-fold decrease, from 4.56 to 1.51 ng/g. However, JH I injection had no significant effect on the JH III levels, which remained within a titre range of around 0.84 ng/g. Conversely, the injection of JH II significantly increased the JH III titre, causing an approximately 2.8-fold increase from 1.01 to 2.88 ng/g. However, JH II injection did not have a significant effect on the JH I levels, which remained in a range of approximately 0.91 ng/g. The JH III injection did not significantly affect the JH I and JH II levels (Figure 5).
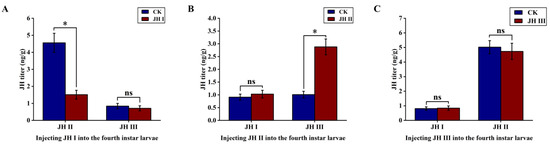
Figure 5.
(A): Effect of injection of JH I into fourth instar larvae on JH II and JH III; (B): Effect of injection of JH II into fourth instar larvae on JH I and JH II; (C): Effect of injection of JH III into fourth instar larvae on JH I and JH II. The data shown in the figures are means ± standard errors. Those with significant expression differences between the different treatment groups and control groups are indicated using asterisks: * p < 0.05; ns represents no significant difference (independent sample t-test).
We observed a negative correlation between JH I and JH II levels and a positive correlation between JH II and JH III titres in the fourth-instar larvae of S. frugiperda. Moreover, increasing the JH III titre did not cause changes in the JH I and JH II levels. As reported in Section 3.1, we found that the JH I titre gradually decreased with the age of the larvae, and when it reached a specific range, the JH II levels began to increase. Based on these observations, we hypothesise that high JH I titres in the larval stage may inhibit JH II synthesis. As the JH I titre decreased to a specific level, the antagonistic effect on JH II was weakened, leading to the promotion of JH II synthesis.
3.4. Effect of JH on 20E
Both JHs and 20E are essential for the moulting and pupation of S. frugiperda larvae. Building on the interactions reported in Section 3.3, we aimed to understand how JHs regulate the synthesis of 20E and, subsequently, affect insect development. In order to investigate their promoting or antagonistic effects on 20E, we selected fourth-instar larvae and prepupal stages with relatively high 20E levels and injected them with different JHs.
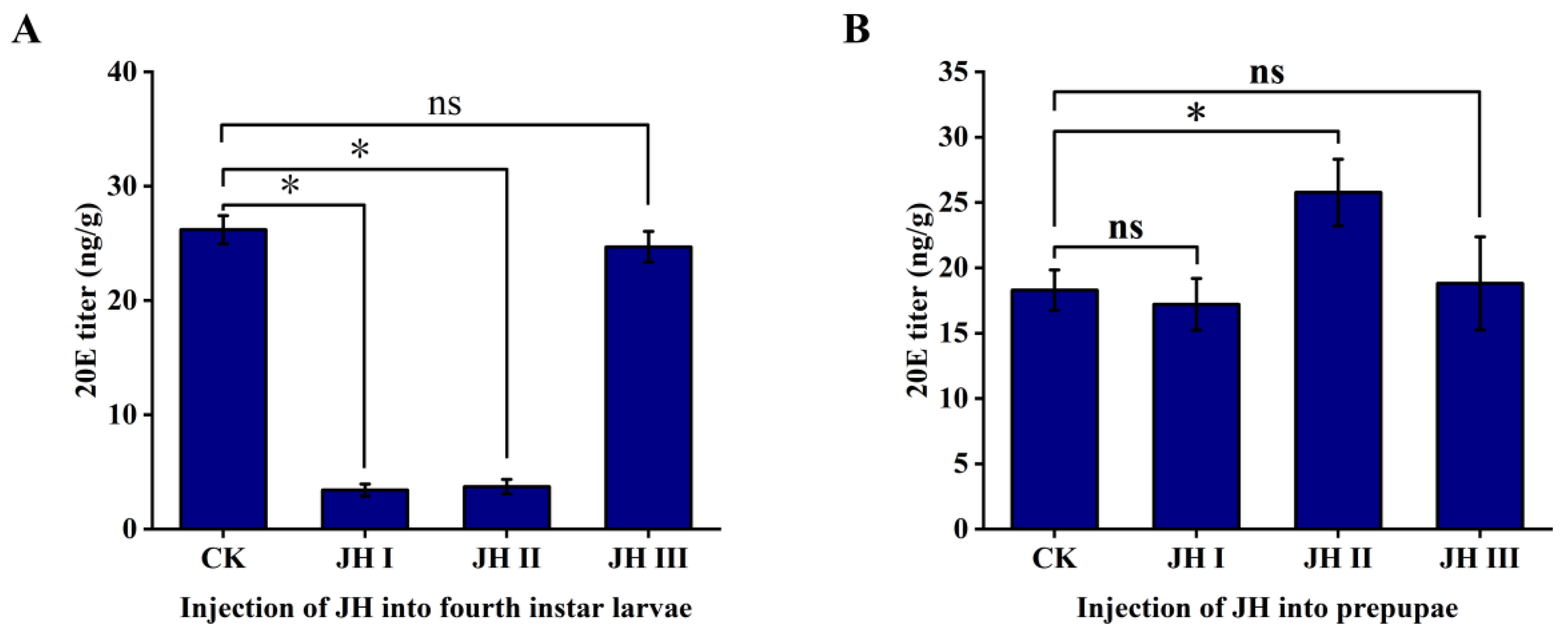
The injection of JH I and JH II in the fourth-instar larvae resulted in a significant decrease in the 20E titre. The JH I injection led to an approximate eight-fold decrease in the 20E levels from 26.2 to 3.42 ng/g. Similarly, JH II injection resulted in an approximate seven-fold decrease in 20E levels from 26.2 to 3.72 ng/g. However, the injection of JH III had no effect on the 20E titre, which remained at approximately 26.2 ng/g (Figure 6A).
