Abstract
Legumes have important nutritional and economic values, but their production faces continuous cropping obstacles that seriously affect their yield formation. In order to reduce the negative impact of the continuous cropping obstacles of legumes, it is necessary to understand the response mechanisms of legumes to continuous cropping, the causes of continuous cropping obstacles and the measures to alleviate continuous cropping obstacles. This review aimed to identify the current knowledge gap in the field of continuous cropping obstacles of legumes and provide direction and focus for future research. The continuous cropping obstacles of legumes start with soil degradation, leading to oxidative stress in the plants. This triggers the expression of plant-hormone- and signal-molecule-related genes, activating the defense system and causing continuous cropping obstacles. Although there has been progress in researching these challenges in legume crops, many questions remain. We believe that the exploration of molecular mechanisms of legume crops responding to continuous cropping, rhizosphere signal exchange and soil environment repair mechanisms after long-term continuous cropping of soybean, and the excavation of candidate genes and functional loci related to continuous cropping obstacles in legume crops are breakthroughs for proposing effective continuous cropping obstacle management strategies in the future.
1. Introduction
Legumes are no less important than major cereals as an essential source of protein and multiple nutrients in the human diet [1,2,3]. However, despite the large variety of legumes, only about 20 legumes meet the criteria of high protein, high fiber and low fat for human consumption [1,4]. As the population grows, the demand for protein increases, but the production of legume crops is affected by low yields, leading to increased challenges in their production [5]. Therefore, it is important to identify the possible causes affecting the yield of legume crops in order to increase their production potential at a later stage.
China is one of the major producers of legume crops [1], but due to the limited area of arable land and the reduction in arable land area, the phenomenon of continuous cropping is common in the production of legume crops [6]. Continuous cropping is defined as growing the same crop on the same plot for two or more consecutive years [7]. For example, Heilongjiang Province in China is a major soybean (Glycine max L.) production area, where the soybean continuous cropping area accounts for more than 40% of the province, and in the northern soybean production areas, this proportion is as high as 70–80% [8]. Similarly, for peanut in China’s main production areas, there is also a widespread phenomenon of continuous cropping [9], where some places even reached 10–20 years of continuous cropping [10]. However, legume crops are sensitive to continuous cropping and are prone to incur continuous cropping obstacles, which seriously affect their yield formation [8,10,11,12]. Therefore, an in-depth understanding of and solutions to the problem of continuous cropping obstacles in legume crops are not only important for guaranteeing global food security but also key to promoting sustainable agricultural development.
Continuous cropping obstacles are the result of the modern intensive and large-scale agricultural production mode, and it is also an inevitable practical problem in agricultural production [6,13]. According to statistics from Ma et al., the number of research findings related to continuous cropping has shown an increasing trend from 2010 to 2021 [13]. This means that the problem of crop yield reduction caused by continuous cropping obstacles is being taken seriously. At present, research on continuous cropping obstacles mainly focuses on medicinal plants [14,15], melon (Cucumis melo L.) [16] and facility vegetables [17], but there are relatively few comprehensive studies and reviews on the continuous cropping obstacles of legumes. This situation highlights the urgency and importance of more in-depth research in the field of the continuous cropping obstacles of legumes. Therefore, we used Google Scholar, PubMed and other academic resources to collect relevant research results on the continuous cropping obstacles of legumes, and conducted a systematic synthesis and analysis. It is shown that the soil environment deteriorates after continuous cropping, resulting in both biotic and abiotic stresses to legumes. These stresses trigger the response mechanisms of legumes at the physiological, biochemical and molecular levels. With the deepening of research, the measures used to alleviate the continuous cropping obstacles of legumes are more and more in line with the development of sustainable agriculture. In addition, we also discuss the shortcomings in the current research and future research directions. These contents will not only help us to understand the complex mechanisms of continuous cropping obstacles but also promote the development of more effective agricultural management measures, thereby enhancing the production potential of legumes.
2. Effects of Continuous Cropping Obstacles on the Growth of Legume Crops
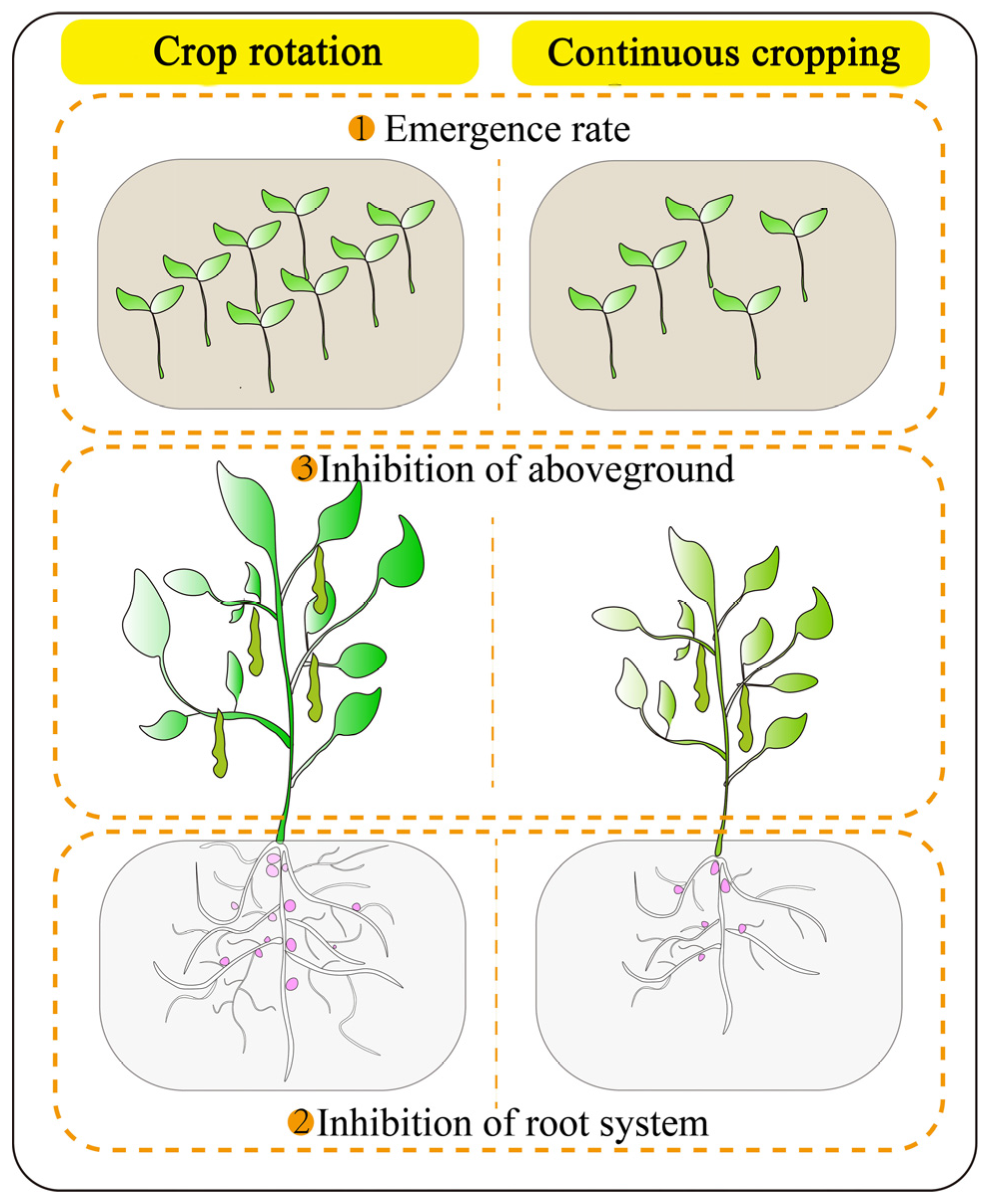
There are differences in the response of legume crops to continuous cropping obstacles, which are influenced by crop type, genotype and number of years of continuous cropping, but yield reduction is a common result of continuous cropping in most legume crops. The occurrence of this hazard form is mainly related to changes in the seedling emergence rate, plant root system and aboveground growth capacity (Figure 1) [18]. Studies showed that continuous cropping reduces the germination and emergence of legumes [19,20,21], indicating that legume crops are sensitive to a severe continuous cropping environment during the germination period, and the reduced emergence inevitably leads to reduced crop yield.
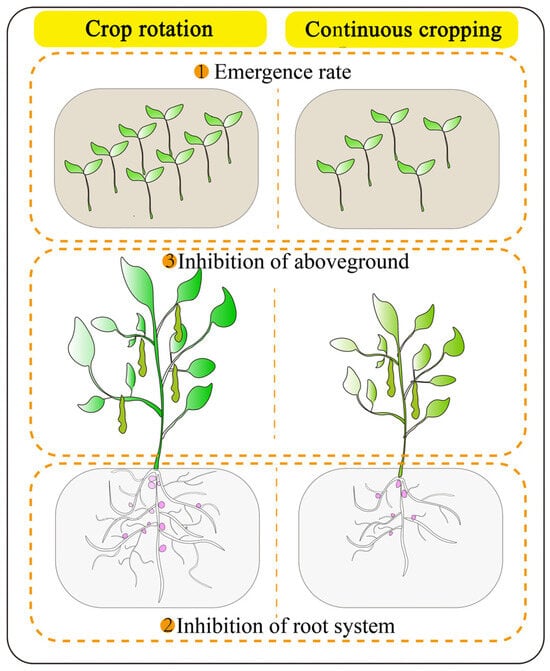
Figure 1.
Effect of continuous cropping on the growth of legume crops.
Crop roots are in direct contact with the soil and can exchange signals and interact with soil microorganisms and other crops, as well as provide nutrients and water for their growth. Crop root systems have a high degree of plasticity in their growth and development, allowing them to adapt to changes in the surrounding environment. Root elongation of legume crops is hindered in continuous cropping systems, resulting in reduced root activity and biomass [22,23]. This undoubtedly limits the root distribution in the soil and the uptake of nutrients and water from the surrounding environment. As a unique organ of legume roots, the number and dry weight of nodules decrease significantly after continuous cropping [24,25]. The decrease in nodule number affects the nitrogen fixation capacity of legumes, which, in turn, negatively affects legume growth [26,27].
The poor development of crop roots inevitably affects the growth of aboveground parts, and changes in plant height and biomass can directly reflect the degree of stress on crops under adverse conditions. The continuous cropping system has a negative effect on the plant height and biomass of legume crops [28,29]. This indicates that root growth is impeded, resulting in limited nutrient and water uptake, leading to nutrient and water stress, which triggers a series of physiological and biochemical responses, resulting in growth retardation and biomass reduction. Continuous cropping obstacles also affect the ability of legumes to flower, pod and fruit, ultimately reducing the yield. For example, a study on peanuts (Arachis hypogaea) found that continuous cropping for 2 years significantly reduces the main stem height, lateral branch length, 100-grain weight and kernel yield of peanuts, ultimately leading to a significant 9.74% reduction in the yield [18]. After more than 5 years of continuous cropping, the main stem height, lateral branch length, number of green leaves, number of pods per plant, 100-grain weight and kernel yield of peanuts are significantly lower than those of continuous cropping for 2 years, and the yield is significantly reduced by 14.26% compared with a rotation crop [18]. In the continuous cropping system, the lack of soil nutrients, the decrease in enzyme activity, the increase in pathogenic bacteria and other factors lead to the deterioration of the soil environment, which causes legume crops to grow under adversity stress and inhibits their growth. The above research results show the inhibitory effect of continuous cropping on the growth and yield formation of legume crops.
3. Mechanisms of Legume Crops Responding to Continuous Cropping Obstacles
Legume crop growth and development are affected to varying degrees in continuous cropping systems. Improving the productivity of legume crops under continuous cropping conditions requires an understanding of their response mechanisms to continuous cropping.
3.1. Responses of the Physiological and Biochemical Levels of Legume Crops Caused by Continuous Cropping Obstacles
3.1.1. Oxidative Stress Induced by Reactive Oxygen Species (ROS)
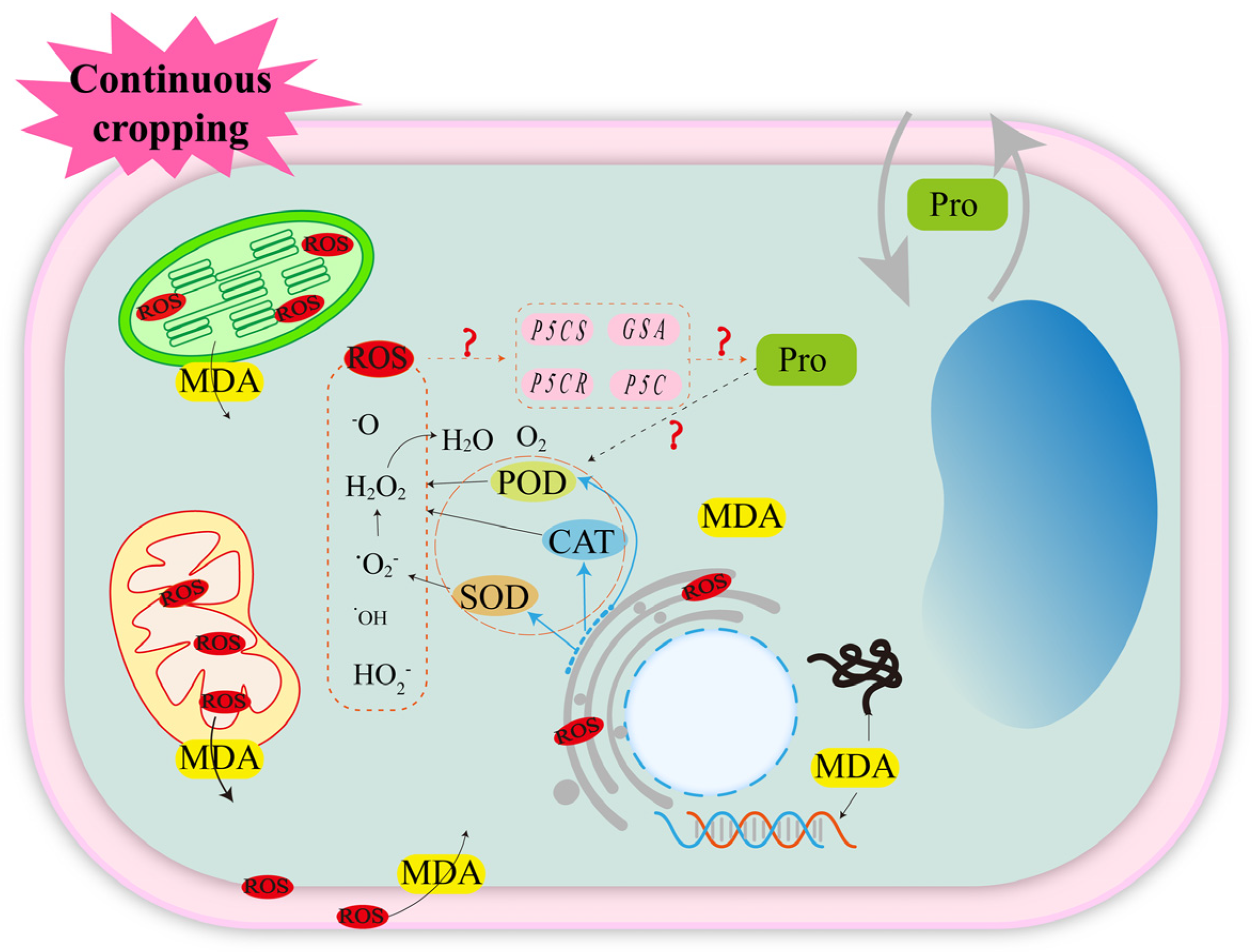
Adversity tends to disrupt the dynamic equilibrium and ion distribution in plant cells, thereby affecting normal plant growth [30,31,32,33]. Complex biotic and abiotic stresses in continuous cropping systems cause changes in the activities of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), in legume crops [34,35,36]. However, due to the influence of research methods, crop types, genotypes and other factors, there is no consistent conclusion on the changes in antioxidant enzyme activities in the continuous cropping system of legume crops. However, antioxidant enzymes are the major antioxidants in plants and the first line of defense against free radicals [37,38,39]. In plants, ROS, such as superoxide radical (-O2−), hydroxyl radical (-OH), perhydroxyl radical (HO2−) and hydrogen peroxide (H2O2), are present [31,40,41]. The antioxidant enzyme SOD catalyzes the generation of H2O2 and O2 from -O2−, and CAT and POD catalyze the generation of 2H2O and O2 from H2O2 by synergizing with SOD [31,32,42]. This indirectly suggests that continuous cropping induces ROS accumulation in legume crops. The production and accumulation of ROS in the plant body can severely damage the organelles and cause membrane peroxidation [41,43,44]. The cell membrane is an important barrier for the exchange of matter and energy between the cell and the external environment. Legume crops protect the cell membrane structure by modulating antioxidant enzyme activities and scavenging ROS in vivo, but only against mild stresses caused by continuous cropping. When the continuous cropping system causes severe stress to legume crops, the ROS scavenging mechanism is abnormal, and the accumulation of large amounts of ROS leads to the collapse of the plant defense system such that the phospholipids and membrane receptor proteins on the cell membrane undergo lipid peroxidation reaction with ROS and generate the lipid peroxidation product malondialdehyde (MDA), which destroys the integrity, fluidity, and selective permeability of the membrane system and affects the normal physiological function of cells [45,46] (Figure 2).
