Genetic Improvements in the Root Traits and Fertilizer Tolerance of Soybean Varieties Released during Different Decades
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Varieties and Experimental Design
2.2. Measurements of RTs
2.3. Statistical Analysis
3. Results
3.1. Analysis of Variance
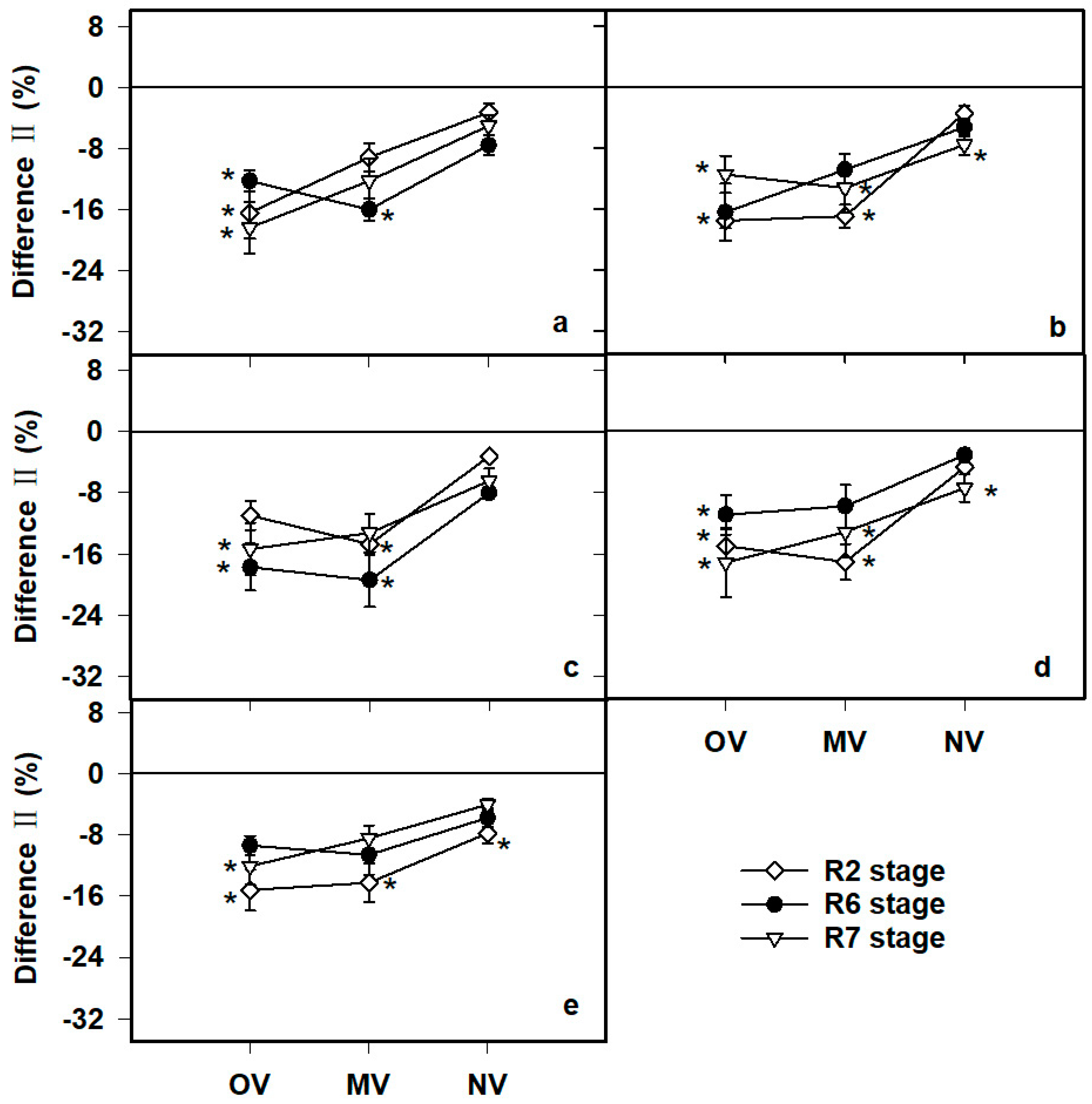
3.2. Genetic Improvement in RTs
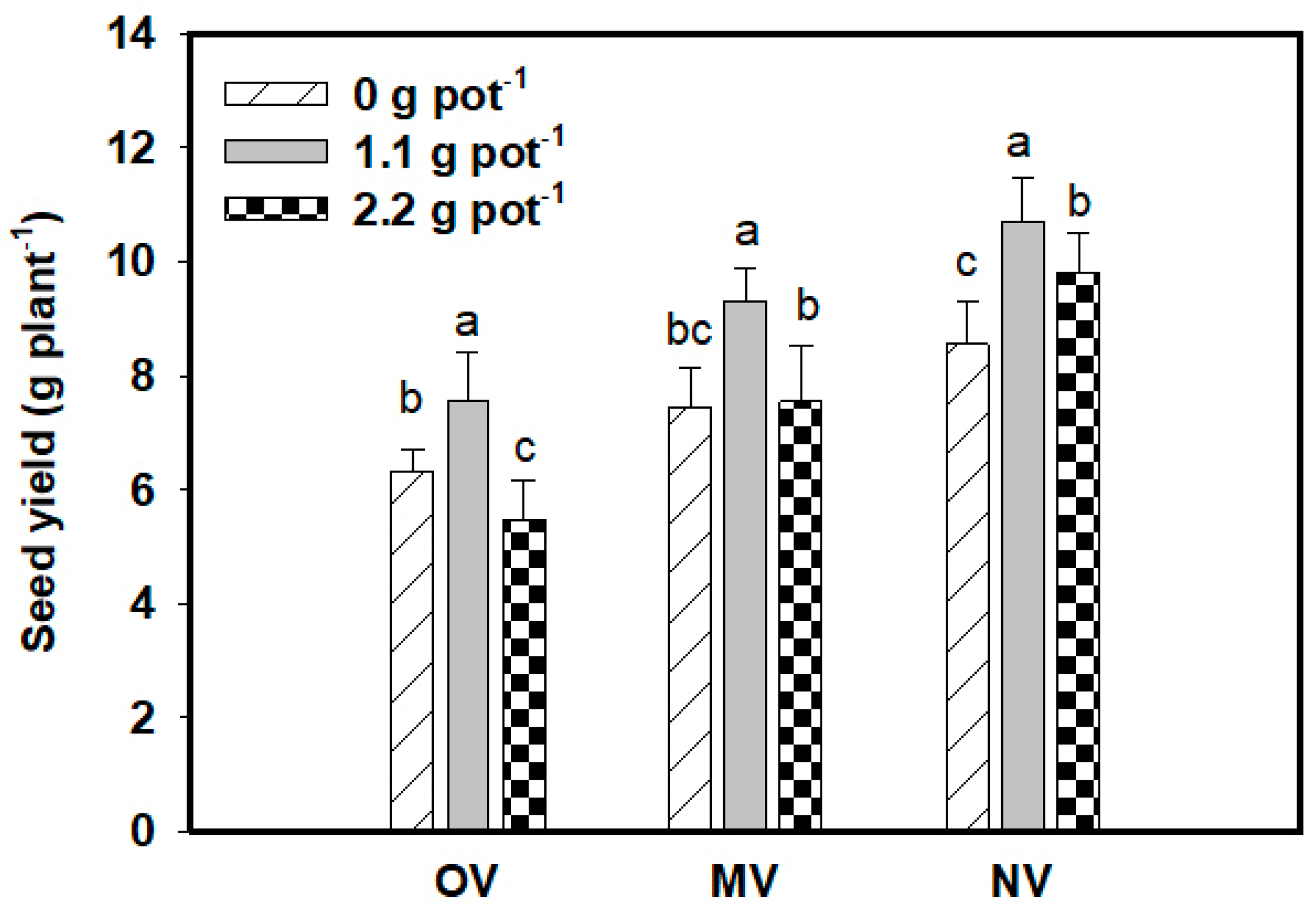
3.3. Effects of N Fertilizer on RTs
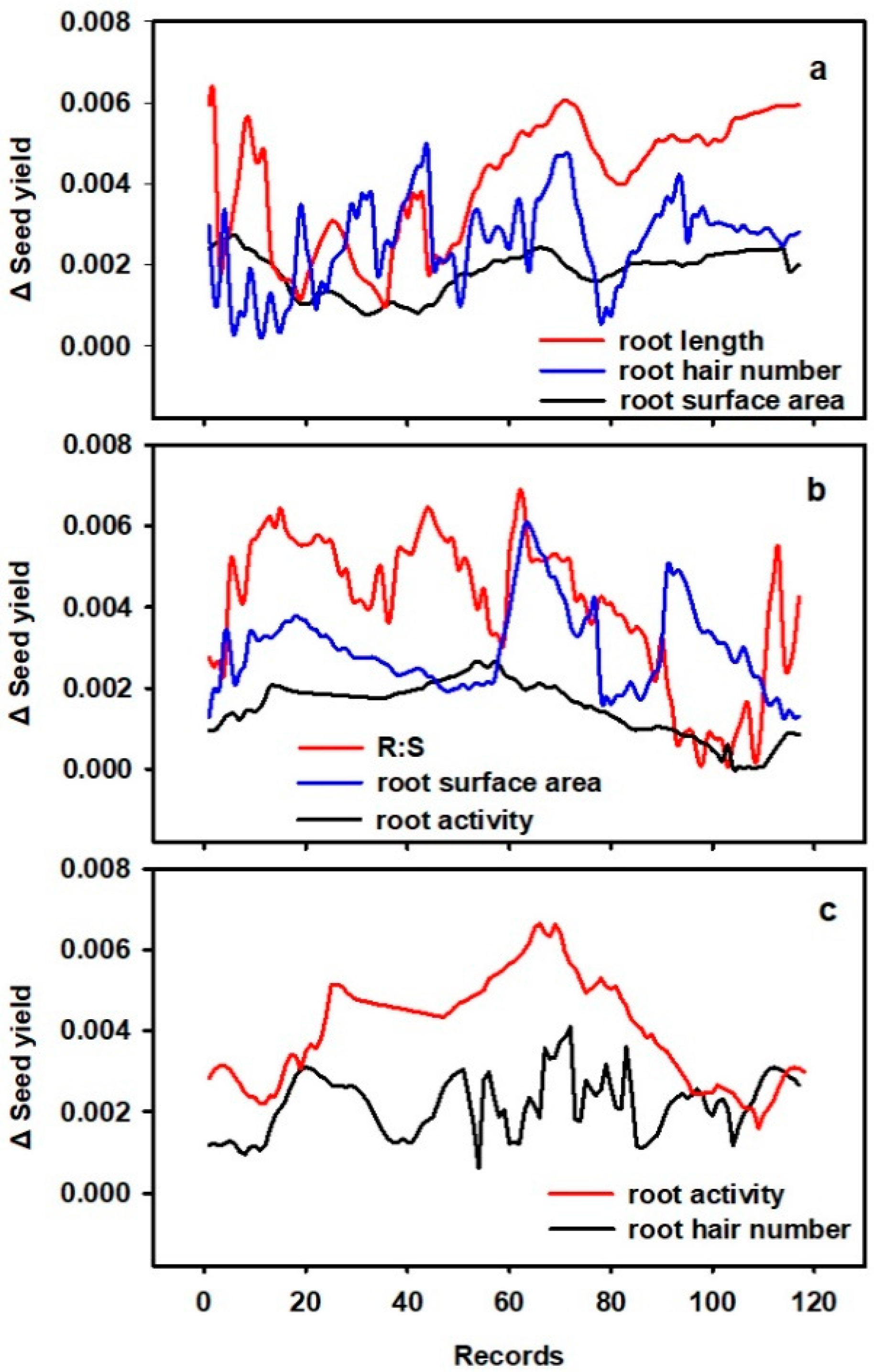
3.4. Relationship between Seed Yield and RTs
4. Discussion
4.1. Changes in RTs
4.2. Root Traits and Fertilization
4.3. R:S
4.4. RTs and Seed Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vogel, J.T.; Liu, W.; Olhoft, P.; Crafts-Brandner, S.J.; Pennycooke, J.C.; Christiansen, N. Soybean Yield Formation Physiology—A Foundation for Precision Breeding Based Improvement. Front. Plant Sci. 2021, 12, 719706. [Google Scholar] [CrossRef] [PubMed]
- Rincker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, T.; Cianzio, S.R.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic Improvement of U.S. Soybean in Maturity Groups II, III, and IV. Crop Sci. 2014, 54, 1419–1432. [Google Scholar] [CrossRef]
- Kahlon, C.S.; Board, J.E.; Kang, M.S. An Analysis of Yield Component Changes for New vs. Old Soybean Cultivars. Agron. J. 2011, 103, 13–22. [Google Scholar] [CrossRef]
- Qin, X.; Feng, F.; Li, D.; Herbert, S.J.; Liao, Y.; Siddique, K.H.M. Changes in yield and agronomic traits of soybean cultivars released in China in the last 60 years. Crop Pasture Sci. 2017, 68, 973–984. [Google Scholar] [CrossRef]
- Todeschini, M.H.; Milioli, A.S.; Rosa, A.C.; Dallacorte, L.V.; Panho, M.C.; Marchese, J.A.; Benin, G. Soybean genetic progress in South Brazil: Physiological, phenological and agronomic traits. Euphytica 2019, 215, 124. [Google Scholar] [CrossRef]
- Kumudini, S.; Hume, D.J.; Chu, G. Genetic Improvement in Short Season Soybeans: I. Dry Matter Accumulation, Partitioning, and Leaf Area Duration. Crop Sci. 2001, 41, 391–398. [Google Scholar] [CrossRef]
