As a Transitional Host, Weed Solanum nigrum L. Increases the Population Base of Root-Knot Nematode Meloidogyne enterolobii for the Next Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Nematode Extraction
2.2. Morphological Observation
2.3. Molecular Identification
2.3.1. DNA Extraction, PCR Amplification, and Sequencing
2.3.2. Sequence Alignment and Phylogenetic Analysis
2.4. Transitional Host Identification
2.4.1. Development Progress and Histopathological Observation
2.4.2. Host Suitability Assay
2.5. Population Density Survey
3. Results
3.1. Disease Symptoms
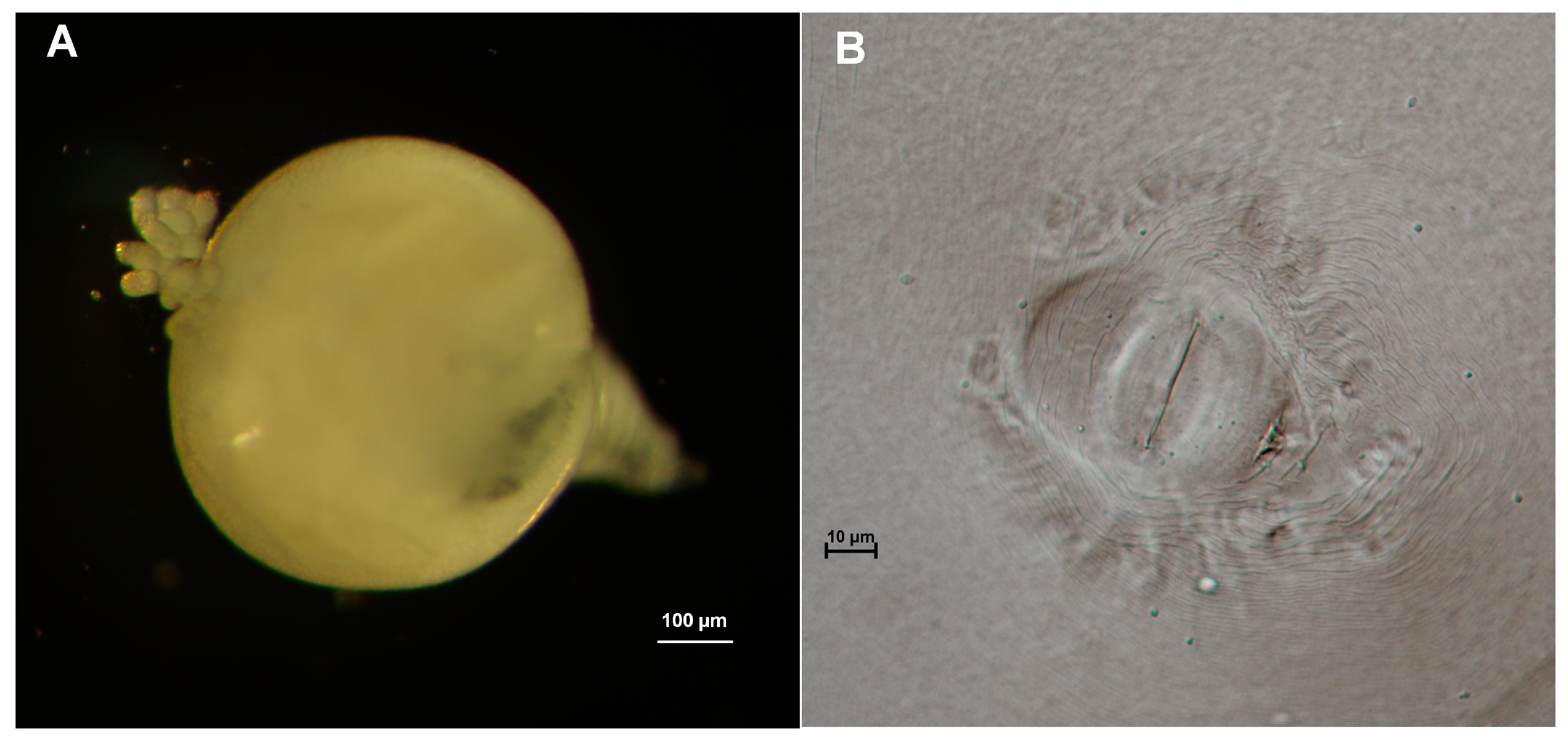
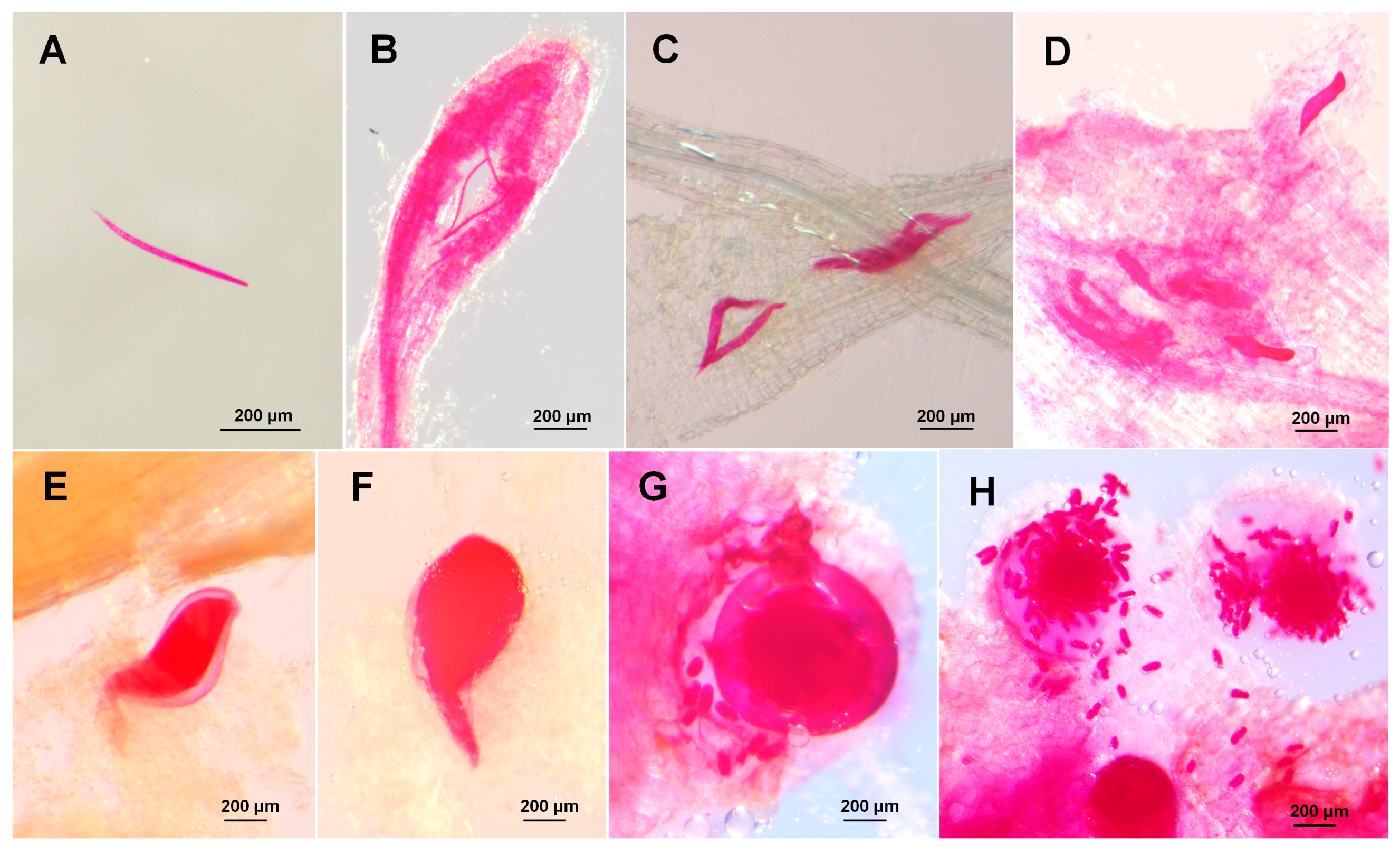
3.2. Morphological Characters of the Nematodes
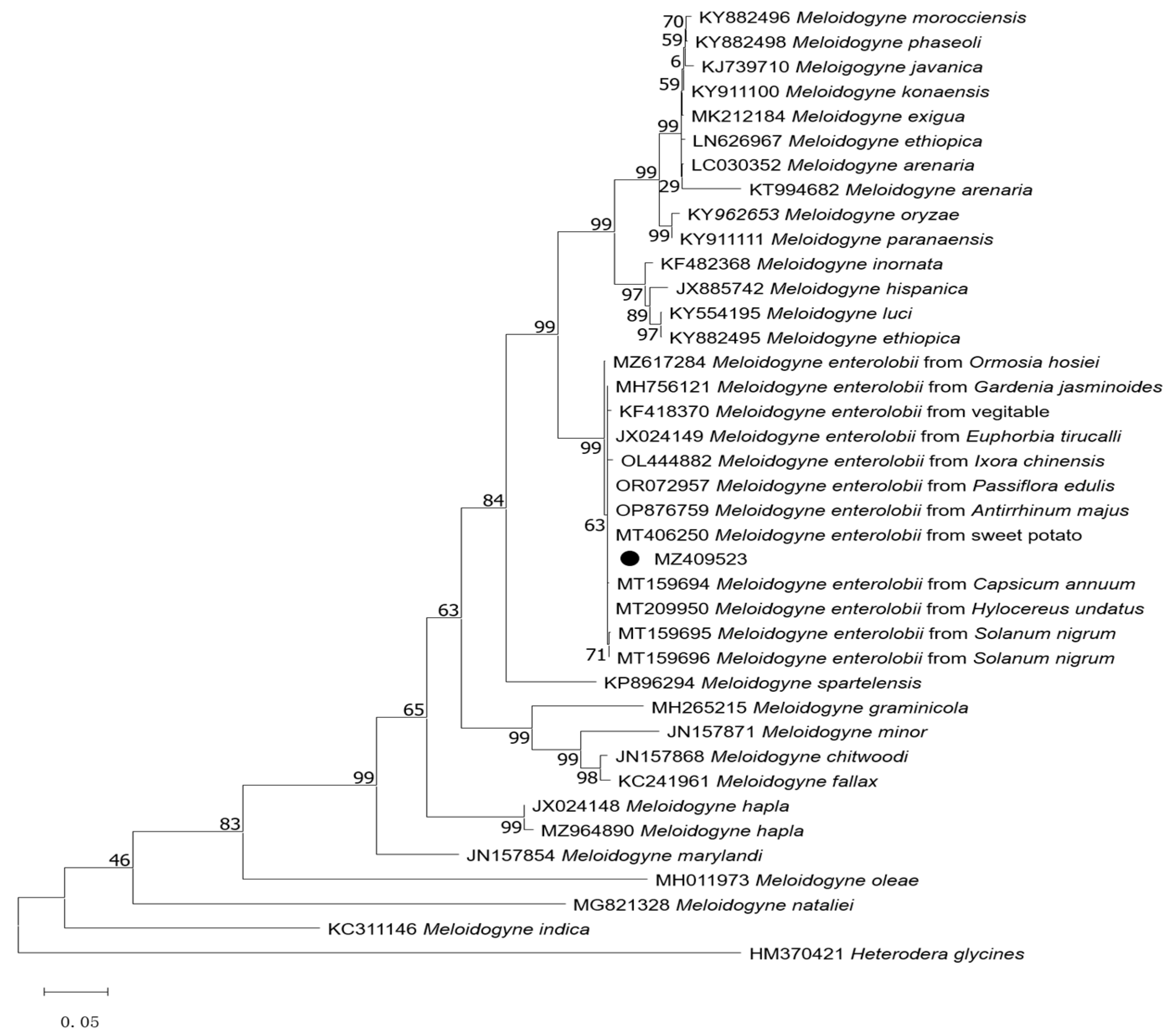
3.3. Molecular Profiles and Phylogenetic Relationship
3.4. Transitional Host Identification
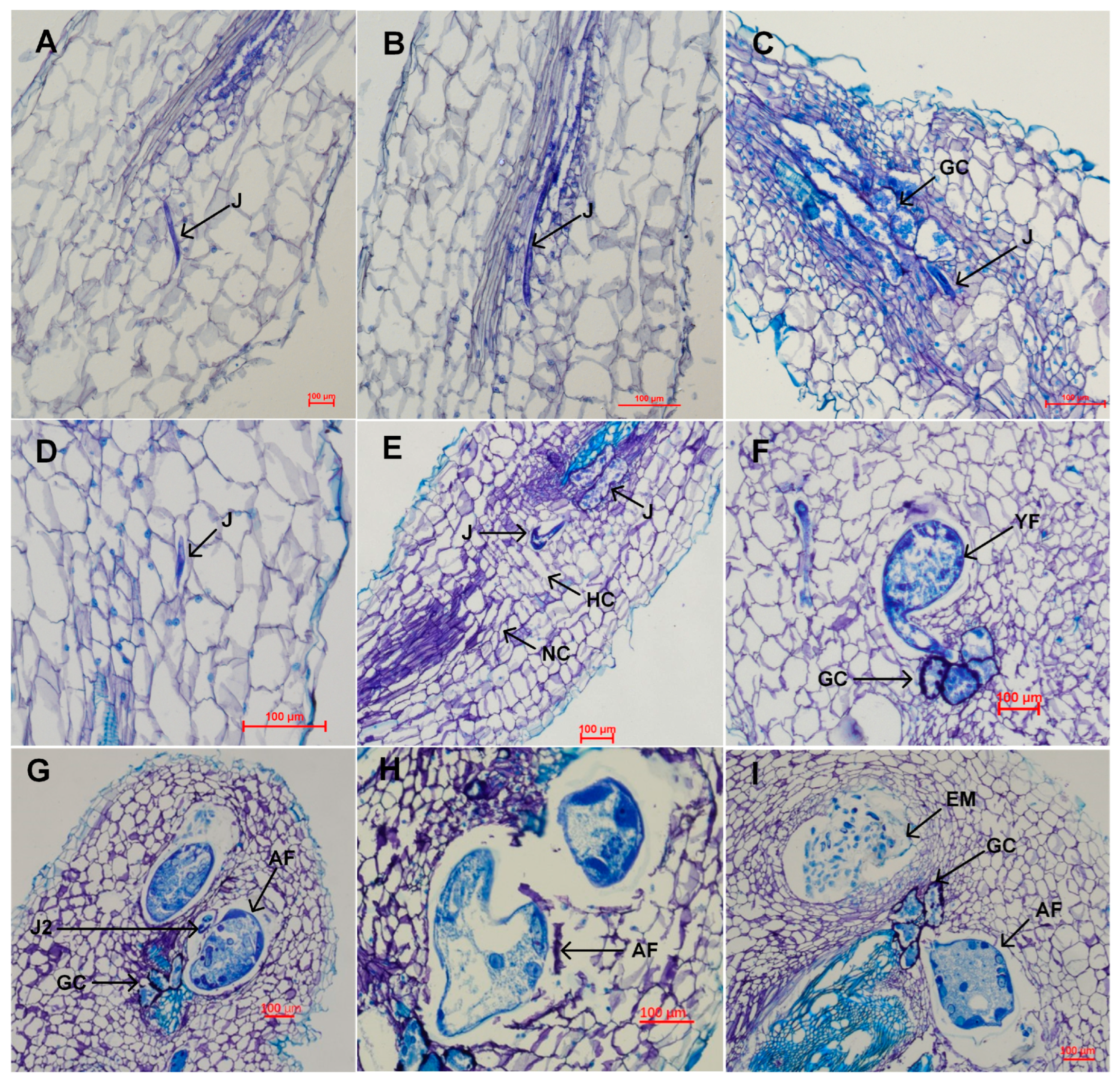
3.4.1. Developmental Progress and Histopathological Observations
3.4.2. Host Suitability of S. nigrum L. for M. enterolobii
3.5. Effect of S. nigrum L. on the Population Densities of M. enterolobii in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a Major Threat to Tomato Production: Current Status and Future Prospects for Its Management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef] [PubMed]
- Castagnone-Sereno, P. Meloidogyne enterolobii (=M. mayaguensis): Profile of an emerging, highly pathogenic, root-knot nematodes species. Nematology 2012, 14, 133–138. [Google Scholar] [CrossRef]
- Yang, B.; Eisenback, J.D. Meloidogyne enterolobii n. sp. (Meloidogynidae), a Root-knot Nematodes Parasitizing Pacara Earpod Tree in China. J. Nematol. 1983, 15, 381–391. [Google Scholar] [PubMed]