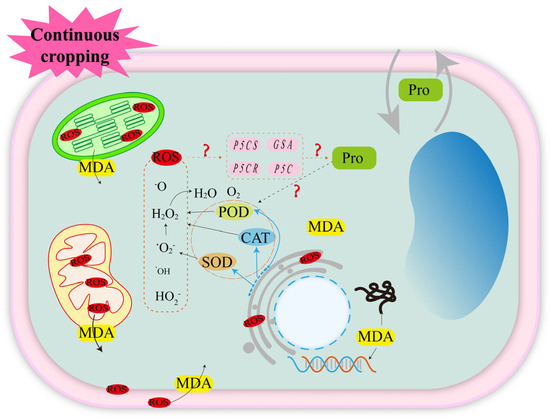
Figure 2.
Continuous cropping causes oxidative stress response in legume crops. The solid lines are confirmed by research; the dashed lines are potential mechanisms that require further study. ? indicates that it has not been proven in continuous cropping abstacle of leguminous crops, but there may be an association, and further research is needed in the future.
The osmotic defense mechanism is an important strategy for plants to initiate a response to abiotic stress. Plants synthesize a large number of osmoregulatory substances through the regulation of in vivo metabolism, and these substances maintain cell expansion pressure by regulating cellular water potential [47]. In a continuous cropping system, the accumulation of proline [47] in legume crops is beneficial to improving the osmotic adjustment ability of cells [34,35]. In addition, ROS were reported to be key signaling substances that induce the expression of relevant genes in the proline synthesis pathway (P5Cs, P5C, P5CR, GSA, etc.) and enable their accumulation under adversity stress [48,49]. In addition to osmotic regulation, proline accumulation can also reduce lipid oxidation by protecting the redox potential of cells and scavenging free radicals [50]. On the other hand, proline can indirectly scavenge ROS by enhancing the oxidative defense system [51]. For example, under salt stress, the exogenous addition of proline increases SOD and CAT activities in mung bean (Vigna radiata L.) [52] and soybean (Glycine max L.) [53], and increases the mRNA levels of CAT-related genes in tobacco (Nicotiana tabacum L.) [54]. These results suggest that proline has a variety of biological functions to cause plants to respond positively to abiotic stress (Figure 2). However, in the continuous cropping obstacle of legume crops, only the increase in proline content was detected, but its synthesis mechanism and action mechanism have not been reported in the study of continuous cropping obstacles. Studying the changes in proline content in legume crops in continuous cropping systems has a certain guiding significance for revealing its occurrence mechanism.
3.1.2. Changes in the Capacity of Photosynthesis
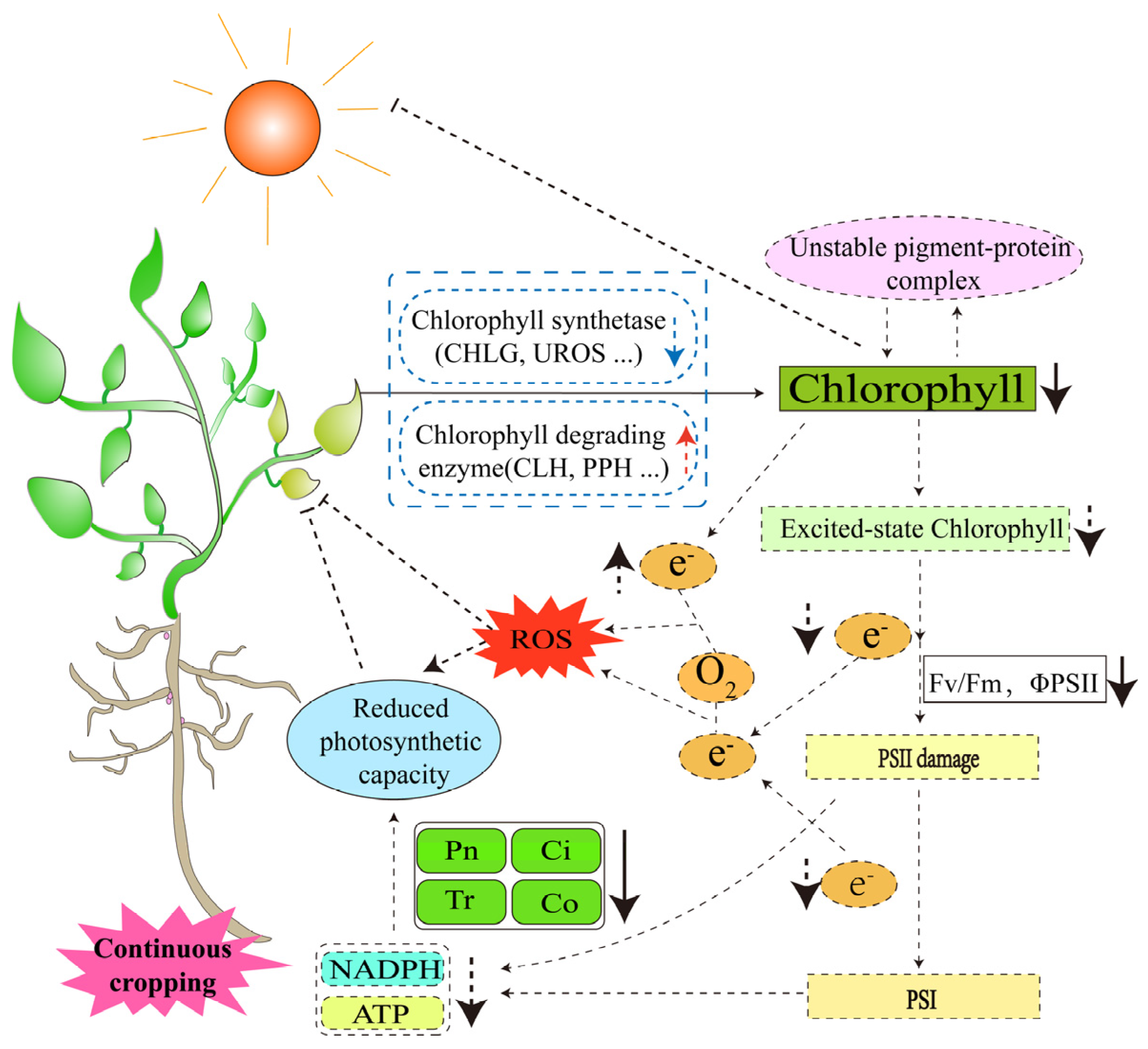
Photosynthesis is a major determinant of crop productivity, and chloroplasts, which are the sites of its response, are highly sensitive to stressful environments and influence the photosynthetic process in plants by affecting the cellular ultrastructure, photosynthetic pigment content and light absorption efficiency [55,56,57,58,59,60]. The chlorophyll contents of soybean, peanut and pea (Pisum sativum L.) leaves were reduced in the continuous cropping system [9,28,61], which may have been due to the blocked expression of chlorophyll synthase genes (CHLG, UROS, etc.) [62,63] or high expression of chlorophyll-degrading enzyme genes (CLH, PPH, etc.) [64,65,66] as a result of continuous cropping. Chlorophyll is the main photosynthetic pigment, and its decreased content causes the instability of pigment–protein complexes, which accelerates the degradation of photosynthetic pigments and affects the light absorption capacity and electron transport capacity [67,68]. In the study of simulating pea continuous cropping obstacles with autotoxic substances, it was found that the maximum photochemical electron efficiency (FV/FM) and the actual photochemical electron efficiency (ΦPSII) of the PSII of pea seedlings were significantly reduced [69]. This suggests that continuous cropping reduces the ability of photosynthetic pigment molecules in legume crops to absorb light energy, reduces the rate of light energy transfer and electron transfer, and thus, inhibits photosynthesis. By determining the photosynthetic rate of soybean and peanut leaves in the continuous cropping system [18,70], as well as the net photosynthetic rate, stomatal conductance, intercellular carbon dioxide concentration and transpiration rate of mature pea [28], it was found that these indices were significantly reduced. This further suggests that continuous cropping may reduce the chlorophyll content, resulting in limited excited-state chlorophyll molecules. Excited-state chlorophyll molecules are electron donors that transfer high-energy electrons to nearby electron acceptors [71]. The decrease in their number will inevitably affect the transfer of electrons to photosystem I, reduce the production of ATP and NADPH, and decrease the photosynthetic efficiency of legume crops. At the same time, the decrease in photosynthetic capacity will interfere with electron transfer in photosynthesis, resulting in the accumulation of electrons in the chlorophyll body, and some of the electrons will react with oxygen molecules to form ROS [72]. Excessive accumulation of ROS leads to oxidative stress, which further adversely affects the growth and development of legume crops (Figure 3). In summary, the essence of continuous cropping leading to the decrease in the photosynthetic capacity of legume crops is the change in photosynthetic pigments, and the synthesis and metabolism of photosynthetic pigments are complex. We believe that studying the molecular mechanisms of physiological changes caused by it has important theoretical guidance significance for improving the photosynthetic efficiency of leguminous crops in continuous cropping systems, which will promote the efficient operation of photosynthetic products, and thus, improve the yield of legume crops.
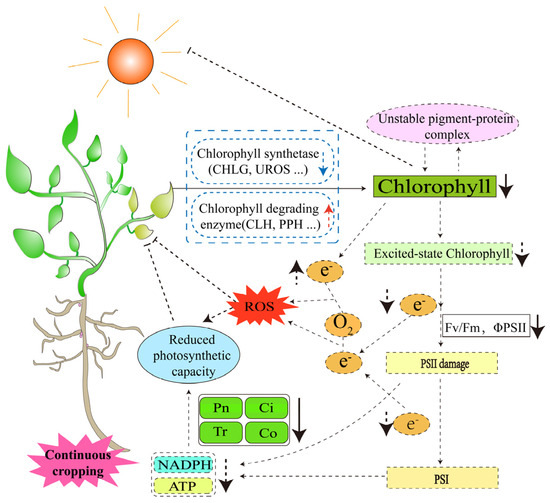
Figure 3.
Response of the photosynthetic system of legume crops to continuous cropping. The solid lines are confirmed by research; the dashed lines are potential mechanisms that require further study.
3.2. Molecular Responses of Legume Crops to Continuous Cropping Obstacles
Changes in plant phenotype and physiological and biochemical levels are regulated by gene expression. Therefore, studying how legume crops respond to continuous cropping at the molecular level is conducive to further understanding their response mechanisms.