- Jin, J.; Liu, X.; Wang, G.; Mi, L.; Shen, Z.; Chen, X.; Herbert, S.J. Agronomic and physiological contributions to the yield improvement of soybean cultivars released from 1950 to 2006 in Northeast China. Field Crops Res. 2010, 115, 116–123. [Google Scholar] [CrossRef]
- Bao, X.; Li, Z.; Yao, X. Changes in photosynthetic traits and their responses to increasing fertilization rates in soybean (Glycine max (L.) Merr.) during decades of genetic improvement. J. Sci. Food Agric. 2021, 101, 4715–4723. [Google Scholar] [CrossRef]
- Koester, R.P.; Skoneczka, J.A.; Cary, T.R.; Diers, B.W.; Ainsworth, E.A. Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J. Exp. Bot. 2014, 65, 3311–3321. [Google Scholar] [CrossRef]
- Keep, N.; Schapaugh, W.; Prasad, P.; Boyer, J. Changes in Physiological Traits in Soybean with Breeding Advancements. Crop Sci. 2016, 56, 122–131. [Google Scholar] [CrossRef]
- Somerville, C.; Briscoe, J. Genetic Engineering and Water. Science 2001, 292, 2217. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Ordóñez, R.A.; Archontoulis, S.V.; Martinez-Feria, R.; Hatfield, J.L.; Wright, E.E.; Castellano, M.J. Root to shoot and carbon to nitrogen ratios of maize and soybean crops in the US Midwest. Eur. J. Agron. 2020, 120, 126130. [Google Scholar] [CrossRef]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, J.P.; Foyer, C.H. Field Phenotyping of Soybean Roots for Drought Stress Tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhong, W.; Zhang, G. Evolution of root characters of soybean varieties of different ages. Sci. Agric. Sin. 2001, 34, 292–295, (In Chinese with English abstract). [Google Scholar]
- Jin, J.; Wang, G.; Liu, X.; Mi, L.; Li, Y.; Xu, Y.; Herbert, S.J. Genetic improvement of yield shapes the temporal and spatial root morphology of soybean (Glycine max) grown in north-east China. N. Z. J. Crop Hortic. Sci. 2010, 38, 177–188. [Google Scholar] [CrossRef]
- Cui, X.; Dong, Y.; Gi, P.; Wang, H.; Xu, K.; Zhang, Z. Relationship between root vigour, photosynthesis and biomass in soybean cultivars during 87 years of genetic improvement in the northern China. Photosynthetica 2016, 54, 81–86. [Google Scholar] [CrossRef]