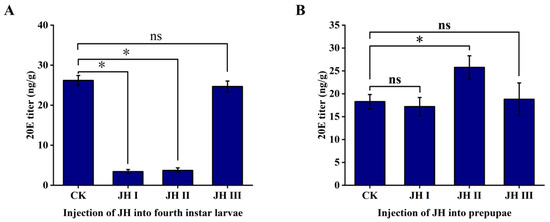
Figure 6.
(A): The effect of injection of JH into 4th instar larvae on the titer of 20E. (B): The effect of injection of JH into prepupae on the titer of 20E. The data shown in the figures are means ± standard errors. The significant expression differences between the different treatment groups and control groups are indicated using asterisks: * p < 0.05; ns represents no significant difference (independent sample t-test).
During the prepupal stage, different JHs were injected into S. frugiperda. The injection of JH II significantly increased the 20E titre in prepupae, causing the 20E level to increase from 18.29 to 25.77 ng/g. In contrast, the injection of JH I and JH III did not produce notable effects on the 20E levels.
3.5. Effects of JHs and 20E on Emergence and Ovarian Development
In Section 3.1, we reported that all three JHs were almost undetectable at the beginning of the pupal stage. However, toward the end of the pupal stage, the JH I and JH II levels increased by varying degrees. In order to explore the relationship between these JHs and emergence, we selected pupae of the same size and divided them into five groups, each containing 50 pupae. We then injected JH I, JH II, JH III (all at concentrations of 50 mg/L), and a control solvent (2 μL) into the abdomen of the pupae.
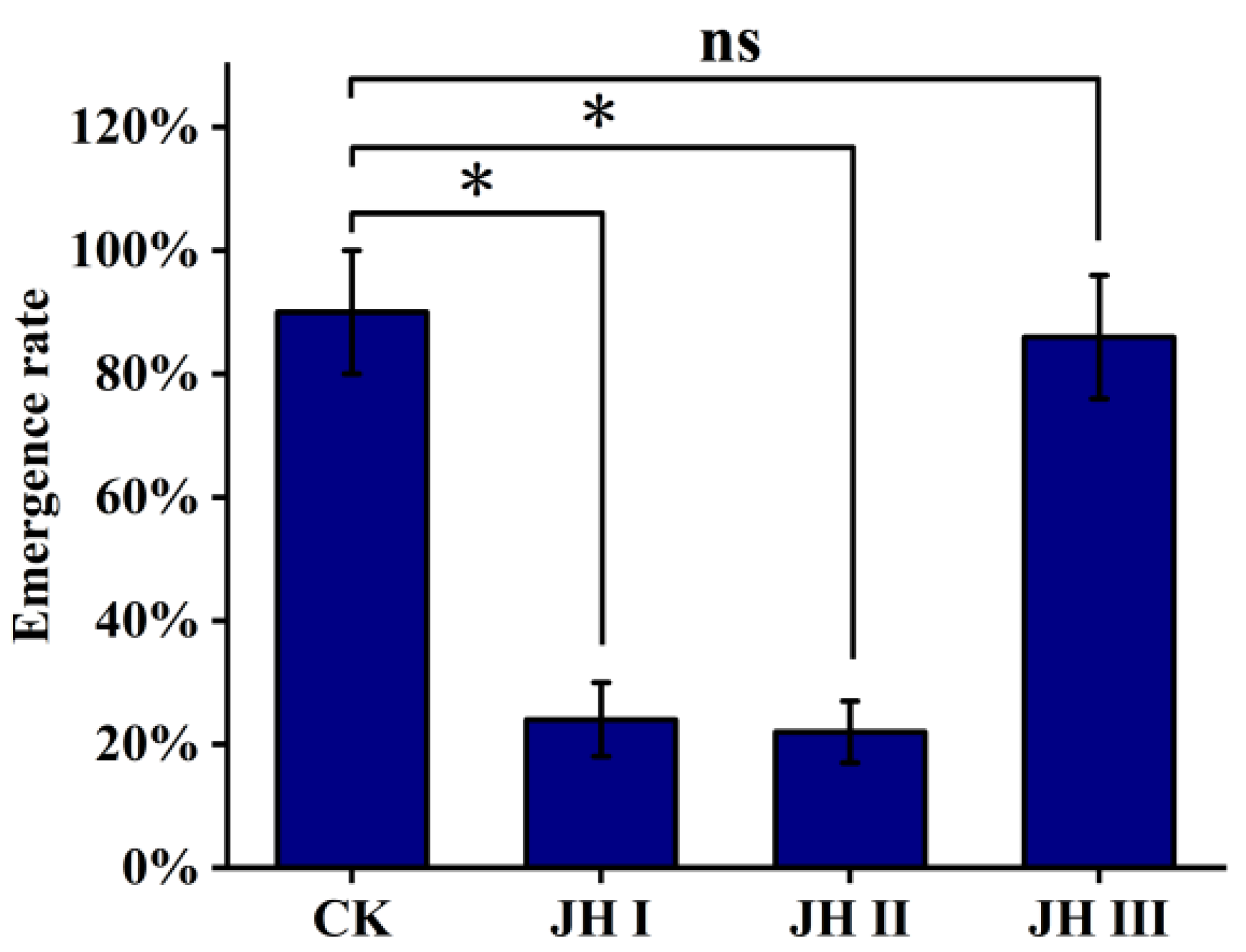
We found that the emergence rate of the pupae injected with JH I and JH II was significantly reduced (22–24%), whereas the emergence rates of pupae injected with JH III and the control group remained high (approximately 90%). Additionally, in the groups injected with JH I and JH II, the pupae emerged 2.5 days earlier than those in the JH III and control groups (Figure 7).