3.2.1. Effects of Continuous Cropping Obstacle on Hormone Signaling Pathways in Legume Crops
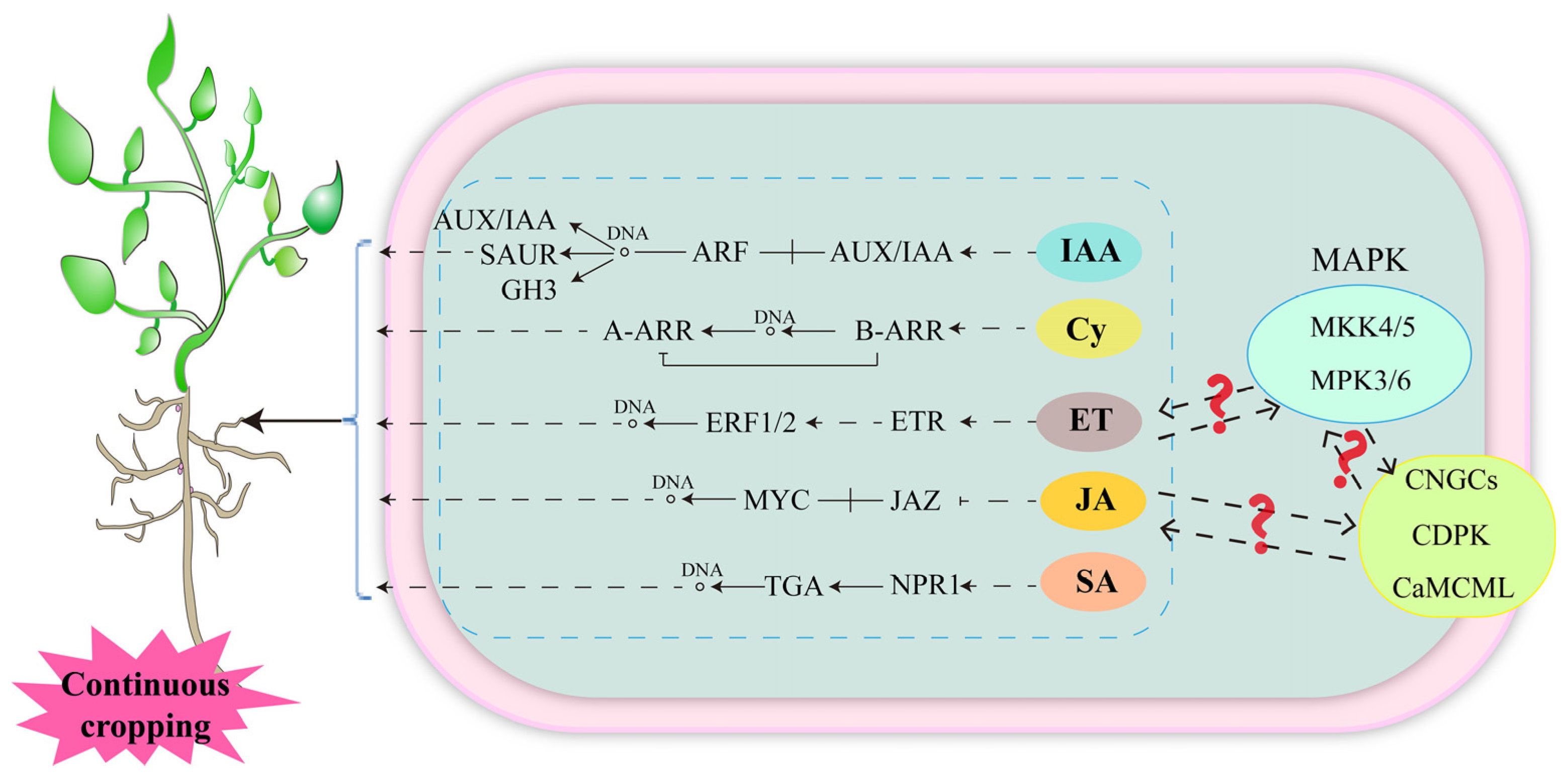
Plant hormones are endogenous small signaling molecules that play a central role in plant growth and development and in response to biotic and abiotic stresses [73]. Upon sensing stress signals, plant hormones activate downstream signaling pathways and synergistic signaling pathways to respond or adapt to the occurrence of specific stress responses [74]. The regulation of auxin on plant growth and development mainly depends on its signal transduction pathway [75]. Auxin/indole-3-acetic acid (Aux/IAA), auxin response factor (ARF) [76], small auxin up-regulated RNA (SAUR) and auxin responsive gretchen hagen 3 (GH3) are important families in the auxin signaling pathway [77,78]. Among them, ARF and AUX/IAA are essential for auxin-mediated transcriptional regulation [79,80]. The peanut root Aux/IAA, ARF, SAUR and GH3 family genes are all down-regulated in a continuous cropping system [81], implying that auxin-regulated crop traits are inhibited. Cytokinin plays a key role in the regulation of plant growth and development and the response to stress [82,83,84]. Studies confirmed that the genes encoding type-A arabidopsis response regulators (A-ARRs) and type-B arabidopsis response regulators (B-ARRs) are downregulated in the cytokinin signaling pathway of peanut roots in the continuous cropping system [81]. Among them, B-ARRs receive the cytokinin signal and its DDK domain (DDK refers to a conserved amino acid residue sequence in the N-terminal signaling region of B-ARRs, which consists of two aspartic acids (D) and one lysine (K)) is phosphorylated and activated, thereby initiating the transcription of downstream target genes, including A-ARRs, thereby regulating plant growth and development [85,86,87,88], and its downregulated expression may have a negative effect on plant growth. Ethylene is a versatile plant hormone that regulates plant growth, senescence and fruit ripening, among others [89]. The genes that encode ethylene response (ETR) and ethylene response factor 1/2 (ERF1/2) in the ethylene signaling pathway were found to be upregulated in the continuous cropping of pea and peanut [81,90]. ETR is an ethylene receptor, and its upregulated expression implies that ethylene signals are sensed and transduced [89]. Ethylene response factors (ERFs) are located downstream of the ethylene signaling pathway, and regulate downstream related genes through interactions with promoters, such as the GCC-box, which play important roles in plant growth and development, metabolism and adversity adaptation [89,91]. In addition, jasmonic acid (JA) [92,93] and salicylic acid (SA) [94] are also important plant hormones that regulate plant growth and development, and their signaling pathways are also significantly responsive to continuous cropping. Among them, the transcription factor MYC in the JA signaling pathway is upregulated in peanut under continuous cropping for 3 years and in pea roots under continuous cropping for 1 year [81,90]. Jasmonate-ZIM domain (JAZ) is not differentially expressed in peanut but is upregulated in pea roots under continuous cropping for 2 years [90]. This indicates that the JA signaling pathway plays different roles by continuous cropping degree and crop species. The regulation of plant growth and development and environmental stress response by SA is achieved by altering the SA concentration and downstream gene expression. Nonexpresser of pathogenesis-related genes 1 (NPR1) is an important regulator in the SA signal transduction pathway [95,96]. SA affects the transcriptional activation activity of NPR1 through a variety of protein modifications to regulate downstream gene expression [97,98]. And NPR1 interacts with transcription factor TGA family proteins [99,100], while class II TGA negatively regulates the expression of SA downstream genes and plays a key role in plant response to SA signaling [101]. Continuous cropping induces the upregulated expression of genes encoding NPR1 and TGA in peanut roots, which inevitably affects peanut growth. In addition, excessive ET, JA and SA has inhibitory effects on plant growth [102,103,104], suggesting that the growth inhibition of legume crops in continuous cropping systems is closely related to hormone synthesis and signaling (Figure 4). Plant hormone synthesis, metabolism and signaling processes are complex, and at the same time, each hormone signaling pathway crosstalks with each other to function. At present, only a few studies have revealed the expression of key genes in the hormone signaling pathway in the study of continuous cropping obstacles of legume crops, and future studies on hormone synthesis, metabolism and signaling crosstalk among hormones are crucial for revealing the mechanism of continuous cropping obstacles of legume crops.
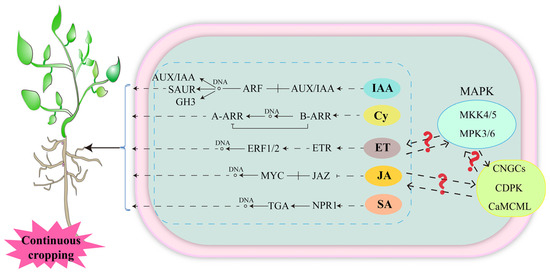
Figure 4.
Response of hormone signal transduction of legume crops to continuous cropping obstacles. Solid line: molecular interaction or relation. Dashed line: indirect link or unknown reaction. ? indicates that it has not been proven in continuous cropping abstacle of leguminous crops, but there may be an association, and further research is needed in the future.
Plant hormone signal transduction pathways not only have a direct regulatory effect on plant growth but also indirectly regulate plant growth and development by affecting other signals. It was reported that plant hormone signal transduction pathways can crosstalk with calcium signaling and mitogen-activated protein kinase (MAPK) cascades [77,105,106]. Genes involved in calcium signaling (CNGCS, CDPK, CaMCML) and key genes in MAPK signaling (MKK4/5, MPK3/6) are differentially expressed in pea roots sensitive to continuous cropping [90], indicating that these signaling pathways are strongly responsive to continuous cropping (Figure 4). However, there is no direct evidence for their crosstalk with plant hormone signal transduction pathways. Plant hormones, calcium signaling, and MAPK signaling all play important roles in plant growth, development and adaptation to adversity. Studying the interaction between them can allow us to better understand the molecular mechanism of legume growth and development and response to continuous cropping, provide theoretical support for the adaptation of legumes to continuous cropping, and provide new ideas and methods to improve their tolerance to continuous cropping.
3.2.2. Chemical Defense Response of Legume Crops to Continuous Cropping Obstacles
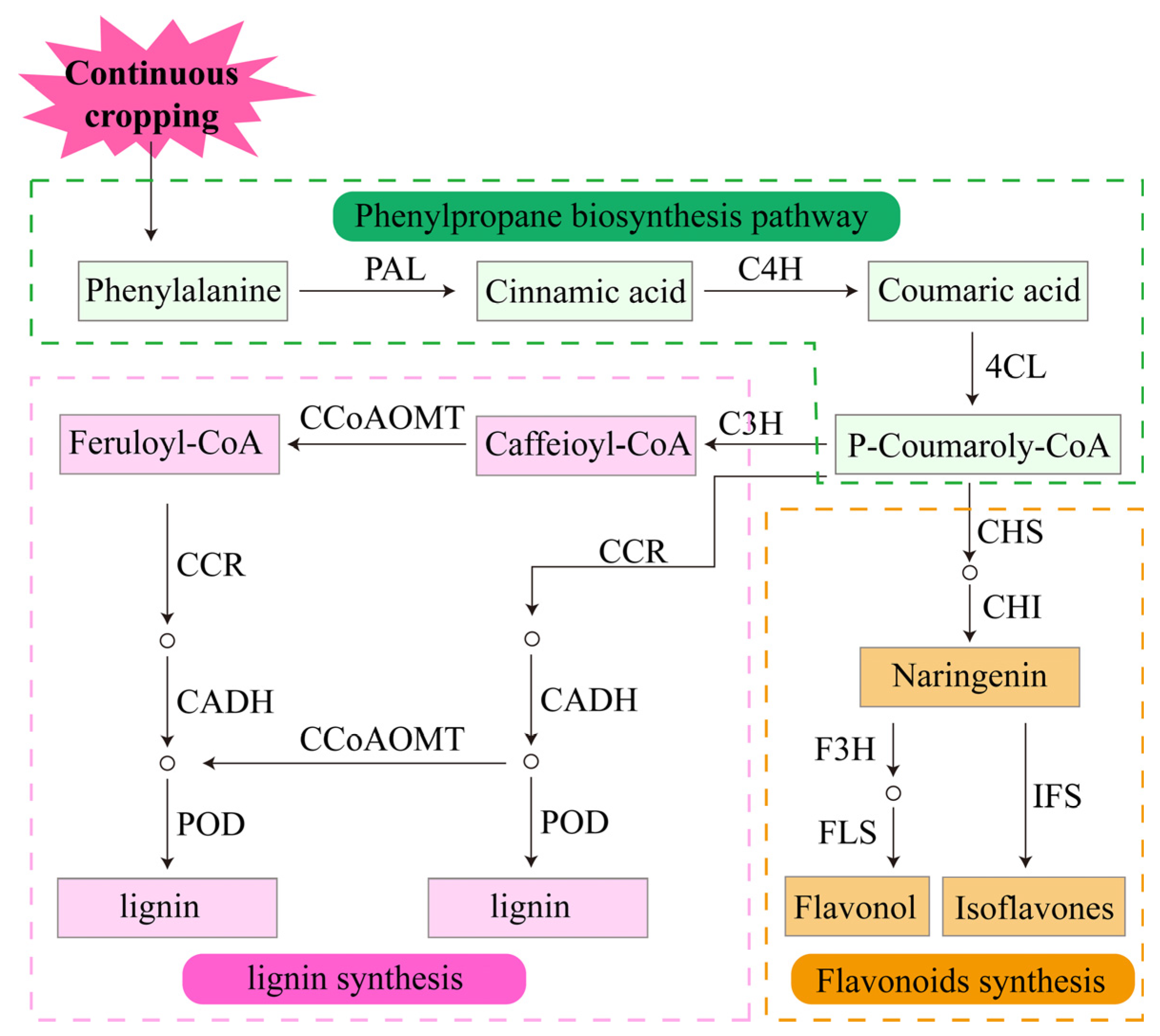
Plants cannot escape biotic and abiotic stresses in nature. To adapt to adverse environments, plants have evolved defense systems to protect themselves and reduce the extent of damage [107]. Plants produce defense responses to biotic and abiotic stresses by synthesizing a variety of secondary metabolites [76]. These secondary metabolites are essential for plant growth and development and include alkaloids, phenolics, terpenoids and other compounds [76]. The phenolic substances lignin and flavonoids in the roots of legume crops respond significantly to continuous cropping [90]. Both of them are branch synthesis products in the phenylpropanoid biosynthetic pathway. Phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H) and 4-coumarate: COA ligase (4CL) are key enzymes in the synthesis pathway [108,109]. After a series of catalytic reactions, 4-coumaroyl coenzyme A is formed and catalyzed by chalcone synthase (CHS) and chalcone isomerase (CHI) to form naringenin [109,110]. Naringenin is the common precursor of most of the end products in the process of flavonoid synthesis [108]. It is catalyzed by flavanone 3-hydroxylase (F3H), anthocyanidin synthase (ANS), flavonol synthase (FLS) and cytochrome p450 (CYP) to form various types of flavonoid compounds [108,111]. After 2 years of continuous cropping, the genes encoding key enzymes in the flavonoid biosynthetic pathway are upregulated in pea roots [90], increasing the synthesis of the enzymes and promoting the synthesis of flavonoids (Figure 5). Studies confirmed that flavonoids can activate antioxidant enzyme activities, enhance the antioxidant properties of low molecular weight antioxidants and indirectly scavenge ROS under adverse conditions [108,112]. At the same time, flavonoid compounds can provide H+ to directly scavenge ROS, thereby reducing the damage of adversity to plants [108]. In addition, flavonoids can be exuded from roots and have an indirect effect on plant growth by mediating underground interactions, including attracting rhizobia, promoting nodulation and nitrogen fixation in legume crops, and inhibiting root pathogens [113,114]. Therefore, the positive response of key genes in the flavonoid biosynthesis pathway to continuous cropping may be the primary means by which legume crops defend against continuous cropping obstacles.
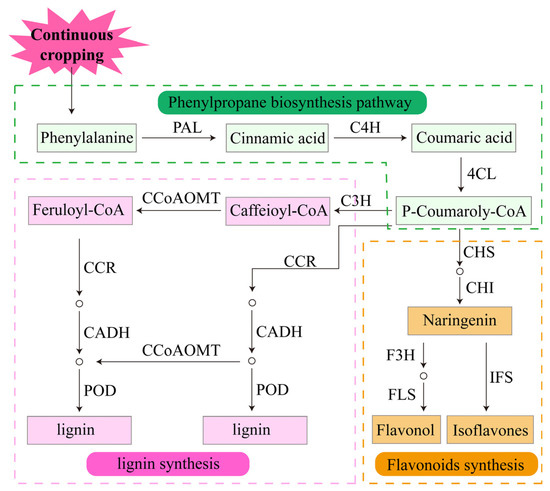
Figure 5.
Response of the defense system of legume crops to continuous cropping.
Another important branch of the phenylpropanoid biosynthetic pathway is lignin biosynthesis. Specifically, p-coumarate 3-hydroxylase (C3H), cinnamyl alcohol dehydrogenase (CADH), caffeoyl-COA O-methyltransferase (CCOAOMT) and peroxidase (POD) are the key enzymes in the lignin biosynthesis pathway [115]. Under the severe continuous cropping treatment of legume crops, C3H, CADH, CCOAOMT and POD are upregulated [90]; PAL and POD activities are increased [116,117,118,119]; and the lignin monomers p-hydroxyphenyl lignin, guaiacyl lignin, syringyl lignin and total lignin are increased [116,117] (Figure 5). It is well known that lignin is the main component of the cell wall and is used to build the first barrier for plants to resist stress [119,120]. Under stress conditions, plants limit cell expansion and maintain growth by increasing lignin content in the roots. The above studies indicate that the stress environment of the continuous cropping system causes an increase in enzyme activity associated with the phenylpropanoid biosynthetic pathway in legume roots, which, in turn, increases the synthesis of lignin monomers, resulting in a large accumulation of lignin. Increased lignin causes root lignification in legume crops, which solidifies root cells and inhibits their growth [121,122]. These results suggest that root lignification is an important strategy for legume crops to respond to continuous cropping.
Currently, research on the mechanism of the response of legume crops to continuous cropping obstacles is mainly focused on morphological indicators, yield indicators, protective enzyme activity, MDA content and photosynthetic physiological indicators. A small number of studies used transcriptomics and metabolomics to analyze gene expression and metabolic pathways involved in response to continuous cropping in legume crops. These methods only revealed physiological characteristics and potential molecular mechanisms of growth inhibition of legume crops by continuous cropping. And there is a lack of continuity and direct evidence between studies. For example, the production of ROS is a key factor in the occurrence of continuous cropping obstacles of legume crops, but the content of ROS is not directly determined. And changes in ROS will trigger a series of signaling and gene expression changes. These changes involve multiple signaling pathways and gene networks, including the hormone signaling pathway, MAPK signaling pathway, Ca2+ signaling pathway and transcription factors, but there is no direct evidence to show and clarify the interactions between them. In addition, the defense system of plants includes physical and chemical defenses. However, whether the epidermal hardness and thickness of roots, stems and leaves of legume crops change after continuous cropping has not been reported, and only lignin and flavonoids have been investigated in the chemical defense system, while whether alkaloids, terpenoids, etc., respond to continuous cropping has also not been investigated. Moreover, the regulatory relationships between the plant defense system and ROS, plant hormone signaling, MAPK signaling pathway and Ca2+ signaling pathway are also unknown. Therefore, there are still many gaps in the research on the mechanism of legume crops responding to continuous cropping obstacles, and if we want to dig deeper into the physiological potential and molecular mechanism of legume crops responding to continuous cropping, it is necessary to integrate traditional physiological indexes with modern bioinformatics to realize the precise analysis and precise regulation in the later stage.