- Caliskan, S.; Ozkaya, I.; Caliskan, M.; Arslan, M. The effects of nitrogen and iron fertilization on growth, yield and fertilizer use efficiency of soybean in a Mediterranean-type soil. Field Crops Res. 2008, 108, 126–132. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.; Specht, J.; Walters, D.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Wang, S.Q.; Han, X.Z.; Qiao, Y.F.; Yan, J.; Xiao-Hui, L.I. Root morphology and nitrogen accumulation in soybean (Glycine max L.) under different nitrogen application levels: Root morphology and nitrogen accumulation in soybean (Glycine max L.) under different nitrogen application levels. Chin. J. Eco-Agric. 2009, 17, 1069–1073, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Gai, Z.; Zhang, J.; Li, C. Effects of starter nitrogen fertilizer on soybean root activity, leaf photosynthesis and grain yield. PLoS ONE 2017, 12, e0174841. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of Nitrate on Nodule and Root Growth of Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Delfim, J.; Dameto, L.S.; Moraes, L.A.C.; Moreira, A. Nitrogen and Nickel Foliar Application on Grain yield, Yield Components, and Quality of Soybean. Commun. Soil Sci. Plant Anal. 2018, 53, 1226–1234. [Google Scholar] [CrossRef]
- McCoy, J.M.; Kaur, G.; Golden, B.R.; Orlowski, J.M.; Cook, D.R.; Bond, J.A.; Cox, M.S. Nitrogen Fertilization of Soybean Affects Root Growth and Nodulation on Two Soil Types in Mississippi. Commun. Soil Sci. Plant Anal. 2018, 49, 181–187. [Google Scholar] [CrossRef]
- Passioura, J.B. Roots and Drought Resistance. Dev. Agric. Manag. For. Ecol. 1983, 12, 265–280. [Google Scholar]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Iowa State University: Ames, IA, USA, 1977. [Google Scholar]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Noulas, C.; Liedgens, M.; Stamp, P.; Alexiou, I.; Herrera, J.M. Subsoil root growth of field grown spring wheat genotypes (Triticum aestivum L.) differing in nitrogen use efficiency parameters. J. Plant Nutr. 2010, 33, 1887–1903. [Google Scholar] [CrossRef]