- Tian, X.X.; Jiang, B.Z.; Cao, Z.M.; Liu, Z.J.; Ling, P.; Xie, S.Q.; Zhu, J. Identification of Capsicum chinense germplasms resistant to Meloidogyne enterolobii and preliminary analysis on resistance mechanism. Chin. J. Trop. Crops 2022, 43, 165–172. [Google Scholar]
- Li, Z.R.; Long, H.B.; Sun, Y.F.; Pei, Y.L.; Chen, Y.; Feng, T.Z. Occurrence and distribution of the root-knot nematode species in vegetables in Hainan Province. Plant Prot. 2020, 46, 213–216, 245. [Google Scholar]
- Becker, J.O.; Ploeg, A.; Nuñez, J.J. Multi-Year Field Evaluation of Fluorinated Nematicides Against Meloidogyne incognita in Carrots. Plant Dis. 2019, 103, 2392–2396. [Google Scholar] [CrossRef]
- Hunt, J.R.; Browne, C.; McBeath, T.M.; Verburg, K.; Craig, S.; Whitbread, A.M. Summer fallow weed control and residue management impacts on winter crop yield through soil water and N accumulation in a winter-dominant, low rainfall region of southern Australia. Crop Pasture Sci. 2013, 64, 922–934. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Rosskopf, E.N. Susceptibility of Several Common Subtropical Weeds to Meloidogyne arenaria, M. incognita, and M. javanica. J. Nematol. 2012, 44, 142–147. [Google Scholar]
- Chen, J.; Gao, F.; Ma, J.; Yang, D.; Zhang, C.; Tang, W.; Xie, Y.; Sun, H. First report of Meloidogyne enterolobii infecting Solanum nigrum in China. Plant Dis. 2023. [Google Scholar] [CrossRef]
- Pinheiro, J.B.; Silva, G.O.; Biscaia, D.; Macedo, A.G.; Correia, N.M. Reaction of weeds, found in vegetable production areas, to root-knot nematodes Meloidogyne incognita and M. enterolobii. Hortic. Bras. 2019, 37, 445–450. [Google Scholar] [CrossRef]