4. Deterioration of the Soil Environment Leads to the Occurrence of Continuous Cropping Obstacles of Legume Crops
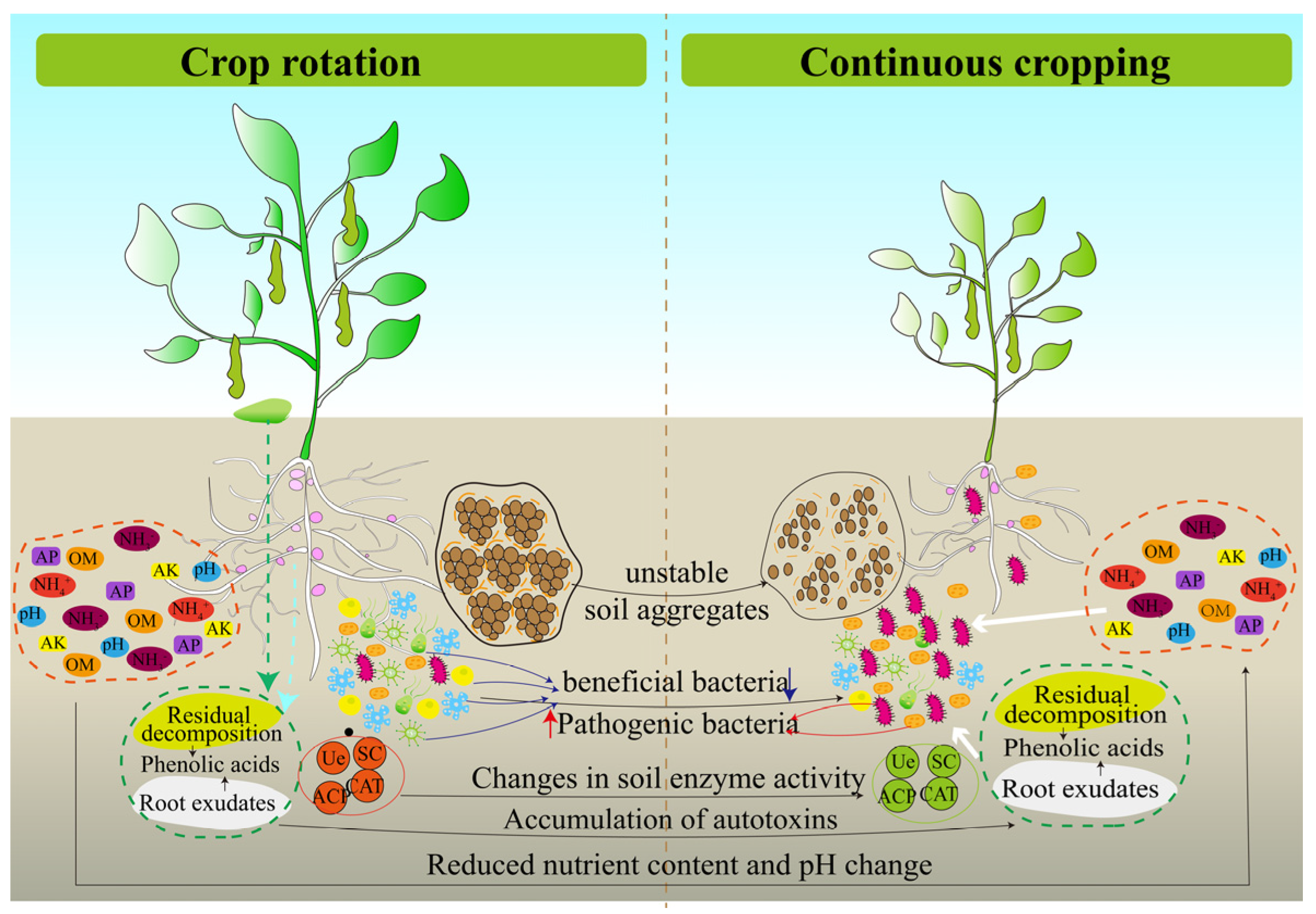
Crops respond to continuous cropping by regulating their physiological, biochemical and other metabolic pathways as a result of crop–soil interaction [6,123]. Among them, soil is an important agricultural production resource and a place where continuous cropping obstacles are triggered. Therefore, researchers and farmers are paying more attention to soil health. A large number of studies showed that continuous cropping has deteriorated the soil environment and threatened soil health. Among them, the change in soil physical and chemical properties, the decrease in soil enzyme activities, the accumulation of autotoxin substances, the increase in insect pests and the change in microbial community structure are the main reasons for the occurrence of continuous cropping obstacles [124,125,126,127,128,129] (Figure 6).
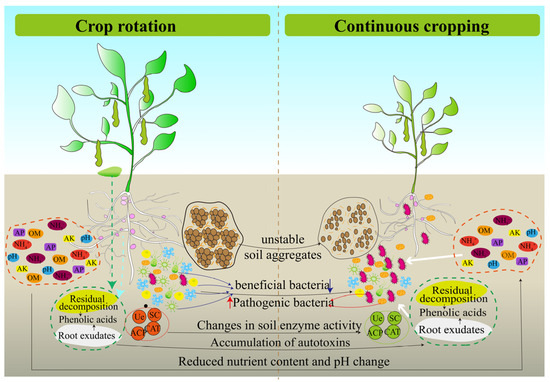
Figure 6.
Soil environment deterioration in continuous cropping system of legume crops.
4.1. Response of Soil Physicochemical Properties of Legume Crops to Continuous Cropping
Continuous cropping tends to cause changes in soil physicochemical properties, including soil aggregates, organic matter, nutrients and pH. These indicators not only influence each other but also regulate the soil physicochemical processes and biodiversity, resulting in soil degradation and reduced crop yields [130,131,132,133]. The soil physical properties are related to the soil structure [132], and soil aggregates are the most fundamental structural units of soil [134], where their stability is an important physical indicator for assessing the soil structure and soil health [135,136]. However, they are susceptible to the influence of the intrinsic soil properties and external environmental factors, including the cropping system [137]. The study found that the stability of soil aggregates decreased after continuous cropping of soybean [138,139], which means that continuous cropping may lead to soil degradation by destroying the soil structure. However, in the research results on the effects of legume crops on soil environment after continuous cropping, it was found that there are few reports on soil physical properties, but soil physical properties determine the water and nutrient absorption capacity of crop roots, and affect the types and activities of soil organisms [140,141]. Therefore, the lack of research has limited the comprehensive analysis of the continuous cropping obstacles of legume crops.
Soil organic matter, nutrient content and pH are the key physical and chemical indices that reflect soil health and fertility and are the key research objects of continuous cropping obstacles. Typically, continuous cropping negatively affects soil organic matter, nutrients and pH. In the study of legume crops, it was found that short-term (<5–6 years) continuous cropping reduces the contents of nitrate nitrogen, ammonium nitrogen, available phosphorus, and available potassium in pea, peanut, soybean and cowpea (Vigna unguiculata L.) soils [11,142,143]. In addition, the variation in pH in different types of legume crops is different. It shows that short-term continuous cropping has a negative effect on the physical and chemical properties of legumes in the soil and further affects the growth of legumes.
4.2. The Role of Autotoxic Substances in the Initiation of the Continuous Cropping Obstacles of Legume Crops
Crops produce large amounts of secondary metabolites that are secreted into the soil during growth [144], and some crop secretions were shown to be potentially autotoxic [123]. In the continuous cropping system of legume crops, these autotoxic substances are repeatedly released into the soil, and their high accumulation will inhibit crop growth and lead to yield losses [145]. These autotoxic substances enter the soil mainly through root secretion, residue decomposition and above-ground leaching [146]. Through identification, phenolic acids, such as cinnamic acid, coumaric acid, ferulic acid and benzoic acid, were found in the aboveground tissues, culture matrix extracts and root exudates of legume crops [147,148,149,150,151,152]. Phenolic compounds are considered to be some of the main autotoxic substances in plants [153,154]. In studies on legume crops, the number of phenolic acids in the soil was found to increase in type [152] and content [155,156] as the number of years of continuous cropping increased. The autotoxicity of these phenolic acids was also widely verified in legume crops, and it was found that autotoxic substances generally have a concentration effect of low promotion and high inhibition on the growth of legume crops [33,157,158,159,160]. This shows that autotoxic substances may be an important cause of continuous cropping obstacles of legume crops. The verification of the above autotoxic effects was achieved via a simulation experiment conducted by artificially adding autotoxic substances in a controlled environment, which can reflect the general rules of autotoxic effects of phenolic acids. However, in a field continuous cropping system, there is a mixture of various autotoxic substances in the soil, and the autotoxic effect verification of a single substance cannot fully reflect the results of autotoxic substances in the soil. And it is also believed that phenolic acids in soil are susceptible to environmental factors, and their content is not sufficient to cause autotoxic effects [161,162,163]. Therefore, the direct inhibitory effect of autotoxic substances in field soils on legume growth needs to be further investigated.
4.3. The Continuous Cropping System of Legume Crops Causes a Change in Soil Enzyme Activity
Soil enzymes are involved in a series of biochemical processes, such as organic matter decomposition and nutrient cycling in soil, and their activities affect the rate of material transformation and cycling in soil, but they are easily affected by environmental factors [164]. Therefore, soil enzyme activities can be an important indicator of soil fertility and sustainable soil use [165,166]. Soil enzyme activities of legume crops are affected by continuous cropping. Urease, acid phosphatase, catalase and invertase tend to decrease in most legume soils after short-term (<5–6 years) cropping. Enzyme activities increase after 2 years of continuous cropping of peanut [164,167,168]. However, there is a significant decrease in urease, phosphatase and sucrase activities and no change in catalase in soils cropped for more than 5 years [167,168]. The uncertain changes in soil enzyme activities in continuous cropping systems are related to changes in soil physical and chemical properties, such as pH, can change the base point and stability of soil enzyme reactions, and enzyme reactions are sensitive to changes in pH, and the optimal pH of each enzyme is different, leading to different changes in the activities of different enzymes in soils of the same crop. At present, the research results on the mechanism of soil enzyme activity change after the continuous cropping of legume crops are few and not deep enough, but the important role of enzyme activity in the soil system cannot be ignored. Therefore, the study of soil enzyme activity changes and its mechanism of action is of great significance in the study of continuous legume cropping obstacles in the later stage to reveal the mechanisms of the material cycle and soil system degradation in continuous cropping systems.
4.4. Response of Legume Soil Nematodes to Continuous Cropping
Soil nematodes are involved in the decomposition of soil organic matter and the mineralization of nutrients, which are important for promoting nutrient cycling, energy flow and maintaining the stability of soil ecosystems [169,170]. Soil nematodes include plant-parasitic nematodes, bacterial-feeding nematodes and fungal-feeding nematodes. Among them, plant-parasitic nematodes have the potential to cause severe plant diseases [171]. Studies found that cyst nematodes in plant-parasitic nematodes are related to the occurrence of soybean continuous cropping obstacles [172]. In a study of peanut, it was found that the number of fungal nematodes in the soil of continuous cropping for 3 years, 6 years and 20 years decreased, while the number of plant-parasitic nematodes significantly increased with the increase in continuous cropping years [173]. The increase in nematode species and numbers in the soil after continuous cropping is related to the change in the soil environment. For example, allelochemicals secreted by roots can incubate the eggs of secondary plant parasitic nematodes [174,175]. In addition, the soil microbial community also influences the type of soil nematodes. It is suggested that changes in the soil environment in continuous cropping systems further influence the alteration of nematode species and populations in the soil, which, in turn, threatens the growth of legume crops.
4.5. The Change in Microbial Community Structure in Legume Crop Soil in Continuous Cropping System Is the Key Factor Causing Continuous Cropping Obstacles
Plant growth and development depend on interactions with soil microorganisms [176,177], which constantly undergo metabolic crosstalk, forming a complex symbiotic relationship that plays an important role in maintaining healthy plant growth and productivity [178]. Studies have shown that planting patterns are the main factors that cause changes in soil microbial community structure [179,180]. In a continuous cropping system, the rhizosphere soil microflora changes, the soil changes from bacterial to fungal type, and harmful microorganisms become the dominant community, which is an important reason for the occurrence of continuous cropping obstacles [181].
The rhizosphere soil has the largest number of bacteria, and their interaction with plants plays a key role in soil fertility, sustainability, and plant growth and development [182]. Tang et al. reported that continuous cropping increased Acidobacteria and Firmicutes and decreased Actinobacteria in soybean rhizosphere soil [183]. Among them, Acidobacteria can cause soil acidification, and most Firmicutes can form drought-tolerant spores. Actinomycetes play an important role in decomposing organic matter and antagonizing plant pathogens [183]. Pan et al. found that continuous cropping reduces the expression of amoA in soybean rhizosphere soil [184]. amoA encodes the alpha subunit of ammonia oxidase, which is a key gene in ammonia-oxidizing bacteria and anaerobic ammonia-oxidizing bacteria [185]. It participates in the ammonia oxidation process, oxidizing ammonia to nitrite and synthesizing amino acids and other nitrogenous compounds through a series of reactions [186]. Therefore, the decrease in amoA expression leads to a decrease in the rate of ammonia oxidation, affecting the conversion and removal of ammonia nitrogen. Another study showed that the bacterial diversity in peanut rhizosphere soil decreases after continuous cropping, and the abundance of Actinobacteria, Firmicutes and Bacteroidetes decreases [81]. There are no significant changes in dominant and common bacteria, but rare bacteria are significantly enriched [81]. The extraction of peanut rhizosphere soil bacterial extract was found to significantly inhibit peanut growth [81]. Liu et al. found higher bacterial abundance and diversity in soybean rotational systems than in continuous cropping [187]. However, Ma et al. found that the bacterial diversity of pea rhizosphere soil was not affected by short-term continuous cropping [90]. The above studies show that the effect of continuous cropping on soil bacteria varies with the type of legume crops, but the decrease in beneficial bacteria and the increase in harmful bacteria produce the main effects on legume crops in response to continuous cropping.
Fungi are an important part of the soil microbial community and play important roles in the soil ecosystem as pathogens and symbionts of plants and animals [188]. A study found that short-term (<5–6 years) continuous cropping increases the relative abundance of fungi in soybean rhizosphere soil [187,189]. An analysis of fungal community structure showed that the abundance of Fusarium oxysporum increases significantly after continuous cropping. Fusarium oxysporum is a soil-borne pathogenic fungus with widespread and severe pathogenicity worldwide [190]. Fusarium oxysporum infects crops through the roots and can cause disease throughout the growing season. It manifests itself mainly as brown necrotic spots in the root cortex of the plant. In severe cases, the main root and a large number of lateral roots rot, and the branches decrease until the plant dies, which is called Fusarium wilt and root rot [191]. In peanut studies, continuous cropping was also found to increase the abundance of soil fungal, and the relative abundance of pathogens, such as Fusarium, Aspergillus, Acrophialophora and Neocosmospora, increase in the rhizosphere under continuous cropping [192]. The above results showed that the abundance of fungi in the rhizosphere soil of legume crops increased after continuous cropping, and the increase in the number of pathogenic bacteria is the key to the continuous cropping obstacles of legume crops.
In addition, an interesting phenomenon occurs in soybean continuous cropping. The researchers found that soybean faces serious continuous cropping obstacles in short-term (<5–6 years) continuous cropping, but as the number of continuous cropping years increases, the soybean yield shows an upward trend (but significantly lower than crop rotation) [193], and organic matter, alkali-hydrolyzable nitrogen, available phosphorus, available potassium, and soil enzyme activity increases and approaches rotational soils [140,194]. The density of soybean cyst nematodes decreases while the abundance of fungal-feeling nematodes increases [195]. In addition, the abundance of soil fungi approaches that of rotation, and the relative abundance of potentially beneficial bacteria Bradyrhizobium and Gemmatimonadetes and pathogenic bacteria Mortierella sp. and Paecilomyces sp. in the soil increases, while the relative abundance of pathogenic fungi Fusarium decreases [187]. This shows that soybean soil produces some disease resistance after long-term continuous cropping, and the soil’s physicochemical properties are improved, which weakens the degree of inhibition of soybean growth. However, it is still unknown why the soybean soil environment improves after long-term continuous cropping. An investigation of its mechanism is likely to be an important breakthrough in effectively alleviating soybean continuous cropping obstacles in the future.