- Tian, P.Z. Ecotypes of root system in soybean cultivars. Acta Agron. Sin. 1984, 10, 173–177, (In Chinese with English abstract). [Google Scholar]
- Robinson, D. Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. Plant Soil 2001, 232, 41–50. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbágy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-B.; Guo, C.; Li, F.-M.; Li, M.; He, J. High Soybean Yield and Drought Adaptation Being Associated with Canopy Architecture, Water Uptake, and Root Traits. Agronomy 2020, 10, 608. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-L.; Wang, T.; Turner, N.C.; Yang, R.-P.; Jin, Y.; Xi, Y.; Zhang, C.; Cui, T.; Fang, X.-W.; et al. Conserved water use improves the yield performance of soybean (Glycine max (L.) Merr.) under drought. Agric. Water Manag. 2017, 179, 236–245. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Laghari, A.H.; Lashari, A.A. Role of Nitrogen for Plant Growth and Development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Wang, X.W.; Yan, C.; Wan, T.; Ma, C.M.; Gong, Z.P.; Dong, S.K. Effects of nitrogen application on yield and photosynthesis in soybean. Crops 2011, 141, 49–52, (In Chinese with English abstract). [Google Scholar]
- Kubar, M.S.; Shar, A.H.; Kubar, K.A.; Rind, N.A.; Ullah, H.; Kalhoro, S.A.; Wang, C.; Feng, M.; Gujar, A.; Sun, H.; et al. Optimizing nitrogen supply promotes biomass, physiological characteristics and yield components of soybean (Glycine max L. Merr.). Saudi J. Biol. Sci. 2021, 28, 6209–6217. [Google Scholar] [CrossRef]
- Sinclair, T. Water and nitrogen limitations in soybean grain production I. Model development. Field Crops Res. 1986, 15, 125–141. [Google Scholar] [CrossRef]
- Bonifas, K.D.; Lindquist, J.L. Effects of Nitrogen Supply on the Root Morphology of Corn and Velvetleaf. J. Plant Nutr. 2009, 32, 1371–1382. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Gibson, A.H.; Harper, J.E. Nitrate Effect on Nodulation of Soybean by Bradyrhizobium japonicum. Crop Sci. 1985, 25, 497–501. [Google Scholar] [CrossRef]