- Almeida, E.J.; Alves, G.C.S.; Santos, J.M.; Martins, A.B.G. Assinalamentos de Meloidogyne enterolobii em goiabeira e em plantas invasoras no estado de São Paulo, Brasil. Nematol. Bras. 2011, 35, 50–52. [Google Scholar]
- Carneiro, R.G.; Mônaco, A.P.A.; Moritz, M.P.; Nakamura, K.C.; Scherer, A. Identificação de Meloidogyne mayaguensis em goiabeira e em plantas invasoras, em solo argiloso, no Estado do Paraná. Nematol. Bras. 2006, 30, 293–298. [Google Scholar]
- Souza, R.M.; Nogueira, M.S.; Lima, I.M.; Melarato, M.; Dolinski, C. Manejo do nematóide das galhas da goiabeira em São João da Barra (RJ) e relato de novos hospedeiros. Nematol. Bras. 2006, 30, 165–169. [Google Scholar]
- Riekert, H. A modified sodium hypochlorite technique for the extraction of root-knot nematode eggs and larvae from maize root samples. Afr. Plant Prot. 1995, 1, 41–43. [Google Scholar]
- Xie, H. Taxonomy of Plant Nematodes, 1st ed.; Anhui Science and Technology Press: Hefei, China, 2000; pp. 21–29. [Google Scholar]
- Williams, B.D.; Schrank, B.; Huynh, C.; Shownkeen, R.; Waterson, R.H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 1992, 131, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Vrain, T.; Wakarchuk, D.; Laplantelevesque, A.; Hamilton, R.H. Intraspecific rDNA restriction fragment length polymorphisms in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Long, H.; Liu, H.; Xu, J.H. Development of a PCR diagnostic for the root-knot nematodes Meloidogyne enterolobii. Acta Phytopathol. Sin. 2006, 36, 109–115. [Google Scholar]
- Liu, W.Z. Plant Nematology Research Techniques, 1st ed.; Liaoning Science and Technology Publishing House: Shenyang, China, 1995; pp. 71–72. [Google Scholar]