In summary, changes in soil physical and chemical properties, accumulation of autotoxic substances, soil enzyme activity, plant parasitic nematodes and microbial community structure in soil are all important factors that cause continuous cropping obstacles of legume crops (Figure 6). In continuous cropping systems, these factors not only affect legume crop growth directly but also act through indirect regulation. For example, soil pH and nutrients are important environmental factors that drive the structure of microbial communities in continuous cropping soils [187,196]. In addition, crop root exudates can shape the microbial community structure of the rhizosphere [197], and some compounds can directly stimulate or inhibit the growth of soil pathogens [198]. The biomass of rhizosphere fungi increases after root exudates from legume crops enter the soil [199,200]. It was suggested that the regulation of rhizosphere soil microbial community structure by root exudates may be an important reason for the inhibition of crop growth in a continuous cropping system. Meanwhile, the mechanism of legume sensitivity to continuous cropping may be related to root secretions, which deserves further investigation at a later stage. These results suggest that there is a correlation between the factors that cause continuous cropping obstacles, and microorganisms play an important role in many factors. At present, soil microorganisms are the research hotspot of continuous cropping obstacles, but the research on legume crops is relatively scarce and not comprehensive enough. In the future, 16s rDNA, ITS and macrogenomics technologies should be used to reveal the structure, diversity and function of microbial communities in continuous cropping soils to screen out bacteria and fungi that significantly respond to continuous cropping, to explore new microbial resources in combination with legume crops phenotypes, to develop new microbial fertilizers, to provide basic data and theoretical support for mitigating the barriers of continuous cropping, and to provide scientific basis and technical support to promote the sustainable development of the legume crops industry.
5. The Main Measures to Alleviate the Continuous Cropping Obstacles of Legume Crops and the Change of Ideas
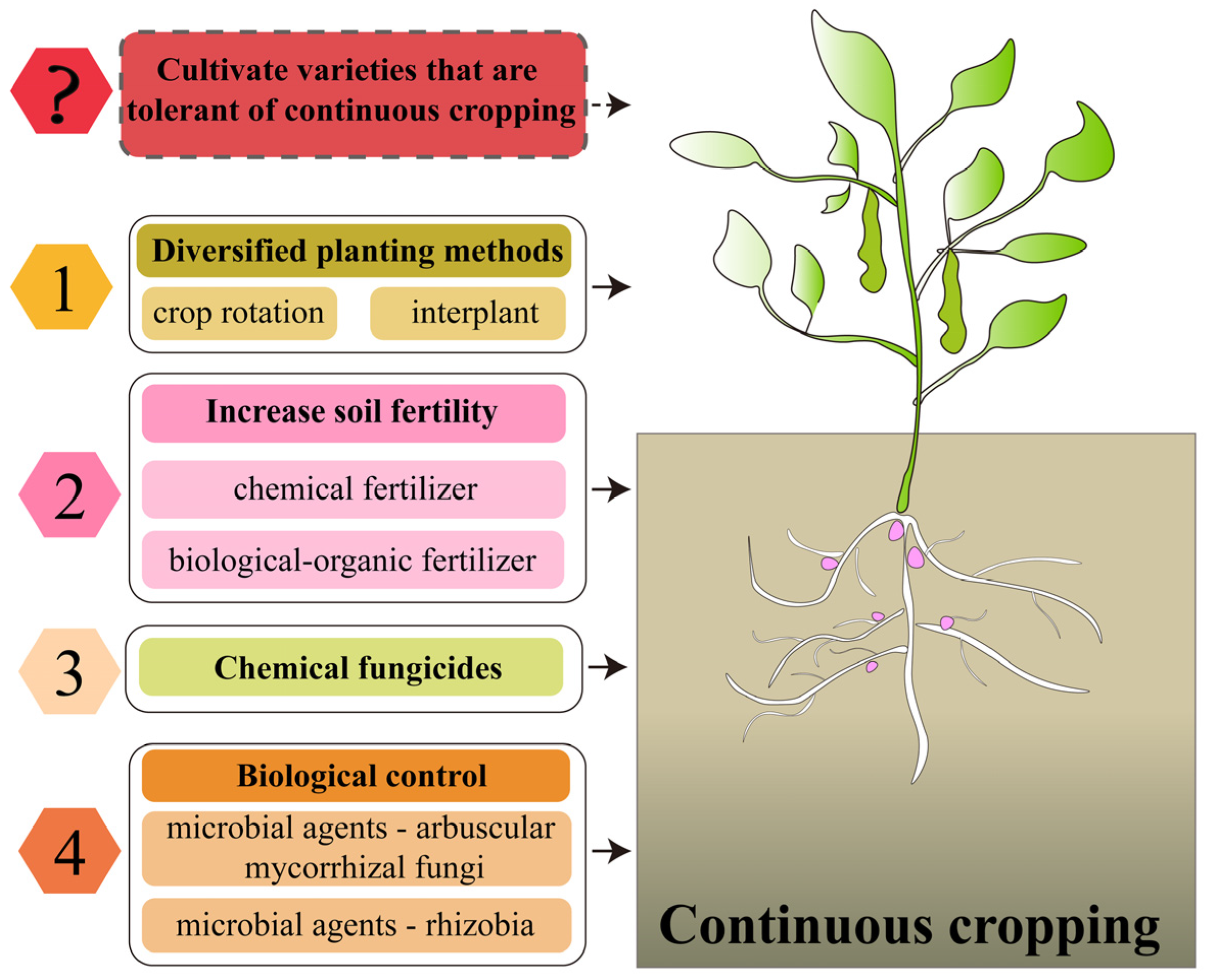
The production of legume crops is facing a serious threat from continuous cropping obstacles. How to alleviate the problem of yield reduction caused by continuous cropping is also a hot spot in the study of the continuous cropping obstacles of legume crops. To this end, many scholars have explored a variety of measures to alleviate continuous cropping obstacles, as follows (Figure 7).
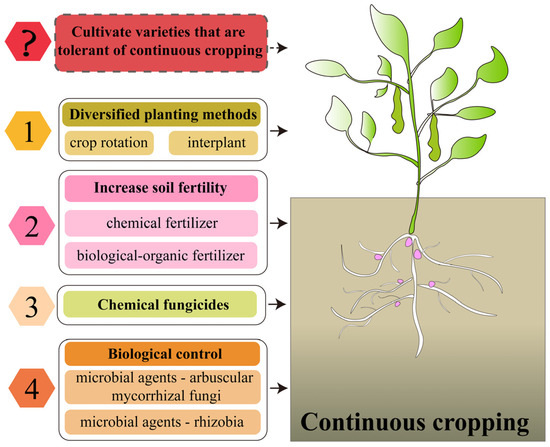
Figure 7.
Measures to alleviate the continuous cropping obstacles of legume crops. ?: Elements that need to be studied at a later date.
5.1. Diversification of Planting Patterns
As early as 300 BC, people recognized that continuously growing the same crop on the same land leads to its growth inhibition [7]. To mitigate this continuous cropping obstacle, crop rotation and intercropping were introduced, especially in the cultivation of legume crops, and these methods were shown to improve degraded soil environments and promote crop growth by increasing ecosystem diversity [194,201,202,203]. However, the application of these cropping patterns is affected by market demand, technical and knowledge constraints, land and resource constraints, economic considerations and labor requirements. Moreover, for some legumes, crop rotation cycles of up to 10 years may be required to significantly improve the soil environment, presenting a challenge in terms of meeting growing protein demand and developing low-input agroecosystems [204]. Therefore, although crop rotation and intercropping are theoretically effective ways to improve the soil environment, in practice, more economic and market considerations need to be taken into account. With advances in agricultural technology, and in-depth research into the mechanisms of continuous cropping obstacles, new solutions may emerge in the future to meet market demands while maintaining soil health and productivity.
5.2. Increase Soil Fertility
Nutrient deficiency is one of the causes of continuous cropping obstacles. People supplement soil nutrients to achieve the purpose of alleviating continuous cropping obstacles. In legume continuous cropping systems, the application of N, P and K fertilizers can increase soil nutrients, influence soil microbial community structure and promote yield formation [205,206]. However, the long-term use of chemical fertilizers not only leads to the decomposition of soil organic matter, degradation of soil structure and reduction in soil aggregates but also to nutrient loss and reduced fertilizer efficiency [207]. Special attention should be paid to the fact that the application of chemical fertilizers not only leads to soil acidification but may also cause the deficiency of some trace elements and the accumulation of heavy metals (Cu, Zn, Cd), which affects the activity of soil enzymes, the structure of soil microbial communities and ecological functions [207,208]. Therefore, although chemical fertilizers can rapidly replenish soil nutrients, their long-term use has a negative impact on soil ecosystems. Therefore, bio-organic fertilizers have been applied for the mitigation of continuous cropping obstacles. Bio-organic fertilizers are organic fertilizers with added functional microorganisms, which increase the soil microbial activity and nutrient utilization [209]. It was found that the application of bio-organic fertilizers increases the soil nutrients and enzyme activities of legume crops in a continuous cropping system and increases the number of beneficial microorganisms in the soil, which greatly improves the yield of legume crops in the continuous cropping system [210,211,212,213], and the mitigation effect was significantly superior to that of chemical fertilizer application [211,213]. Another study concluded that the application of organic fertilizer increases the contents of copper, zinc and cadmium in peanut continuous cropping soil, which may cause metal pollution in the soil [214]. Therefore, whether the use of organic fertilizer to mitigate continuous cropping causes secondary pollution in soil needs to be further investigated.
5.3. Application of Chemical Fungicides
The increase in pests and diseases in the soil after continuous cropping is an important factor causing the continuous cropping obstacles of legumes. In order to alleviate these challenges, chemical agents, especially fungicides, are often used to reduce the occurrence of pests and diseases and reduce the continuous cropping obstacles of legumes [215,216]. However, reliance on fungicides can only promote crop growth in the short term. The widespread use of these chemicals can cause serious environmental pollution [217]. At the same time, it has a negative impact on soil health, especially on the microbial balance necessary for healthy ecosystems, leading to a reduction in soil biodiversity. In addition, fungicides can increase the resistance of pathogens, especially when fungicides are used repeatedly or at high doses [218]. Therefore, although fungicides can provide short-term mitigation effects against the continuous cropping obstacles of legumes, the long-term effects on environmental health and pathogen resistance have forced us to explore effective mitigation measures that are premised on sustainable development.
5.4. Biological Control
With the revelation of the important role of soil microorganisms in the occurrence of continuous cropping obstacles, the measures to alleviate continuous cropping obstacles have also entered the stage of biological control. Using beneficial microorganisms to inhibit the growth of pathogenic bacteria and harmful organisms in the soil reduces the infection of pathogenic bacteria to the root system and achieves the purpose of alleviating continuous cropping obstacles. For example, rhizobacterial inoculation increases the nodulation capacity of legume crops in continuous cropping systems while altering the composition of the soil microbial community and increasing soil enzyme activity, thereby promoting the growth of legume crops [28,32,142,219,220]. The inoculation of the endophytic fungus Phomopsis liquidambari B3 in continuous cropping soybean can effectively increase the number of beneficial bacteria and fungi in the soil [221], thus alleviating the continuous cropping obstacles, while the inoculation of the arbuscular mycorrhizal fungus Funneliformis mosseae significantly reduces the occurrence of continuous cropping soybean root rot [222]. Therefore, the use of beneficial bacteria not only effectively mitigates the continuous cropping obstacle of legume crops, but also avoids chemical fertilizer inputs and secondary soil environmental degradation, which plays an important role in the development of sustainable agriculture. In summary, changing planting patterns and applying chemical fertilizers, organic fertilizers and biological control are the main measures to alleviate the continuous cropping obstacles. These measures promote the growth of legume crops by improving the soil environment in the continuous cropping system. However, there is a lack of research on improving the ability of legume crops themselves to withstand continuous cropping through the selection and breeding of continuous cropping tolerant varieties and molecular breeding to mitigate continuous cropping obstacles, which needs to be further explored by researchers.
6. Future Perspectives and Research to Advance Continuous Cropping of Legumes
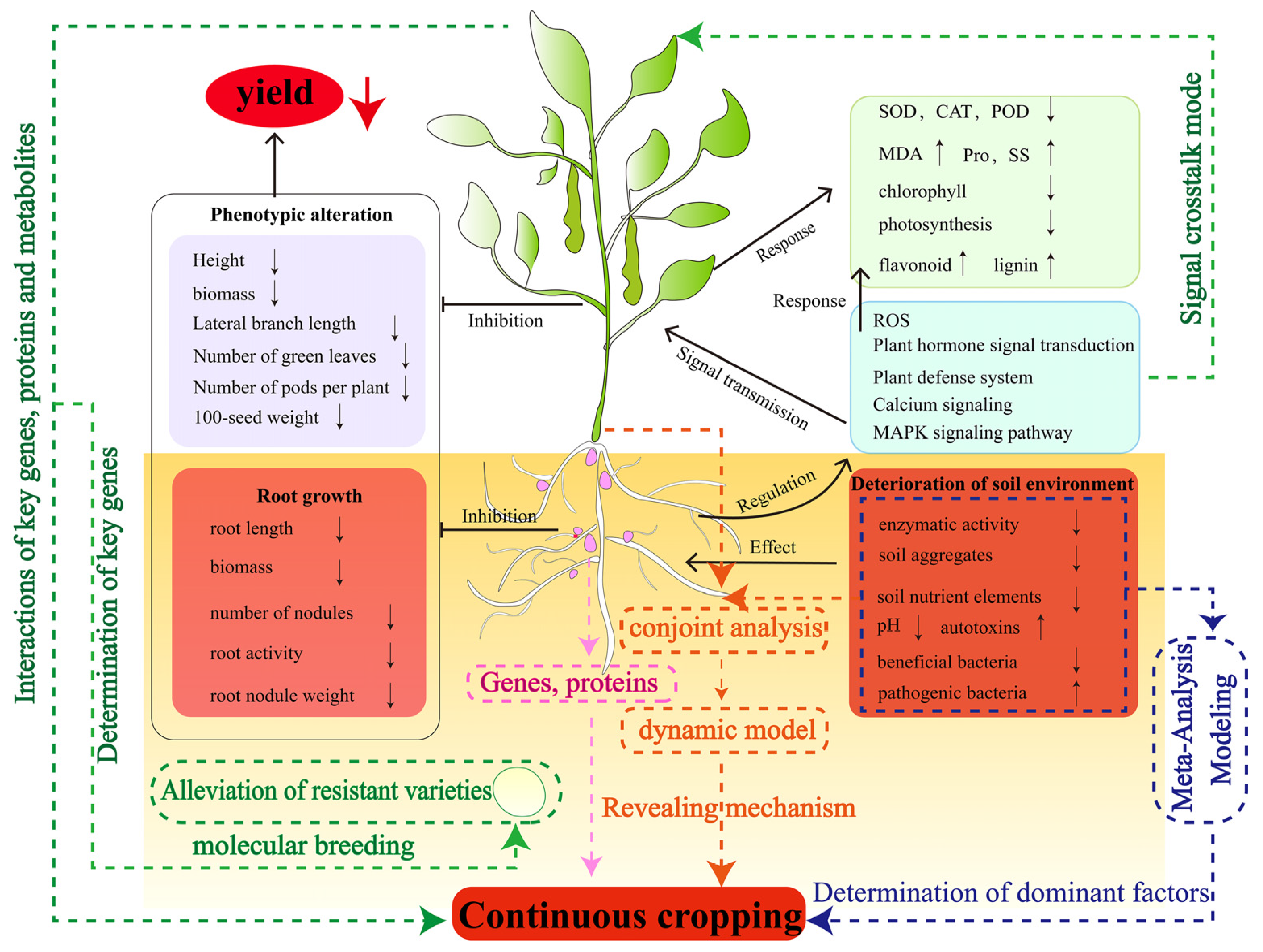
The occurrence of continuous cropping obstacles is a very complex problem that limits agricultural production and it has been extensively studied in legume crops. In this review, it was found that the continuous cropping obstacle of legume crops is caused by the deterioration of the soil environment. The root system first senses the change in the soil environment and regulates the expression of specific genes through a series of signal transduction pathways, initiates the antioxidant system and produces secondary metabolites in response to continuous cropping. At the same time, the root system transmits the stress signal to the aboveground part, causing physiological and biochemical reactions, such as antioxidant and photosynthetic systems in the aboveground part, ultimately affecting its growth and development (Figure 8). The above provides a more comprehensive theoretical basis for understanding the occurrence and response of the continuous cropping obstacle of legume crops. However, it is still insufficient to reveal the occurrence mechanism of the continuous cropping obstacle of legume crops, and the following issues need to be elucidated in future research (Figure 8):
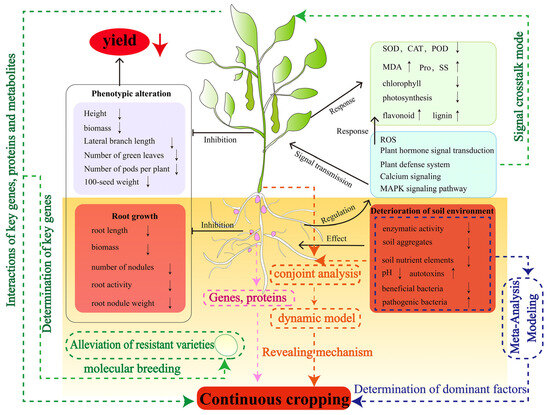
Figure 8.