- Hébert, Y.; Guingo, E.; Loudet, O. The Response of Root/Shoot Partitioning and Root Morphology to Light Reduction in Maize Genotypes. Crop Sci. 2001, 41, 363–371. [Google Scholar] [CrossRef]
- Rogers, H.H.; Prior, S.A.; Runion, G.B.; Mitchell, R.J. Root to shoot ratio of crops as influenced by CO2. Plant Soil 1995, 187, 229–248. [Google Scholar] [CrossRef]
- Nleya, T.; Sexton, P.; Gustafson, K.; Miller, J.M. Soybean growth stages. In IGrow Soybean: Best Management Practices for Soybean Production; Clay, D.E., Carlson, C.G., Clay, S.A., Wagner, L., Deneke, D., Hay, C., Eds.; South Dakota State University, SDSU Extension: Brookings, SD, USA, 2013. [Google Scholar]
- Cooper, R.L. Influence of Early Lodging on Yield of Soybean [Glycine max (L.) Merr.]. Agron. J. 1971, 63, 449–450. [Google Scholar] [CrossRef]
- Woods, S.J.; Swearingin, M.L. Influence of Simulated Early Lodging upon Soybean Seed Yield and its Components1. Agron. J. 1977, 69, 239–242. [Google Scholar] [CrossRef]
- Stoffella, P.J.; Kahn, B.A. Root System Effects on Lodging of Vegetable Crops. HortScience 1986, 21, 960–963. [Google Scholar] [CrossRef]



| Source of Variations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Root Traits | Y | FR | V | D | Y × FR | Y × V | Y × D | FR × V |
| Root length | 612.38 ns | 1352.39 ** | 169.99 ** | 408.09 ** | 55.54 ns | 194.36 ns | 56.73 ns | 111.5 ** |
| Root surface area | 435.68 ns | 663.14 ** | 309.13 * | 159.30 ** | 39.3 ns | 64.29 ns | 13.42 ns | 62.15 * |
| Root hair number | 1008.43 ns | 853.21 ** | 324.18 ** | 1664.35 * | 141.08 ns | 17.5 ns | 74.48 ns | 5.95 ** |
| R:S | 464.3 ns | 647.57 ** | 402.1 ** | 253.15 * | 19.8 ns | 120.1 ns | 29.43 ns | 51.5 ** |
| Root activity | 618.37 ns | 205.31 *** | 1113.3 * | 398.38 * | 69.41 ns | 79.12 ns | 51.39 ns | 184.75 * |
| Seed yield per plant | 1065.22 ns | 282.49 ** | 439.09 ** | / | 57.13 ns | 112.12 ns | / | 37.10 ** |