- Li, X.; Zhao, W.; Zhou, X.; Feng, J.; Gao, Y.; Yao, X.; Liu, Y.; Liu, J.; Yang, R.; Zhao, F.; et al. The Use of Toluidine Blue Staining Combined with Paraffin Sectioning and the Optimization of Freeze-thaw Counting Methods for Analysing Root-Knot Nematodes in Tomato. Hortic. Environ. Biotechnol. 2017, 58, 620–626. [Google Scholar] [CrossRef]
- Taylor, A.L.; Sasser, J.N. Biology, Identification, and Control of Root-Knot Nematodes (Meloidogyne Species); North Carolina State University Graphics: Raleigh, NC, USA, 1978; p. 111. [Google Scholar]
- Sasser, J.N.; Carter, C.C.; Hartman, K.M. Standardization of Host Suitability Studies and Reporting of Resistance to Root-Knot Nematodes; North Carolina State University: Raleigh, NC, USA, 1984. [Google Scholar]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A Diverse Group of Novel and Important Plant Parasites. In Root-Knot Nematodes, 1st ed.; Perry, R.N., Moens, M., Starr, J., Eds.; CAB International: Oxfordshire, UK, 2009; pp. 1–13. [Google Scholar]
- Hu, M.X.; Zhuo, K.; Liao, J.L. Multiplex PCR for the simultaneous identification and detection of Meloidogyne incognita, M. enterolobii, and M. javanica using DNA extracted directly from individual galls. Phytopathology 2011, 101, 1270–1277. [Google Scholar] [CrossRef]
- Brito, J.A.; Kaur, R.; Cetintas, R.; Stanley, J.D.; Mendes, M.L.; Powers, T.O.; Dickson, D.W. Meloidogyne spp. infecting ornamental plants in Florida. Nematropica 2010, 40, 87–103. [Google Scholar]
- Freitas, V.M.; Silva, J.G.P.; Gomes, C.B.; Castro, J.M.C.; Correa, V.R.; Carneiro, R.M.D.G. Host status of selected cultivated fruit crops to Meloidogyne enterolobii. Eur. J. Plant Pathol. 2017, 148, 307–319. [Google Scholar] [CrossRef]
- Long, H.B.; Bai, C.; Peng, J.; Zeng, F.Y. First Report of the Root-Knot Nematodes Meloidogyne enterolobii Infecting Jujube in China. Plant Dis. 2014, 98, 1451. [Google Scholar] [CrossRef] [PubMed]