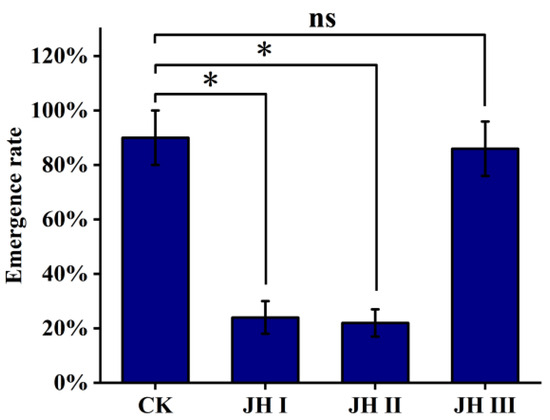
Figure 7.
The effect of pupal stage on eclosion via the injection of JH. The data shown in the figures are means ± standard errors. The significant expression differences between the different treatment groups and control groups are indicated using asterisks: * p < 0.05; ns represents no significant difference (independent sample t-test).
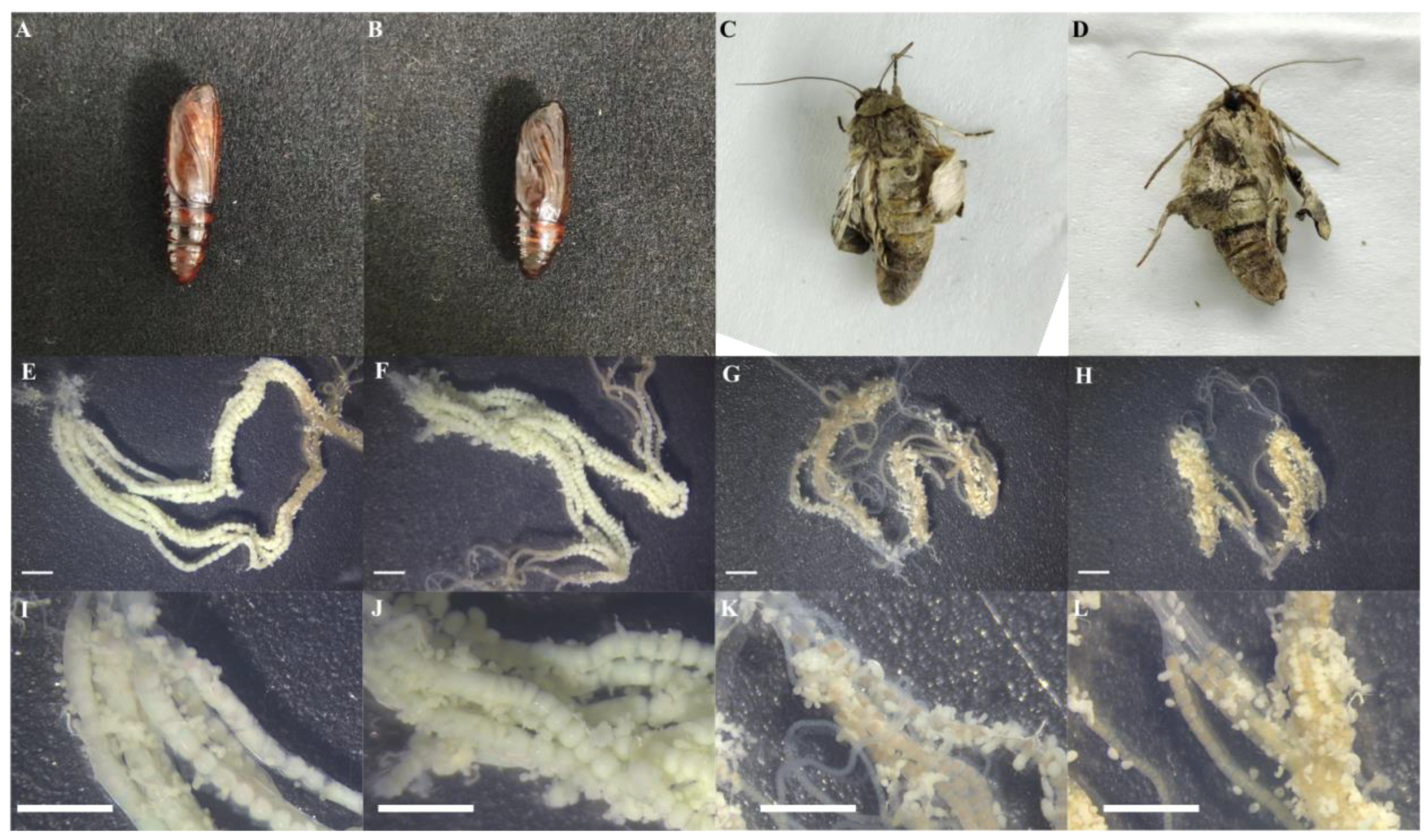
In the groups injected with JH I and JH II, some of the adult moths that failed to emerge from the pupal shell exhibited curled wings and were unable to fly normally. Their thoracopods were incompletely developed, and they were unable to walk in a straight line. The pupae that failed to complete eclosion after JH I and JH II injection became black and hardened. The dissection of these pupae revealed that JH I and JH II injection caused advanced ovarian development, with the female egg mass showing substantial growth, appearing large and green, in contrast to the dark red and smaller egg masses observed in the JH III and control groups (Figure 8).