Mechanisms of legume crops responding to continuous cropping obstacles. The dotted lines are the content that needs to be studied in the future. The arrows following the morphological, physiological and biochemical indicators indicate an increase or decrease in the value of the corresponding indicator, respectively.
- At present, research on the mechanism of continuous cropping obstacles in legume crops mainly focuses on the characteristics of changes in their phenotypes and physiological and biochemical levels, which need to be analyzed in depth from the molecular level by combining with advanced biological techniques. For example, “omics” technology plays an important role in understanding the crop response to continuous cropping and can guide people to find new methods to influence the phenotypic, physiological and biochemical changes. Future studies can use the combination of multi-omics technology and bioinformatics to find key genes, proteins, metabolic pathways and products involved in legumes that significantly respond to continuous cropping obstacles. It will be helpful to further understand the molecular mechanism of legume responses to continuous cropping. Nodulation and nitrogen fixation are the characteristics of legume crops, but how the continuous cropping system affects the genetic pathway of legume crop nodule formation and the mechanism of their symbiotic behavior is still unknown. Clarifying the ecological adaptability and physiological and molecular mechanisms of nodules in continuous cropping is helpful for understanding the relationship between rhizobia and continuous cropping obstacles, and provides a scientific basis for solving the problem of continuous cropping obstacles. In addition, through large-scale genomic data and continuous cropping test data, the mining of candidate genes and functional sites related to continuous cropping obstacles of legume crops is the key task for the cultivation of continuous-cropping-resistant germplasm resources of legume crops in the future.
- Changes in the physical and chemical properties and microbial communities in soil ecosystems trigger legume continuous cropping obstacles. The interactions between these factors determine the structure, function and complexity of soil ecosystems. At the same time, it also increases the difficulty of researching the causes of continuous cropping obstacles. In the future, it is necessary to strengthen the detection of the soil environment in the continuous cropping system of legume crops and further analyze the mechanism of soil environment degradation in the continuous cropping system through a meta-analysis, model building and other methods to clarify the leading factors. In addition, soil microorganisms are currently a hot topic in the research of continuous cropping obstacles, but the research of continuous cropping obstacles in legume crops only revealed a decrease in beneficial bacteria and an increase in pathogenic bacteria in the soil. In the future, it is necessary to explore the beneficial bacteria of rhizosphere resistance to continuous cropping according to the existing research results and design specific compound microorganisms for legume crops to effectively alleviate the continuous cropping obstacles of legume crops.
- After long-term (>5–6 years) continuous cropping of soybean, the degree of growth inhibition was weakened, the soil environment was improved, soil nutrients and enzyme activities were increased, and soil pests and diseases were reduced. However, it is not clear why soybean soil self-repaired after long-term continuous cropping. Therefore, in-depth research on the mechanism of soil environment improvement after the long-term continuous cropping of soybean may be an important breakthrough to alleviate the continuous cropping obstacle of legume crops in the future.
- The research on continuous cropping obstacles of legume crops is more focused on the changes in the soil environment, but the research on crop–soil interaction is scarce. The occurrence of continuous cropping obstacles is closely related to the rhizosphere signal exchange of legume crops. The induction effect of root exudates on microorganisms in the continuous cropping system of legume crops and how roots perceive microbial signals are still uncertain. In the future, a systematic dynamic model should be established by linking the changing characteristics of soil environmental factors with the physiological and biochemical characteristics, as well as genes and metabolites of legume crops, to comprehensively and deeply reveal the mechanism of continuous cropping obstacles of legume crops.
Author Contributions
L.M. wrote the manuscript and prepared the figures; S.M. and G.C. revised the manuscript; X.L. revised the English grammar; Q.C. and S.L. designed, supervised, reviewed and edited the writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the major special project in Gansu Province (20ZD7NA007), National Green Fertilizer Industry Technology System (CARS-22-G-12), National Science Fund (31460382), China Agriculture Research System of MOF and MARA-Food Legumes (CARS-08), National Natural Science Foundation of China (32260483) and Natural Science Fund Project of Gansu Province (21JR7RA822).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fouad, A.W.; Fathy, E.E.; Helmy, H.R. Challenges and opportunities for the global cultivation and adaption of legumes. Biotechnol. Bloeng. 2021, 8, 160–172. [Google Scholar]
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common vetch: A drought tolerant, high protein neglected leguminous crop with potential as a sustainable food source. Front Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, H.; Khan, N.; Tian, J.; Wang, L.; Wu, J.; Cheng, X.; Chen, X.; Liu, Y.; He, Y.J.A. Economic assessment of food legumes breeding in China: Evidence using a provincial level dataset. Agronomy 2022, 12, 2297. [Google Scholar] [CrossRef]
- Bhat, K.A.; Mahajan, R.; Pakhtoon, M.M.; Urwat, U.; Bashir, Z.; Shah, A.A.; Agrawal, A.; Bhat, B.; Sofi, P.A.; Masi, A.; et al. Low temperature stress tolerance: An insight into the omics approaches for legume crops. Front Plant Sci. 2022, 13, 888710. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.; Konkin, D.; Polowick, P.; Hodgins, C.L.; Subedi, M.; Xiang, D.; Yu, B.; Patterson, N.; Rajagopalan, N.; Babic, V. CRISPR/Cas9 gene editing in legume crops: Opportunities and challenges. Legume Sci. 2021, 3, e96. [Google Scholar] [CrossRef]
- Tan, G.; Liu, Y.; Peng, S.; Yin, H.; Meng, D.; Tao, J.; Gu, Y.; Li, J.; Yang, S.; Xiao, N.; et al. Soil potentials to resist continuous cropping obstacle: Three field cases. Environ. Res. 2021, 200, 111319. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.; Yang, R.; Yu, L.; Lv, C.; LI, R.R.; Wang, Y. Crop continuous cropping obstacles: Research progress. Chin. Agric. Sci. Bull. 2019, 35, 36–42. [Google Scholar]
- Li, Y. Characteristics of Soil Nematode Community and Its Influencing Factors in Soybean Continuous Cropping in Black Soil Region; Northeast Agricultural University: Harbin, China, 2021. [Google Scholar]
- Wang, H.W.; Tang, M.J.; Su, C.L.; Zhang, W.; Xu, R.S.; Guan, Y.X.; Dai, C.C. The alleopathic compound luteolin from peanut litter affects peanut nodule formation and the rhizosphere microbial community. Agron. J. 2018, 110, 2587–2595. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.L.; Cui, R.Z.; Li, G.S.; Zheng, F.L.; Tan, D.S. Physiological effects of humic acid in peanut growing in continuous cropping soil. Agron. J. 2021, 113, 550–559. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; Li, R.Q.; Tan, S.L.; Wang, S.Q. Effects of continuous cropping obstacle on the characteristics of Ejiangdou No.5 and Ejiangdou No.8. Hubei Agric. Sci. 2019, 58, 72–73,78. [Google Scholar]
- Wang, D.; Yang, X.; Fan, Q.; Liang, H.; Tian, F. Effects of continuous cropping on growth development and quality of protected cowpea. North. Hortic. 2023, 3, 51–55. [Google Scholar]
- Ma, Z.; Guan, Z.; Liu, Q.; Hu, Y.; Liu, L.; Wang, B.; Huang, L.; Li, H.; Yang, Y.; Han, M. Obstacles in continuous cropping: Mechanisms and control measures. Adv. Agron. 2023, 179, 205–256. [Google Scholar]
- Zeeshan Ul Haq, M.; Yu, J.; Yao, G.; Yang, H.; Iqbal, H.A.; Tahir, H.; Cui, H.; Liu, Y.; Wu, Y. A systematic review on the continuous cropping obstacles and control strategies in medicinal plants. Int. J. Mol. Sci. 2023, 24, 12470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Elcin, E.; He, L.; Vithanage, M.; Zhang, X.; Wang, J.; Wang, S.; Deng, Y.; Niazi, N.K.; Shaheen, S.M. Using biochar for the treatment of continuous cropping obstacle of herbal remedies: A review. Appl. Soil Ecol. 2023, 2023, 105127. [Google Scholar] [CrossRef]
- Ku, Y.; Li, W.; Mei, X.; Yang, X.; Cao, C.; Zhang, H.; Cao, L.; Li, M. Biological control of melon continuous cropping obstacles: Weakening the negative effects of the vicious cycle in continuous cropping soil. Microbiol. Spectr. 2022, 10, e01776-22. [Google Scholar] [CrossRef]
- Wang, G.Y.; Guo, W.L.; Chen, B.H.; Fan, F.F.; Huang, X.Q.; Cai, Z.C.; Zhou, J.H.; Gu, G.L. Research progress on mechanism and application of reductive soil disinfection (RSD) in prevention and control of continuous cropping obstacles of facility soil and vegetable. J. Henan Agric. Sci. 2023, 52, 1–10. [Google Scholar]
- Li, Y.H.; Zhang, F.; Yang, X.K.; Yang, C.; Lei, Z.J.; Gao, F.; Wang, Y.Y.; Li, X.D. Effects of continuous cropping on agronomic traits and physiological characteristics of peanut and its regulation under plastic mulching. J. Peanut Sci. 2012, 41, 16–20. [Google Scholar]
- Yan, W.; Wan, T.; Wang, Z. Effects of continuous cropping on seed germination and seedling growth, physiological characters of alfalfa. Legume Res. Int. J. 2022, 45, 1434–1439. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, B.; Wang, C.; Zheng, Y.; Liu, J.; Cheng, D.; Gao, X. Effect of continuous cropping on peanut seedling physiological characteristics and pod yield. J. Peanut Sci. 2006, 35, 29–33. [Google Scholar]
- Su, Y.; Yang, Z.; Cai, J.; Li, G.; Qu, S. The effect of continuous cropping on the germination and seedling formation of alfalfa. Heilongjiang Anim. Sci. Vet. 2018, 21, 167–171. [Google Scholar]
- Ruan, W.B.; Li, X.M.; Wang, Y.F.; Wang, J.G.; Zhang, F.S. Study of soybean (Glycine max L.) root growth in monocultural conditions with root-splitting equipment. Soybean Sci. 2001, 20, 183–186. [Google Scholar]
- Zhao, Q.; Chen, L.; Dong, K.; Dong, Y.; Xiao, J.X. Cinnamic acid inhibited growth of faba bean and promoted the incidence of fusarium wilt. Plants 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.Q.; Wang, S.B.; Jiang, S.Q.; Cheng, B.; Li, H.; Chu, C.J.; Wang, C.B. Dffert of continuous cropping on photosynthesis and accumulation of dry matter in peanut. J. Peanut Sci. 2007, 36, 35–37. [Google Scholar]
- Qiao, Y.F.; Han, X.Z. Effects of different continuous cropping patterns on biological nitrogen fixation of soybean. J. Anhui Agric. Sci. 2008, 36, 13588–13589. [Google Scholar]
- Bekuzarova, S.A.; Kozyrev, A.K.; Shabanova, I.A.; Lushenko, G.V.; Weissfeld, L.I. Enhancing of nitrogen fixation by legumes. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2020; p. 02006. [Google Scholar]
- O’Hara, G. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 2001, 41, 417–433. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ma, S.Y.; Ma, L.; Yang, N.; Wang, N.; Zhang, W.X.; Lian, R.F.; Li, S.; Chai, Q. Effects of rhizobium inculation on the growth and photosynthetic characteristics of pea plants. Acta Agrestia Sin. 2021, 29, 1234–1241. [Google Scholar]
- Jani, A.D.; Motis, T.N.; Longfellow, J.M.; Lingbeek, B.J.; Aiuto, C.J. Continuous cropping legumes in semi-arid southern africa: Legume productivity and soil health implications. Exp. Agric. 2022, 58, e15. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Yang, N.; Ma, S.; Ma, L.; Zhang, X.; Wang, N.; Li, S.; Chai, Q. Physiological response of continuously cropped pea seedlings to inoculation with compound rhizobia preparations. Acta Agrestia Sin. 2020, 29, 144–152. [Google Scholar]
- Wang, N.; Ma, S.Y.; Ma, L.; Yang, N.; Zhang, X.H.; Zhang, W.X. Allelopathy effects of cinnamic acid and palmitic acid on seed germination and seedling growth of pea. Plant Physiol. J. 2021, 57, 1657–1667. [Google Scholar]
- Hang, C.M. Effect of vanillic acid on vigna sesquipedatis wight seed germination and anti-oxidant enzyme activity. Seed 2013, 32, 65–66. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of plants to water stress: A Meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Yaning, C.; Sushkova, S.; Chapligin, V.A.; Mandzhieva, S. A review on salinity adaptation mechanism and characteristics of populus euphratica, a boon for arid ecosystems. Acta Ecologica Sin. 2016, 36, 497–503. [Google Scholar] [CrossRef]
- Herbette, S.; de Labrouhe, D.T.; Drevet, J.R.; Roeckel, P. Transgenic tomatoes showing higher glutathione peroxydase antioxidant activity are more resistant to an abiotic stress but more susceptible to biotic stresses. Plant Sci. 2011, 180, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bloch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2020, 11, 552969. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Li, C.; Yu, G. Modulatory role of reactive oxygen species in root development in model plant of arabidopsis thaliana. Front. Plant Sci. 2020, 11, 485932. [Google Scholar] [CrossRef] [PubMed]
- Somal, M.K.; Sachan, R.S.K.; Bhagat, D.; Khusbhoo; Bala, R.; Kumar, M. An Update on Reactive Oxygen Species Synthesis and Its Potential Application; Springer Nature Singapore: Singapore, 2023; pp. 1–15. [Google Scholar]
- Janku, M.; Luhova, L.; Petrivalsky, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Morales, M.; Munne, B.S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, K.; Abdelly, C.; Savoure, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Villamor, J.G.; Lin, W.; Sharma, S.; Verslues, P.E. Proline Coordination with fatty acid synthesis and redox metabolism of chloroplast and mitochondria. Plant Physiol. 2016, 172, 1074–1088. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Front. Agric. China 2011, 5, 1–14. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.G. Effect of exogenous proline on SOD and POD activity for soybean callus under salt stress. Acta Agric. Boreali-Sin. 2002, 17, 37–40. [Google Scholar]