- Long, H.B.; Sun, Y.F.; Bai, C.; Guo, J.R.; Zeng, F.Y. Identification of the Root Knot Nematodes Meloidogyne enterolobii in Hainan Province. Chin. J. Tropic. Agric. 2015, 36, 371–376. [Google Scholar]
- Rodriguez, M.G.; Sanchez, L.; Rowe, J. Host status of agriculturally important plant families to the root-knot nematodes Meloidogyne mayaguensis in Cuba. Nematropica 2003, 33, 125–130. [Google Scholar]
- Zhang, P.; Shao, H.D.; You, C.P.; Feng, Y.; Xie, Z.W. Characterization of root-knot nematodes infecting mulberry in Southern China. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Kaspary, T.E.; Júnior, I.T.S.; Ramos, R.F.; Bellé, C. Host status of morning-glory (Ipomoea spp.) to Meloidogyne species. J. Nematol. 2021, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.J.; de Oliveira, G.H.; Pastoriza, R.J.; Maranhão, E.H.; Pedrosa, E.M.; Maranhão, S.R.; Boiteux, L.S.; Pinheiro, J.B.; de Carvalho Filho, J.L.S. Search for sources of resistance to Meloidogyne enterolobii in commercial and wild tomatoes. Hortic. Bras. 2019, 37, 188–198. [Google Scholar] [CrossRef]
- Marques, M.L.S.; Chadud, J.V.G.; Oliveira, M.F.; Nascimento, A.R.; Rocha, M.R. Identification of Chili Pepper Genotypes (Capsicum spp.) Resistant to Meloidogyne enterolobii. J. Agric. Sci. 2019, 11, 165–175. [Google Scholar] [CrossRef]
- Gao, Z.W.; Xue, M.J.; Zhou, S.F.; Li, H.; Wu, W.T.; Wang, Y. Pathogenicity of Meloidogyne enterolobii on the resistant tomato variety VFNT. Acta Phytopathol. Sin. 2022, 52, 191–202. [Google Scholar]