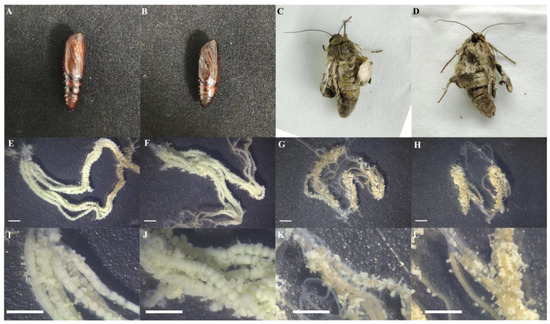
Figure 8.
(A): Solvent control group; (B): pupae with eclosion failure; (C): burrowing the back surface of a failed adult pupa shell; (D): burrowing the ventral surface of a failed adult pupa shell; (E): JH I treatment of emerging adult ovaries; (F): JH II treatment of emerging adult ovaries (G): JH III treatment of emerging adult ovaries; (H): CK treatment of emerging adult ovaries. (I–L) The local magnification of the ovaries of (E–H), respectively, with the white line segments representing 2 mM in length.
3.6. Molecular Mechanism Underlying the Effect of JH on 20E
As reported in Section 3.3, we found that JH I and JH II inhibited the synthesis of 20E, leading to the prolongation of the fourth-instar larval stage, whereas a transient rise in JH II levels was required to promote 20E synthesis during the prepupal stage. In order to further investigate the regulatory effect of JHs on 20E, we injected different JHs into fourth-instar larvae and prepupae and conducted fluorescence quantitative analysis to evaluate the molecular mechanism underlying the JH-mediated regulation of 20E.
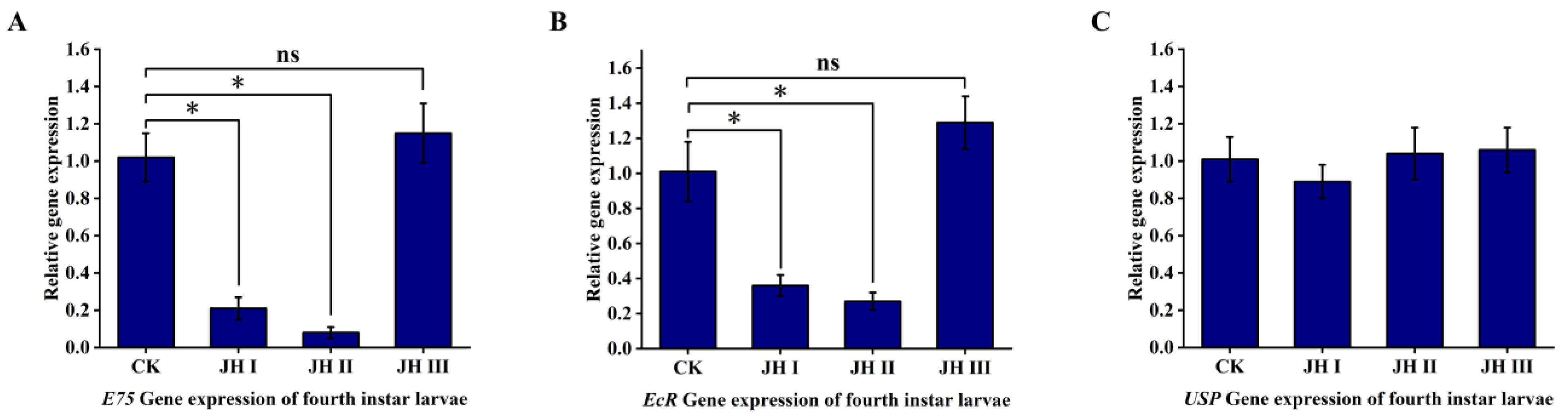
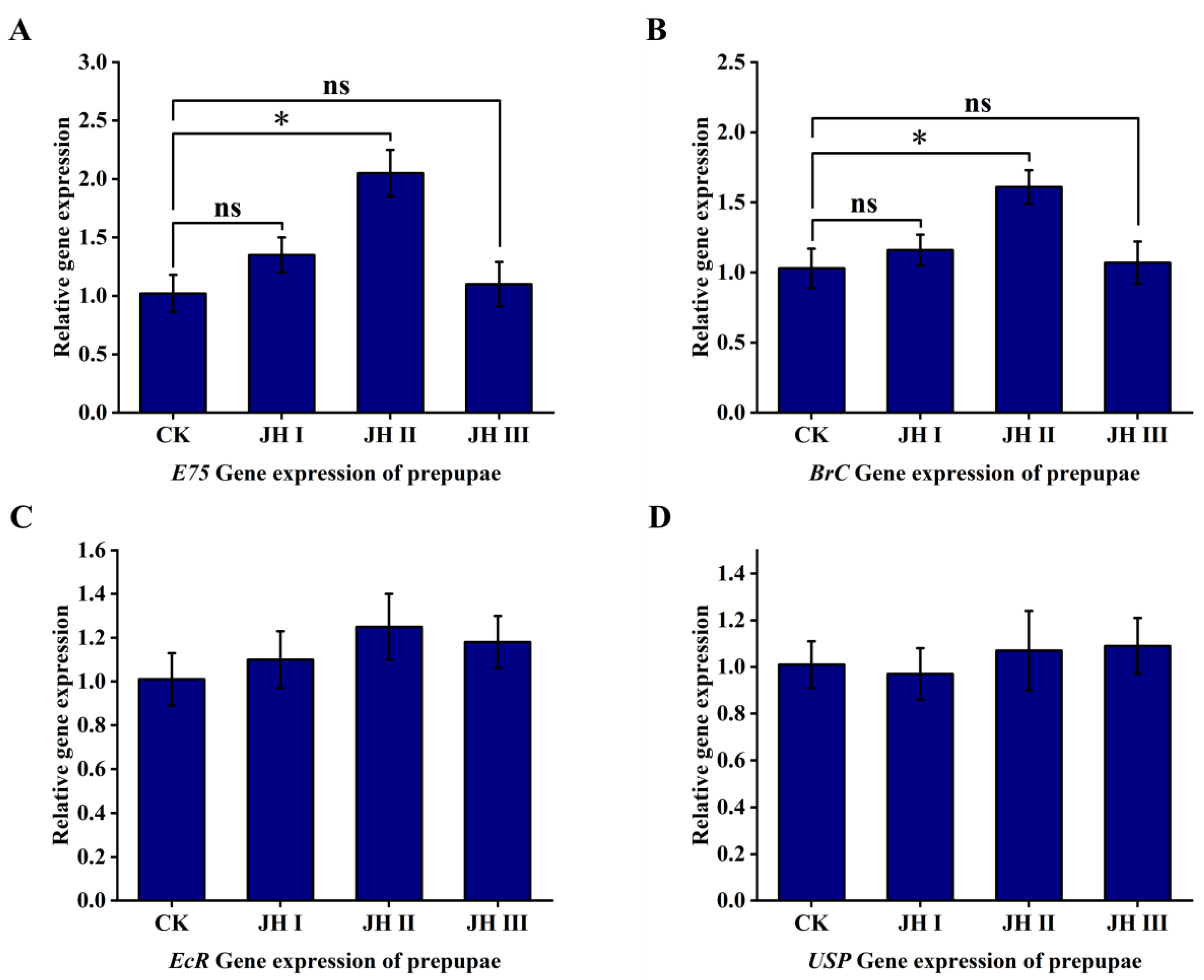