- Banu, N.A.; Hoque, A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2020, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Stewart, J.J.; Baker, C.R.; Adams, W.W. Optimization of photosynthetic productivity in contrasting environments by regulons controlling plant form and function. Int. J. Mol. Sci. 2018, 19, 872. [Google Scholar] [CrossRef] [PubMed]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A. Modulation of the functional components of growth, photosynthesis, and anti-oxidant stress markers in cadmium exposed Brassica juncea L. Plants 2019, 8, 260. [Google Scholar] [CrossRef]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Rossi, M.L.; Martinelli, A.P.; Peres, L.E.P.; Medici, L.O.; Lea, P.J.; Azevedo, R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 2009, 67, 387–394. [Google Scholar] [CrossRef]
- Patterson, D.T. Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max). Weed Sci. 2017, 29, 53–59. [Google Scholar] [CrossRef]
- Zheng, X.X.; Zhu, Y.; Liang, H.B.; Zhuan, J.P.; Shi, J.Q.; Wang, X.F. Cloning and functional analysis of uroporphyrinogen Ⅲ synthase gene bnHemd from brassica napus. Chin. J. Oil Crop Sci. 2020, 42, 380–389. [Google Scholar]
- Rudiger, W.; Benz, J.; Guthoff, C. Detection and partial characterization of activity of chlorophyll synthetase in etioplast membranes. Eur. J. Biochem. 1980, 109, 193–200. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hortensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell. 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, S.S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; Hortensteiner, S.; Gidoni, D.; Gal-On, A.; Goldschmidt, E.E.; et al. Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell. 2007, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Grimm, B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Figueiredo, P.; Duarte, I.F.; Gil, A.M.; Santos, C. Different responses of young and expanded lettuce leaves to fungicide mancozeb: Chlorophyll fluorescence, lipid peroxidation, pigments and proline content. Photosynthetica 2014, 52, 148–151. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, G.W.; Hu, W.Z.; Xu, S.C.; Gun, Y.M. Effects of cinnamic acid on growth and chlorophyll fluorescence parameters of Pisum sativum L. seedlings. China Veg. 2013, 2013, 44–49. [Google Scholar]
- Du, C.Y.; Li, D.M.; Pang, Q.G. Study on the effect of successive planting soybean to nutrient chlorophyll photosynthetic efficiency and photosynthesis of soybean plants. Soybean Sci. 2003, 22, 146–150. [Google Scholar]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research progress in improving photosynthetic efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Liszkay, A.K. Redox and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Tang, T.; Zhang, D.P.; Cheng, Y.; Lu, B. Recent advances in auxin–cytokinin interactions involved in shaping architecture of rice root system. Plant Physiol. J. 2020, 56, 2495–2509. [Google Scholar]
- Zaynaba, M.; Fatimab, M.; Abbasc, S.; Sharifd, Y.; Umairc, M.; Zafare, M.H.; Bahadarf, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A. Crosstalk among Phytohormone Signaling Pathways during Abiotic Stress; National Institute ofPlant Genome Research: NewDelhi, India, 2019. [Google Scholar]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Dreher, K.A.; Brown, J.; Saw, R.E.; Callisa, J. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell. 2006, 18, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef]
- Li, X.; Jousset, A.; Boer, W.; Carrion, V.J.; Zhang, T.; Wang, X.; Kuramae, E.E. Legacy of land use history determines reprogramming of plant physiology by soil microbiome. ISME J. 2019, 13, 738–751. [Google Scholar] [CrossRef]
- Yang1, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohnishi, A.; Sasaki, D.; Fujii, S.; Iwase, A.; Sugimoto, K.; Masuda, T.; Wada, H. Shoot removal induces chloroplast development in roots via cytokinin signaling. Plant Physiol. 2017, 173, 2340–2355. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.; Huang, L.; O’Neil, R.C.; Shokhirev, M.N.; Ecker, J.R. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat. Commun. 2018, 9, 1604. [Google Scholar] [CrossRef]
- Yan, Z.W.; Liu, X.; Ljung, K.; Li, S.; Zhao, W.; Yang, F.; Wang, M.; Tao, Y. Type B response regulators act as central integrators in transcriptional control of the auxin biosynthesis enzyme TAA1. Plant Physiol. 2017, 175, 1438–1454. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.J.; Cheng, Z.J.; Sang, Y.L.; Zhang, M.M.; Rong, X.F.; Wang, Z.W.; Tang, Y.Y.; Zhang, X.S. Type-b Arabidopsis response regulators specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017, 29, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Zhang, S.W.; Cheng, J.J.; Wang, X.C. Functional analysis of the ARR21 gene in Arabidopsis thaliana during growth and development and in vitro regeneration. J. Shanxi Agric. Sci. 2021, 49, 689–693. [Google Scholar]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.L.; Feng, L.Z.; Dong, Y.B.; Li, Y.L. Progress on ethylene signal transduction pathway and related genes in plants. Curr. Biotechnol. 2019, 9, 449–454. [Google Scholar]
- Ma, L.; Ma, S.; Chen, G.; Lu, X.; Wei, R.; Xu, L.; Feng, X.; Yang, X.; Chai, Q.; Zhang, X.; et al. New insights into the occurrence of continuous cropping obstacles in pea (Pisum sativum L.) from soil bacterial communities, root metabolism and gene transcription. BMC Plant Biol. 2023, 23, 226. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Tian, H.; Wang, S.; Chen, J.G. The small ethylene response factor ERF96 is involved in the regulation of the abscisic acid response in Arabidopsis. Front. Plant Sci. 2015, 6, 1064. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasoo, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Amornsiripanitch, N.; Dong, X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006, 2, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Scott, A.; BowLing, A.; Gordon, S.; Dong, X.N. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 1994, 6, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Dong, X.N. Posttranslational modifications of NPR1: A single protein playing multiple roles in plant immunity and physiology. PLoS Pathog. 2016, 12, e1005707. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Withers, J.; Mohan, R.; Marques, J.; Gu, Y.; Yan, S.; Zavaliev, R.; Nomoto, M.; Tada, Y.; Dong, X. Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 2015, 18, 169–182. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Fan, W.H.; Kinkema, M.; Li, X.; Dong, X.N. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6523–6528. [Google Scholar] [CrossRef]
- Zhou, J.M.; Trifa, Y.; Klessig, D.F.; Silva, H.; Pontier, D.; Lam, E.; Shah, J.; Klessig, D.F. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol. Plant Microbe Interact. 2000, 33, 191–202. [Google Scholar] [CrossRef]
- Kesarwani, M.; Yoo, J.; Dong, X. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol. 2007, 144, 336–346. [Google Scholar] [CrossRef]
- Yadav, V.; Mallappa, C.; Gangappa, S.N.; Bhatia, S.; Chattopadhyay, S. A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell 2005, 17, 1953–1966. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Francini, A.; Khan, M.I.R.; Ferrante, A. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Ludwig, A.A.; Saitoh, H.; Felix, G.; Freymark, G.; Miersch, O.; Wasternack, C.; Boller, T.; Jones, J.D.G.; Romeis, T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Acad. Natl. Sci. USA 2005, 102, 10736–10741. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Howell, S.H. Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J. 2000, 24, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Kroymann, J.; Ballhorn, D.J.; Kautz, S.; Heil, M.; Hegeman, A.D. Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defence in nature. PLoS ONE 2009, 4, e5450. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Hidalgo, S.C.; Bonawitz, N.D.; Franke, R.B.; Chapple, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.K.; Higashi, Y.; Nakabayashi, R. The qrigin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Scherbakov, A.V.; Ivanov, V.B.; Ivanova, A.V.; Usmanov, I.Y. The equifinal achievement of the total antioxidant activity of flavonoids by plants in various habitats. IOP Conf. Ser. Earth Environ. Sci. 2021, 670, 012018. [Google Scholar] [CrossRef]
- Dong, W.; Song, Y. The significance of flavonoids in the process of biological nitrogen fixation. Int. J. Mol. Sci. 2020, 21, 5926. [Google Scholar] [CrossRef]
- Bosse, M.A.; Silva, M.B.D.; Oliveira, N.; Araujo, M.A.; Rodrigues, C.; Azevedo, J.P.; Reis, A.R.D. Physiological impact of flavonoids on nodulation and ureide metabolism in legume plants. Plant Physiol. Biochem. 2021, 166, 512–521. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, D.I.L.; Lima, R.B.; Ferrarese, M.d.L.L.; Bubna, G.A.; Ferrarese-Filho, O. Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ. Exp. Bot. 2009, 66, 25–30. [Google Scholar] [CrossRef]
- Lima, R.; Salvador, V.; Santos, W.; Bubna, G.; Finger-Teixeira, A.; Soares, A.; Marchiosi, R.; Ferrarese, M.L.; Ferrarese, F.O. Enhanced lignin monomer production caused by cinnamic acid and its hydroxylated derivatives inhibits soybean root growth. PLoS ONE 2013, 8, e80542. [Google Scholar] [CrossRef] [PubMed]
- Bubna, G.A.; Lima, R.B.; Zanardo, D.Y.L.; Santos, W.D.; Ferrarese, M.d.L.L.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.D.; Ferrarese, M.L.; Finger, A.; Teixeira, A.C.N.; Ferrarese-Filho, O. Lignification and related enzymes in glycine max root growth-inhibition by ferulic acid. J. Chem. Ecol. 2014, 30, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Bohm, P.A.F.; Zanardo, F.M.L.; Ferrarese, M.L.L.; Ferrarese-Filho, O. Peroxidase activity and lignification in soybean root growth-inhibition by juglone. Biol. Plant. 2006, 50, 315–317. [Google Scholar] [CrossRef]
- Finger, T.A.; Ferrarese, M.d.L.L.; Soares, A.R.; Da Silva, D.; Ferrarese-Filho, O. Cadmium-induced lignification restricts soybean root growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef]
- Rui, H.; Chen, C.; Zhang, X.; Shen, Z.; Zhang, F. Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J. Hazard. Mater. 2016, 301, 304–313. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- She, S.; Niu, J.; Zhang, C.; Xiao, Y.; Chen, W.; Dai, L.; Liu, X.; Yin, H. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 2017, 199, 267–275. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Du, J.F.; Li, Y.; Tang, H.; Yin, Z.Y.; Yang, L.; Ding, X.H. Evolutions and managements of soil microbial community structure drove by continuous cropping. Front. Microbiol. 2022, 13, 839494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Yang, L.; Zheng, Y.L.; Yang, W.W.; Zhang, L.M.; Deng, L.L.; Gao, Q.; Li, J.R.; Mi, Q.; Li, X.M.; et al. Autotoxins in continuous tobacco cropping soils and their management. Front. Plant Sci. 2023, 14, 1106033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Cheng, Y.; Lei, Y.F.; Li, J.S.; Shi, F.; Dou, M.M.; Ma, L.H.; Cheng, X.F. Advances in research on allelopathic autotoxicity effects of medicinal plants. Chin. Tradit. Herb. Drugs 2018, 49, 1946–1956. [Google Scholar]
- Zhang, Z.Z.; Wu, J.H.; Xi, Y.P.; Zhang, L.Z.; Gao, Q.; Wang-Pruski, G. Effects of autotoxicity on seed germination, gas exchange attributes and chlorophyll fluorescence in melon seedlings. J. Plant Growth Regul. 2021, 41, 993–1003. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Liu, T.; Wang, H.; Yang, Y.; Chen, X.; Zhu, S. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmeria nivea L. Gaud) fields in different areas in China. Sci. Rep. UK 2020, 10, 3264. [Google Scholar] [CrossRef]
- Yang, J.I.; Ruegger, P.M.; McKenry, M.V.; Becker, J.O.; Borneman, J. Correlations between root-associated microorganisms and peach replant disease symptoms in a California soil. PLoS ONE 2012, 7, e46420. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Li, T.; Zhao, D.; Liao, Y. Tillage practices with different soil disturbance shape the rhizosphere bacterial community throughout crop growth. Soil Tillage Res. 2020, 197, 104501. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.F.; Zhang, Y.F.; Chen, T.Y.; Wu, P.; Yang, L.; Jian, L.Q.; Zhang, J.S.; Guo, W.; Xue, Y.W.; et al. Effects of tillage methods on soil structure and yield of maize in Sanjiang Plain. Soil Fertolizer Sci. China 2021, 6, 95–103. [Google Scholar]
- Almajmaiea, A.; Hardiea, M.; Acunaa, T.; Birchb, C. Evaluation of methods for determining soil aggregate stability. Soil Tillage Res. 2017, 167, 39–45. [Google Scholar] [CrossRef]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Dong, X.L.; Wang, Y.T.; Tian, L.; Lou, B.Y.; Zhang, X.J.; Liu, T.; Liu, X.J.; Sun, Y.Y. Review of relationships between soil aggregates, microorganisms and soil organic matter in salt-affected soil. Chin. J. Eco-Agric. 2023, 31, 364–372. [Google Scholar]
- Wang, L.; Wu, J.G.; Zhang, Z.Y.; Yang, T.Y. Efferts of soybean continuous cropping on the aggregation and combines forms of humus in black soil. J. Soil Water Conserv. 2014, 28, 238–242. [Google Scholar]
- Karlen, D.L.; Hurley, E.G.; Andrews, S.S.; Cambardella, C.A.; Meek, D.W.; Duffy, M.D.; Mallarino, A.P. Crop rotation effects on soil quality at three northern corn/soybean belt locations. Agron. J. 2006, 98, 484–495. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, Q.; Hao, S.P. Advances of studies on the effect of soil physical properties on soil biological activity and crop growth. J. Henan Agric. Univ. 2002, 36, 33–37. [Google Scholar]
- Yang, J.Y.; Gu, S.Y.; Li, Y.H.; He, W.Y.; Tang, Y.; Zhai, C.; Du, L. Effects of deep ploughing-rotary tillage combined with organic fertilizer on black soil physical properties. Chin. J. Soil Sci. 2021, 52, 1290–1298. [Google Scholar]
- Zhang, X.H.; Ma, S.Y.; Li, S.; Zeng, R.F.; Ma, L.; Yang, N.; Pu, Z.H. Effects of rhizobium inoculation on soil nutrients and enzyme activities of continuous crop pea. Chin. J. Soil Sci. 2022, 53, 1360–1367. [Google Scholar]
- Shi, C.R.; Yu, S.L.; Du, B.H.; Yang, Z.; Chen, N.; Pan, L.J.; Wang, T.; Wang, M.; Chi, X.Y.; Chen, M.N. The characteristics variation of soil physical and chemical properties and its correlation with soil microorganisms under continuous peanut croppiing. J. Peanut Sci. 2018, 4, 1–6. [Google Scholar]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Ma, S.Y.; Chen, G.P.; Wang, N.; Ma, L.; Lian, R.F.; Li, S.; Zhang, X.C. Identification of potential autotoxic substances in pea soil and analysis of their autotoxic effects. Acta Pratacul. Turae Sin. 2023, 32, 134–145. [Google Scholar]