- Marques, M.L.S.; Oliveira, M.F.; Pereira, P.S.; Rocha, M.R. Penetration and development of Meloidogyne enterolobii in resistant and susceptible Capsicum spp. Eur. J. Hortic. Sci. 2020, 85, 86–91. [Google Scholar] [CrossRef]
- Freitas, V.M.; Correa, V.R.; Motta, F.C.; Sousa, M.G.; Gomes, A.C.M.M.; Carneiro, M.D.G.; Silva, D.B.; Mattos, J.K.; Nicole, M.; Carneiro, R.M.D.G. Resistant accessions of wild Psidium spp. to Meloidogyne enterolobii and histological characterization of resistance. Plant Pathol. 2014, 63, 738–746. [Google Scholar] [CrossRef]
- Das, S.; DeMason, D.A.; Ehlers, J.D.; Close, T.J.; Roberts, P.A. Histological characterization of root-knot nematodes resistance in cowpea and its relation to reactive oxygen species modulation. J. Exp. Bot. 2008, 59, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Chen, Y.P.; Liu, Q.; Jian, H. Research progresses in occurrence, diagnoses, pathogenic mechanisms and integrated management of vegetable root-knot nematodes in China. J. Plant Prot. 2022, 49, 424–443. [Google Scholar]
- Mônaco, A.P.A.; Carneiro, R.G.; Kranz, W.M.; Gomes, J.C.; Scherer, A.; Santiago, D.C. Reação de espécies de plantas daninhas a Meloidogyne incognita raças 1 e 3, a M. javanica e a M. paranaensis. Nematol. Bras. 2009, 33, 235–242. [Google Scholar]
- Razalia, F.N.; Sinniaha, S.K.; Hussinb, H.; Abidina, N.Z.; Shuib, A.S. Tumor suppression effect of Solanum nigrum polysaccharide fractionon Breast cancer via immunomodulation. Int. J. Biol. Macromol. 2016, 92, 185–193. [Google Scholar] [CrossRef]
- Long, H.B.; Sun, Y.F.; Zeng, F.Y.; Bai, C. Effect of Initial population density of Meloidogyne enterolobii on Cucumber Growth in Greenhouse. Chin. J. Tropic. Agric. 2015, 35, 61–64. [Google Scholar]


| Character | Range | Mean |
|---|---|---|
| Eggs/g a | 22,637.64–37,802.14 | 28,235.92 ± 6585.66 |