In the fourth-instar larvae, the injection of JH I and JH II resulted in the significant downregulation of the ecdysone primary response gene E75 and the receptor gene EcR. Specifically, JH I injection caused an approximate five-fold downregulation of E75 expression and an approximate three-fold downregulation of EcR expression, whereas JH II injection led to an approximate 12-fold downregulation of E75 expression and an approximate three-fold downregulation of EcR expression. However, JH III injection did not induce the downregulation of the ecdysone primary response gene E75 and the EcR receptor gene. Moreover, JH I, JH II, and JH III treatments did not induce the downregulation of the receptor gene USP (Figure 9).
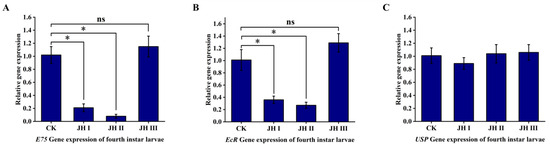
Figure 9.
(A): The effect of injection of JH into fourth instar larvae on the expression of E75 gene. (B): The effect of injection of JH into fourth instar larvae on the expression of EcR gene. (C): The effect of injection of JH into fourth instar larvae on the expression of USP gene. Data shown in the figures are means ± standard errors. With significantly expression differences between different treatment groups and control groups are indicated using asterisks: * p < 0.05; ns represents no significant difference (independent sample t-test).