- Fernandez, C.; Monnier, Y.; Ormeño, E.; Baldy, V.; Greff, S.; Pasqualini, V.; Mévy, J.-P.; Bousquet-Mélou, A. Variations in allelochemical composition of leachates of different organs and maturity stages of pinus halepensis. J. Chem. Ecol. 2009, 35, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.Y.; Lu, X.; Chai, Q.; Zhang, X.C.; Li, S. Isolation, identification and biological effects of allelochemicals from the root exudates of pea. Plant Physiol. J. 2018, 54, 500–508. [Google Scholar]
- Yu, J.Q.; Yoshihisa, M. Autointoxication of root exudates in Pisum sativus. Acta Hortic. Sin. 1999, 26, 175–179. [Google Scholar]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, M. Allelopathic and autotoxic effects of medicago sativa-derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef]
- Zhao, R.L.; Cai, L.Q. The determination of root exudates of Medicago sativa and the study on allelopathic effect of typical exudation 3,5-di-tert-butyl-4-hydroxybenzaldehyde. Chin. Agric. Sci. Bull. 2013, 29, 34–41. [Google Scholar]
- Chai, Q.; Huang, G.B.; HUang, P.; Zhang, E.H.; Fen, F.X. Identification of cicer arietinum root exudation and allelopathy of benzaldhyde. Acta Pratacul. Turae Sin. 2005, 14, 106–111. [Google Scholar]
- Ma, F.M.; Wang, A.; Wu, L.; Wang, C.C.; Li, Y.C.; Liu, C.; Ma, G.W.; Shi, X.Y. Identification of soybean root exudates and cloning of the PAL1, PAL2, C4H Genes. Crops 2011, 2, 65–70. [Google Scholar]
- Carlsen, S.C.K.; Kudsk, P.; Laursen, B.; Mathiassen, S.K.; Mortensen, A.G.; Fomsgaarda, I.S. Allelochemicals in rye (Secale cereale L.): Cultivar and tissue differences in the production of benzoxazinoids and phenolic acids. Nat. Prod. Commun. 2009, 4, 199–208. [Google Scholar]
- Gao, L.L.; Guo, P.Y. Research progress on the inhibitory effects of phenolic acid allelochemicals on algae. Technol. Water Treat. 2012, 38, 1–4. [Google Scholar]
- Huang, Y.Q.; Han, X.R.; Yang, J.F.; Liang, C.H.; Zhan, X.M. Autotoxicity of peanut and identification of phytotoxic substances in rhizosphere soil. Allelopath. J. 2013, 31, 297–308. [Google Scholar]
- Huang, X.X.; Bie, Z.L.; Huang, Y. Identification of autotoxins in rhizosphere soils under the continuous cropping of cowpea. Allelopath. J. 2010, 25, 383–392. [Google Scholar]
- Chen, L.; Zhang, M.L.; Xin, M.Y.; Li, J.D. Effects of exogenous phenolic acids on allelopathy of potted soybean seedlings. ACS Agric. Sci. Technol. 2015, 15, 1151–1154. [Google Scholar]
- Li, Q.K.; Liu, P.; Tang, C.H.; Zhao, H.J.; Wang, J.T.; Song, X.Z.; Yang, L.; Wang, S.B. Effects of two phenolic acids on root zone soil nutrients, soil enzyme activities and pod yield of peanut. Chin. J. Appl. Ecol. 2016, 27, 1189–1195. [Google Scholar]
- Cheng, L.; Zhao, Q.; Dong, K.; Zhi, X.Y.; Dong, Y. Physiological mechanism of faba bean Fusarium wilt promoted by benzoic acid and cinnamic acid. J. Plant Prot. 2019, 46, 298–304. [Google Scholar]
- Yuan, T.T.; Dong, K.; Guo, Z.P.; Dong, Y. Allelopathic effects of ferulic acid inducing fusarium wilt occurrence and abnormal root tissue structure of faba bean. J. Plant Nutr. Fertil. 2020, 26, 914–923. [Google Scholar]
- Blum, U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Blair, A.C.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. A lack of evidence for an ecological role of the putative allelochemical (+/−)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006, 32, 2327–2331. [Google Scholar] [CrossRef]
- Perry, L.G.; Thelen, G.C.; Ridenour, W.M.; Callaway, R.M.; Paschke, M.W.; Vivanco, J.M. Concentrations of the allelochemical (+/−)-catechin in Centaurea maculosa soils. J. Chem. Ecol. 2007, 33, 2337–2344. [Google Scholar] [CrossRef]
- Wan, Z.M.; Song, C.C. Advance on response of soil enzyme activity to ecological environment. Chin. J. Soil Sci. 2009, 40, 951–956. [Google Scholar]
- Liu, G.M.; Zhang, X.C.; Wang, X.P.; Shao, H.B.; Yang, J.S.; Wang, X.P. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Z.; Zou, Y.; Wang, S.; Han, L. Effect of soybean continuous cropping on soil enzyme activity. J. Plant Nutr. Fertil. 1996, 2, 4. [Google Scholar]
- Li, Z.; Jiang, L.G.; Tang, R.H.; Guo, W.F. Effects of long-term continuous peanut cropping on dry matter weight of different peanut varieties, soil nutrient contents and enzyme activities. Soils 2018, 50, 491–497. [Google Scholar]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Han, X.; Zou, W. Effect of spring soybean long-term monoculture on soil nematode community structure and food web. Soybean Sci. 2017, 36, 8. [Google Scholar]
- Cheng, H. Microbial Communities in the Rhizoplane and Rhizosphere as Affected by Soybean Cultivar and Cropping System; Agricultural University: Beijing, China, 2005. [Google Scholar]
- Li, X.G.; Ding, C.F.; Liu, J.G.; Zhang, T.L.; Wang, X.X. Evident response of the soil nematode community to consecutive peanut monoculturing. Agron. J. 2015, 107, 195–203. [Google Scholar] [CrossRef]
- Masamune, T.; Anetai, M.; Takasugi, M.; Katsui, N. Isolation of a natural hatching stimulus, glycinoeclepin A, for the soybean cyst nematode. Nature 1982, 297, 495–496. [Google Scholar] [CrossRef]
- Halbrendt, J. Allelopathy in the management of plant-parasitic nematodes. J. Nematol. 1996, 28, 8. [Google Scholar]
- Bulgarelli, D.; Garrido-Oter, R.; Munch, P.C.; Weiman, A.; Droge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Heijden, M.G.; Roussely, V.P.; Walser, J.C.; Schlaeppi, K. Deciphering composition and function of the root microbiome of a legume plant. Microbiome 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. Response of soil fungal community structure to long-term continuous soybean cropping. Front. Microbiol. 2018, 9, 3316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Z.; Jia, H.; Li, L.; Wu, F. Continuously monocropped Jerusalem artichoke changed soil bacterial community composition and ammonia-oxidizing and denitrifying bacteria abundances. Front. Microbiol. 2018, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, N. Research progress on formation mechanism of plant continuous cropping disorder. Sci. J. Technol. 2022, 4, 8–13. [Google Scholar]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fert. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, C.; Ma, J.; Yu, M.; Li, Y.; Wang, G.; Zhang, L. Prokaryotic diversity in continuous cropping and rotational cropping soybean soil. FEMS Microbiol. Lett. 2009, 298, 267–273. [Google Scholar] [CrossRef]
- Pan, F.; Han, X.; Zou, W.; Wang, C.; Zhang, Z.; Liu, H.; Xu, Y. Shifts of bacterial community structure and function in long-term soybean monoculture. Arch. Agron. Soil Sci. 2020, 67, 793–808. [Google Scholar] [CrossRef]
- Alves, R.J.E.; Minh, B.Q.; Urich, T.; Haeseler, A.; Schleper, C. Unifying the global phylogeny and environmental distribution of ammonia-oxidising archaea based on amoA genes. Nat. Commun. 2018, 9, 1517. [Google Scholar] [CrossRef]
- Gong, G.L.; Zhang, T.; Shi, Z.H.; Wei, X.M.; Wang, L. To clone the ammonia monooxygenase gene of autotrophic bacteria ammonium oxidation capacity. J. Shanxi Univ. Sci. Technol. 2015, 33, 125–129. [Google Scholar]
- Liu, Z.X.; Liu, J.J.; Yu, Z.H.; Yao, Q.; Li, Y.S.; Liang, A.Z.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.B.; et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Zhou, Y.J.; JIa, X.; Zhao, Y.H.; Lu, Y.; Tian, G.; Liu, L. A review on soil fungal community and its affecting factors in forest ecosystem. Ecol. Environ. Sci. 2020, 29, 1703–1712. [Google Scholar]
- Liu, J.; Yao, Q.; Li, Y.; Zhang, W.; Mi, G.; Chen, X.; Yu, Z.; Wang, G. Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a mollisol of northeast PR China. Land Degrad. Dev. 2019, 30, 1725–1738. [Google Scholar] [CrossRef]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Fang, X.L. Study of spatial distribution of alpine meadow in hezuo cit. Chin. J. Grassl. 2021, 43, 106–114. [Google Scholar]
- Chen, M.N.; Zhang, J.C.; Liu, H.; Wang, M.; Pan, L.J.; Chen, N.; Wang, T.; Jing, Y.; Chi, X.; Du, B. Long-term continuously monocropped peanut significantly disturbed the balance of soil fungal communities. J. Microbiol. 2020, 58, 563–573. [Google Scholar] [CrossRef]
- Li, C.G.; Li, X.M.; Kong, W.D.; Wu, Y.; Wang, J.G. Effect of monoculture soybean on soil microbial community in the northeast China. Plant Soil. 2009, 330, 423–433. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, C.H.; Song, X.; Liu, Y.; Gao, Q.X.; Zheng, R.; Li, J.T.; Zhang, P.C.; Liu, X.L. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. UK 2022, 12, 2758. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J. Effects of continuous soybean monoculture on soil nematode community. J. Plant Nutr. Fertil. 2015, 21, 1022–1031. [Google Scholar]
- Yao, Q.; Xu, Y.; Liu, X.; Liu, J.; Huang, X.; Yang, W.; Yang, Z.; Lan, L.; Zhou, J.; Wang, G. Dynamics of soil properties and fungal community structure in continuous-cropped alfalfa fields in northeast China. PeerJ 2019, 7, e7127. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant-microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Ding, C.; Hua, K.; Zhang, T.F.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.; Huang, M.; Li, F.; Si, T.; Wang, Y.; Yu, X.; Zhang, X.; Wang, H.; Shi, P. Rotational strip intercropping of maize and peanut enhances productivity by improving crop photosynthetic production and optimizing soil nutrients and bacterial communities. Field Crops Res. 2023, 291, 108770. [Google Scholar] [CrossRef]
- Knight, J.D. Frequency of field pea in rotations impacts biological nitrogen fixation. Can. J. Plant Sci. 2012, 92, 1005–1011. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Q.Q.; Zhang, K.Z.; Sha, D.J.; Jiang, C.J.; Yang, X.; Liu, X.B.; Zhang, H.; Wang, X.G.; Guo, F.; et al. Maize-peanut rotational strip intercropping improves peanut growth and soil properties by optimizing microbial community diversity. PeerJ 2022, 10, e13777. [Google Scholar] [CrossRef]
- Moussart, A.; Even, M.N.; Lesné, A.; Tivoli, B. Successive legumes tested in a greenhouse crop rotation experiment modify the inoculum potential of soils naturally infested by Aphanomyces euteiches. Plant Pathol. 2013, 62, 545–551. [Google Scholar] [CrossRef]
- Jie, G.S.; Wei, G.D.; Chao, M.M.; Wei, Z.; Jun, L.; Long, S.D. Effects of fertilization on bacterial community under the condition of continuous soybean monoculture in black soil in northeast China. Sci. Agric. Sin. 2017, 50, 1271–1281. [Google Scholar]
- Xue, Q.X. Effects of crop stubbles and fertilization on the percentage of seeds damaged by pest of continuous cropping soybean. Chin. Agric. Sci. Bull. 2012, 28, 131–137. [Google Scholar]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility-A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Yue, S. Effects of heavy metals on microorganisms and enzymes in soils of lead–zinc tailing ponds. Environ. Res. 2022, 207, 112174. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ma, Y.; An, X.; Kan, L.; Xie, C.; Mei, X.; Wang, Z.; Xu, Y.; Dong, C. Effects on the root morphology and mircostructure of young pear (Pyrus pyrifolia) tree by split-root supply of bioorganic and chemical fertilizer. Rhizosphere 2022, 22, 100504. [Google Scholar] [CrossRef]
- Liu, S.X.; Li, X.R.; Ding, F.H.; Wu, Q.Q. Effects of organic fertilizerson yield and quality of cowpeas and avilable nutrients in soil under continuous cropping cultication. Fujian J. Agric. Sci. 2016, 31, 728–732. [Google Scholar]
- Liu, J.G.; Li, X.G.; Wang, X.X. Effects of successive application of organic fertilizers on rhizosphere microbial populations and enzyme activities of monoculture peanut. Soils 2018, 50, 305–311. [Google Scholar]
- Wang, D.C.; WU, J.G.; Li, J.M. Effect of organic manure on nematodes in rhizosphere soil of soybean under continuous cropping. Acta Pedol. Sin. 2018, 55, 490–502. [Google Scholar]
- Chen, D.L.; Wang, X.X.; Zhang, Y.; Yang, Z.; Yao, X.D.; Li, X.G. Effect of persistent application of bioorganic fertilizer on peanut yield and rhizosphere bacterial community. Soils 2021, 53, 537–544. [Google Scholar]
- Wang, X.B.; Liu, W.X.; Li, Z.G.; Teng, Y.; Christle, P.; Luo, Y.M. Effects of long-term fertilizer applications on peanut yield and quality and on plant and soil heavy metal accumulation. Pedosphere 2017, 17, 60457. [Google Scholar] [CrossRef]
- Ge, H.B.; Liu, Z.F.; Ma, Z.W.; Xu, B.Q.; Zeng, X.H.; Huang, H.Y.; Xiong, Q.Y.; Zou, X.; Hu, Y.; Hu, J.H. Effects of different fungicides on leaf spot disease control and yield of continuous cropping peanut. J. Peanut Sci. 2014, 43, 52–55. [Google Scholar]
- Chen, Y.; Yang, D.Q.; Tang, C.H.; Zhang, J.L.; Wang, J.G. Effects of Different fungicides and spraying times on leaf spot disease and yield of continuous cropping peanut in dryland. Shandong Agric. Sci. 2021, 53, 94–97. [Google Scholar]
- Liu, X.M.; Cheng, X.K.; Wang, H.Y.; Wang, K.Y.; Qiao, K. Effect of fumigation with 1, 3-dichloropropene on soil bacterial communities. Chemosphere 2015, 139, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.H.; Hayes, C.; Harman, G.E. Molecular and cellular biology of biocontrol trichoderma spp. Trends Biotechnol. 1994, 12, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Asante, M.; Ahiabor, B.D.K.; Atakora, W.K. Growth, nodulation, and yield responses of groundnut (Arachis hypogaea L.) as influenced by combined application of rhizobium inoculant and phosphorus in the guinea savanna zone of ghana. Int. J. Agron. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Jie, W.G.; Yao, Y.X.; Guo, N.; Zhang, Y.Z.; Qiao, W. Effects of Rhizophagus intraradices on plant growth and the composition of microbial communities in the roots of continuous cropping soybean at maturity. Sustainability 2021, 13, 6623. [Google Scholar] [CrossRef]
- Wu, J.R.; Xu, F.J.; Cao, W.; Zhang, W.; Guan, Y.X.; Dai, C.C. Fungal endophyte Phomopsis Liquidambari B3 enriches the diversity of nodular culturable endophytic bacteria associated with continuous cropping of peanut. Arch. Agron. Soil Sci. 2018, 65, 240–252. [Google Scholar] [CrossRef]
- Bai, L.; Sun, H.B.; Liang, R.T.; Cai, B.Y. iTRAQ proteomic analysis of continuously cropped soybean root inoculated with Funneliformis mosseae. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).