| GI b | 4.00–5.00 | 4.70 ± 0.48 |
| EMI c | 4.00–5.00 | 4.30 ± 0.48 |
| Fecundity | 352.00–454.00 | 409.00 ± 35.00 |
| RF dz | 31.76–69.21 | 48.04 ± 14.71 (good host) |
| Year | Treatment | Population Density (J2/cm3) z | |
|---|---|---|---|
| Pre-Plant | Post-Plant | ||
| 2022 | Planting S. nigrum L. | 0.48 ± 0.25 b | 1.33 ± 0.16 a |
| Unplanting S. nigrum L. | 0.43 ± 0.11 b | 0.49 ± 0.30 b | |
| 2023 | Planting S. nigrum L. | 0.53 ± 0.31 b | 1.56 ± 0.43 a |
| Unplanting S. nigrum L. | 0.44 ± 0.25 b | 0.58 ± 0.16 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Sun, Y.; Chen, Y.; Feng, T.; Che, H.; Long, H. As a Transitional Host, Weed Solanum nigrum L. Increases the Population Base of Root-Knot Nematode Meloidogyne enterolobii for the Next Season. Agronomy 2024, 14, 129. https://doi.org/10.3390/agronomy14010129
Pei Y, Sun Y, Chen Y, Feng T, Che H, Long H. As a Transitional Host, Weed Solanum nigrum L. Increases the Population Base of Root-Knot Nematode Meloidogyne enterolobii for the Next Season. Agronomy. 2024; 14(1):129. https://doi.org/10.3390/agronomy14010129
Chicago/Turabian StylePei, Yueling, Yanfang Sun, Yuan Chen, Tuizi Feng, Haiyan Che, and Haibo Long. 2024. "As a Transitional Host, Weed Solanum nigrum L. Increases the Population Base of Root-Knot Nematode Meloidogyne enterolobii for the Next Season" Agronomy 14, no. 1: 129. https://doi.org/10.3390/agronomy14010129
APA StylePei, Y., Sun, Y., Chen, Y., Feng, T., Che, H., & Long, H. (2024). As a Transitional Host, Weed Solanum nigrum L. Increases the Population Base of Root-Knot Nematode Meloidogyne enterolobii for the Next Season. Agronomy, 14(1), 129. https://doi.org/10.3390/agronomy14010129