At the prepupal stage, the injection of JH II resulted in the two-fold upregulation of the ecdysone primary response gene E75, whereas JH I and JH III injections did not have a significant effect on gene expression. Additionally, the injection of JH II resulted in the 1.6-fold upregulation of the ecdysone pupation-related BrC gene, whereas JH I and JH III injections did not significantly affect the expression levels. Furthermore, the injection of JH I, JH II, or JH III did not induce the upregulation of EcR and USP receptor expression (Figure 10).
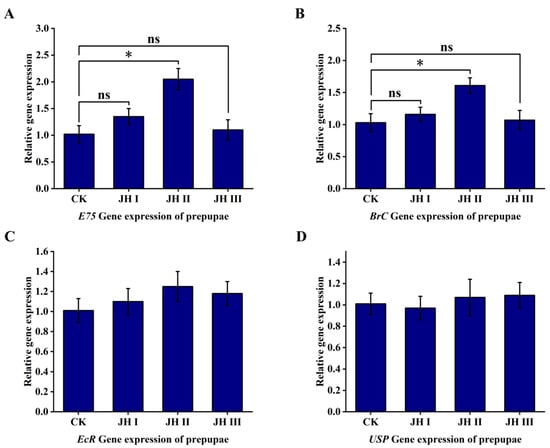
Figure 10.
(A): The effect of injection of JH into prepuae on the expression of E75 gene; (B): The effect of injection of JH into prepuae on the expression of BrC gene; (C): The effect of injection of JH into prepuae on the expression of EcR gene; (D): The effect of injection of JH into prepuae on the expression of USP gene. The data shown in the figures are means ± standard errors. The significant expression differences between the different treatment groups and control groups are indicated using asterisks: * p < 0.05; ns represents no significant difference (independent sample t-test).
4. Discussion
Most insects typically synthesise only JH III, whereas lepidopterans synthesise JH I, JH II, and JH III. However, the specific roles of these JHs in S. frugiperda are not yet well understood. In order to investigate the roles and interactions of JHs and 20E at different developmental stages, we measured the levels of JH I, JH II, JH III, and 20E using UPLC-MS/MS under both food sufficiency and starvation conditions. The results obtained from injecting fourth-instar larvae, prepupae, and pupae with these hormones revealed that high levels of JH I and JH II are crucial for maintaining the larval state and may be important for promoting adult reproduction. However, conventional JH III does not appear to have a significant effect on the metamorphosis of S. frugiperda larvae or adult reproduction.
In the fourth- and fifth-instar larvae of S. frugiperda, the JH I and JH II titres remained high. The injection of JH I and JH II during this period significantly prolonged larval development. Moreover, under starvation stress, the JH I and JH II levels increased significantly, whereas the 20E levels decreased significantly, leading to the production of over-aged larvae. Similar results have been observed in studies on Drosophila, where starvation caused low JH degradation in the haemolymph, leading to increased JH levels [30].
The injection of JH I and JH II into the fourth-instar larvae of S. frugiperda inhibited the synthesis of 20E and affected larval moulting, resulting in a prolonged larval instar development time. Similarly, the implantation of supernumerary active CA in the penultimate larval instar resulted in the formation of a supernumerary larval instar [21]. The concurrent injection of JH I and JH II led to the downregulation of the 20E primary response gene E75 and the 20E receptor gene EcR. Therefore, high titres of JH I and JH II in fourth-instar larvae play a key role in preventing metamorphosis in S. frugiperda. These titres inhibit the synthesis of 20E, maintaining it at a low level, which is crucial for maintaining the larval state.
During the prepupal stage, the reappearance of JH is required to prevent precocious metamorphosis in certain tissues, such as the eye, optic lobe, and abdominal diaphragm [31]. The primary role of JH during the prepupal stage is to activate the prothoracic glands and release 20E, which is essential for larval–pupal transformation [32,33,34]. Numerous studies have shown that a rapid increase in JH is required at the final pupation stage, as it activates the prothoracic glands and promotes 20E synthesis, thereby facilitating normal pupation. Our findings indicate that JH II levels in S. frugiperda significantly increase during the prepupal stage, and 20E levels begin to rise rapidly, peaking in the larval stage. Injecting JH II during the prepupal stage resulted in the upregulation of the ecdysone primary response gene E75 and the key pupation gene BrC, thus prompting the involvement of 20E in the following pupation. Overall, these results suggest that JH II and 20E play important roles in S. frugiperda pupation.
A critical regulatory gene during metamorphosis in holometabolous insects is BrC, which is essential for successful pupal formation [35]. Research on M. sexta has revealed that BrC is regulated by both 20E and JH [36]. In Drosophila, the application of JH at pupariation caused the re-expression of Br-C, leading to the formation of an adult with a pupal abdomen [25]. Studies using a JH analogue, Methoprene, during the prepupal stage resulted in the upregulation of BrC expression [21]. In S. frugiperda, JH II plays a critical role during the larval–pupal transition. Our findings indicate that injecting JH II during the prepupal stage in S. frugiperda induces the upregulation of BrC expression. Therefore, the reappearance of JH II, which was not detected during the larval–pupal transition in S. frugiperda, was necessary and promoted 20E synthesis. Under the combined action of JH II and 20E, S. frugiperda larvae were able to pupate successfully. In terms of pest control, JH inhibitors can be applied during this period to suppress the synthesis of JH II, thereby affecting pupation, reducing the number of adults, and ultimately decreasing the number of the first brood of larvae.
The same treatment of pupae of Spodoptera exigua with JH I and JH II also resulted in a reduction in the eclosion rate, as well as the appearance of dysmorphic adults [37]. Our results revealed that JH I and JH II were present in extremely low titres in the early pupal stage of S. frugiperda, and their increased levels during this stage resulted in a decreased emergence rate. Moths that failed to emerge displayed incomplete claws of thoracic leg development, and their wings were curled, preventing normal flight. Therefore, JH I and JH II can disrupt the expression of the pupal stage programme in S. frugiperda, shortening the pupal stage and developmental time, affecting organ differentiation, and ultimately resulting in unsuccessful emergence.
JH is known to stimulate vitellogenesis and egg development in a variety of insect species [38]. JH is the principal gonadotropic hormone controlling female reproduction in hemimetabolous insects [11,39,40,41]. In adult insects, JH requires Met/Tai to achieve its previtellogenic and vitellogenic effects on fat body competency and Vg synthesis [42,43]. JH promotes PKC-triggered VgR phosphorylation for insect vitellogenesis [44]. In Drosophila, JH stimulates the synthesis of 20E and vitellogenin in the ovaries [45]. The increase in JH levels during the early adult stage of S. frugiperda can cause premature ovarian development [27]. Male insects begin to synthesise and accumulate JH during their pupal stage. After emergence, JH in the gonads is transferred to female insects to promote ovarian development and maturation. Our findings showed that JH I and JH II levels increased rapidly during the final pupal stage of S. frugiperda. Early elevation of JH I and JH II titres during the pupal stage resulted in ovarian development and the rapid growth of egg mass. These results suggest that the presence of JH I and JH II is required only during eclosion and the later reproductive stages, and that the premature elevation of JH I and JH II levels activates the corresponding pathway prematurely, leading to failed eclosion and premature ovarian development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13082177/s1, Table S1: Primer information.
Author Contributions
Writing-review and editing, X.L.; writing-original draft preparation, S.L.; Methodology, W.Z. and G.Y.; Supervision, J.H.; Software, H.L.; Funding acquisition and Investigation, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Key Research and Development Program of Guangxi (Guike AA17204043).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the privacy of the organization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, P.; Zhang, Y.; Liu, T.; Jing, X.; Zhang, S. The Population Growth of Spodoptera frugiperda on Six Cash Crop Species and Implications for Its Occurrence and Damage Potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-X.; Hu, C.-X.; Jia, H.-R.; Wu, Q.-l.; Shen, X.-J.; Zhao, S.-Y.; Jiang, Y.-Y.; Wu, K.-M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Xie, M.; Li, Y.; Yang, J.; Zhang, M.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar]
- Qin, Y.; Yang, D.; Kang, D.; Zhao, Z.; Zhao, Z.; Yang, P.; Li, Z. Potential economic loss assessment of maize industry caused by fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 69–73. [Google Scholar]
- Wan, J.; Huang, C.; Li, C.-y.; Zhou, H.-x.; Ren, Y.-l.; Li, Z.-y.; Xing, L.-s.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.d.; Peterson, J.A.; Hunt, T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Jindra, M. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190064. [Google Scholar] [CrossRef]
- Santos, C.G.; Humann, F.C.; Hartfelder, K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W. The Evolution of Insect Metamorphosis. Curr. Biol. 2019, 29, R1252–R1268. [Google Scholar] [CrossRef] [PubMed]
- Schooley, D.; Judy, K.; Bergot, B.; Hall, M.; Jennings, R. Determination of the physiological levels of juvenile hormones. In The Juvenile Hormones; Springer: Boston, MA, USA, 1976; pp. 101–117. [Google Scholar]
- Trautmann, K.; Suchý, M.; Masner, P.; Wipf, H.-K.; Schuler, A. Isolation and Identification of Juvenile Hormones by Means of a Radioactive Isotope Dilution Method: Evidence for JH III in Eight Species from Four Orders. In The Juvenile Hormones; Springer: Boston, MA, USA, 1976; pp. 118–130. [Google Scholar]
- Schooley, D.; Baker, F.; Tsai, L.; Miller, C.; Jamieson, G. Juvenile hormones O, I, and II exist only in Lepidoptera. In Biosynthesis, Metabolism and Mode of Action of Invertebrate Hormones; Springer: Berlin/Heidelberg, Germany, 1984; pp. 373–383. [Google Scholar]
- Lanzrein, B.; Hashimoto, M.; Parmakovich, V.; Nakanishi, K.; Wilhelm, R.; Lüscher, M. Identification and quantification of juvenile hormones from different developmental stages of the cockroach Nauphoeta cinerea. Life Sci. 1975, 16, 1271–1284. [Google Scholar] [CrossRef]
- Hagenguth, H.; Rembold, H. Identification of juvenile hormone 3 as the only JH homolog in all developmental stages of the honey bee. Z. Naturforschung C 1978, 33, 847–850. [Google Scholar] [CrossRef]
- Westerlund, S.A.; Hoffmann, K.H. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography?mass spectrometry (LC-MS). Anal. Bioanal. Chem. 2004, 379, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Dahm, K.; Bhaskaran, G.; Peter, M.; Shirk, P.; Seshan, K.; Röller, H. On the identity of the juvenile hormone in insects. In The Juvenile Hormones; Springer: Boston, MA, USA, 1976; pp. 19–47. [Google Scholar]
- Li, K.; Jia, Q.Q.; Li, S. Juvenile hormone signaling—A mini review. Insect Sci. 2018, 26, 600–606. [Google Scholar] [CrossRef]
- De Loof, A.; Schoofs, L. From One Site of Insect Juvenile Hormone Synthesis, No Identified Receptors, and a Denomination as “Status Quo Hormone” in the 1960s to Multiple, Sometimes Conflicting, Possibilities to Date. Life Excit. Biol. 2020, 8, 196–238. [Google Scholar]
- Gao, Y.; Liu, S.; Jia, Q.; Wu, L.; Yuan, D.; Li, E.Y.; Feng, Q.; Wang, G.; Palli, S.R.; Wang, J.; et al. Juvenile hormone membrane signaling phosphorylates USP and thus potentiates 20-hydroxyecdysone action in Drosophila. Sci. Bull. 2022, 67, 186–197. [Google Scholar] [CrossRef]
- Smykal, V.; Daimon, T.; Kayukawa, T.; Takaki, K.; Shinoda, T.; Jindra, M. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 2014, 390, 221–230. [Google Scholar] [CrossRef]
- Whisenton, L.; Bowen, M.; Granger, N.; Gilbert, L.; Bollenbacher, W. Brain-mediated 20-hydroxyecdysone regulation of juvenile hormone synthesis by the corpora allata of the tobacco hornworm, Manduca sexta. Gen. Comp. Endocrinol. 1985, 58, 311–318. [Google Scholar] [CrossRef]
- Zhou, X.; Riddiford, L.M. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development 2002, 129, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Non-genomic action of juvenile hormone modulates the synthesis of 20-hydroxyecdysone in Drosophila. Sci. Bull. 2022, 67, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, L.; Jiang, X. Starvation influences allatotropin gene expression and juvenile hormone titer in the female adult oriental armyworm, Mythimna separata. Arch. Insect Biochem. Physiol. 2008, 68, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Yao, L.; Peng, J.; Chen, Z.; Yang, F.; Chen, S.; Tang, Q. Comparative transcriptome analysis reveals key candidate genes mediating ovarian development in Spodoptera frugiperda fed on two host plants. Front. Physiol. 2022, 13, 1056540. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Ba, R.; Luo, J.; Zou, L.; Huang, M.; Li, Y.; Li, H.; Li, X. Simultaneous Detection and Distribution of Five Juvenile Hormones in 58 Insect Species and the Absolute Configuration in 32 Insect Species. J. Agric. Food Chem. 2023, 71, 7878–7890. [Google Scholar] [CrossRef]
- Nur Aliah, N.A.; Ab-Rahim, S.; Moore, H.E.; Heo, C.C. Juvenile hormone: Production, regulation, current application in vector control and its future applications. Trop. Biomed. 2021, 38, 254–264. [Google Scholar] [CrossRef]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef]
- Cymborowski, B.; Stolarz, G. The role of juvenile hormone during larval-pupal transformation of Spodoptera littoralis: Switchover in the sensitivity of the prothoracic gland to juvenile hormone. J. Insect Physiol. 1979, 25, 939–942. [Google Scholar] [CrossRef]
- Safranek, L.; Cymborowski, B.; Williams, C.M. Effects of juvenile hormone on ecdysone-dependent development in the tobacco hornworm, Manduca sexta. Biol. Bull. 1980, 158, 248–256. [Google Scholar] [CrossRef]
- Ohtaki, T.; Yamanaka, F.; Sakurai, S. Differential timing of pupal commitment in various tissues of the silkworm, Bombyx mori. J. Insect Physiol. 1986, 32, 635–642. [Google Scholar] [CrossRef]
- Konopova, B.; Jindra, M. Broad-Complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 2008, 135, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Hiruma, K.; Shinoda, T.; Riddiford, L.M. Juvenile hormone prevents ecdysteroid-induced expression of broad complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev. Biol. 1998, 203, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, D.; Lee, J. Disturbance of adult eclosion by fenoxycarb, a juvenile hormone mimic, in the beet armyworm, Spodoptera exigua. J. Asia Pac. Entomol. 2000, 3, 103–111. [Google Scholar] [CrossRef]
- Roy, A.; Palli, S.R. Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action. BMC Genom. 2018, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of endocrine system in the regulation of female insect reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2021, 8, 593613. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Song, J.; Kang, L.; Zhou, S. An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem. Mol. Biol. 2017, 82, 31–40. [Google Scholar] [CrossRef]
- Gujar, H.; Palli, S.R. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 35546. [Google Scholar] [CrossRef]
- Jing, Y.-P.; Wen, X.; Li, L.; Zhang, S.; Zhang, C.; Zhou, S. The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. USA 2021, 118, e2106908118. [Google Scholar] [CrossRef]
- Bownes, M. The roles of juvenile hormone, ecdysone and the ovary in the control of Drosophila vitellogenesis. J. Insect Physiol. 1989, 35, 409–413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).