| FR × D | V × D | Y × FR × V | Y × FR × D | Y × V × D | FR × V × D | Y × FR × V × D | Error | |
| Root length | 12.89 ns | 109.42 * | 198.35 ns | 92.42 * | 108.37 * | 57.37 ** | 90.36 ns | 27.86 |
| Root area | 83.24 * | 57.63 ** | 38.30 ** | 62.38 ns | 106.39 * | 78.43 * | 46.54 * | 20.47 |
| Root hair number | 47.54 ns | 18.45 ns | 174.34 ** | 74.38 ns | 87.53 ns | 55.32 ** | 22.54 ns | 15.24 |
| R:S | 17.54 ** | 197.47 *** | 21.64 ns | 83.73 * | 93.4 * | 28.49 * | 79.21 * | 11.42 |
| Root activity | 30.26 * | 102.02 ns | 15.37 ns | 33.2 ns | 6.44 ns | 84.12 ** | 32.37 ns | 27.6 |
| Seed yield per plant | / | / | 45.64 ns | / | / | / | / | 16.73 |
| Root Traits | FR | |||||
|---|---|---|---|---|---|---|
| 0 (g pot−1) | 1.1 (g pot−1) | 2.2 (g pot−1) | ||||
| Regression Equation | R2 | Regression Equation | R2 | Regression Equation | R2 | |
| Root length (cm) | y = 250.65x − 485,127 | 0.80 ** | y = 241.95x − 465,234.6 | 0.76 ** | y = 261.35x − 435,009.19 | 0.71 ** |
| Root surface area (cm2) | y = 23.05x − 43,542.27 | 0.75 ** | y = 21.52x − 40,241.34 | 0.70 ** | y = 27.54x − 52,422.23 | 0.69 ** |
| Root hair number | y = 0.0548 exp (0.0066x) | 0.65 ** | y = 183.43x − 335,951.43 | 0.67 ** | y = 0.0049 exp (0.0078x) | 0.87 ** |
| R:S | y = 0.0016x − 2.9432 | 0.78 ** | y = 10−8 exp 0.0083x | 0.69 ** | y = 0.0026x − 4.9768 | 0.76 ** |
| Root activity (μg TTF·g−1 FW·h−1) | y = 0.7589x − 1434.3 | 0.67 ** | y = 10−3 exp 0.0132x | 0.75 ** | y = 1.6165x − 3139.5 | 0.85 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Yao, X. Genetic Improvements in the Root Traits and Fertilizer Tolerance of Soybean Varieties Released during Different Decades. Agronomy 2024, 14, 2. https://doi.org/10.3390/agronomy14010002
Bao X, Yao X. Genetic Improvements in the Root Traits and Fertilizer Tolerance of Soybean Varieties Released during Different Decades. Agronomy. 2024; 14(1):2. https://doi.org/10.3390/agronomy14010002
Chicago/Turabian StyleBao, Xueyan, and Xingdong Yao. 2024. "Genetic Improvements in the Root Traits and Fertilizer Tolerance of Soybean Varieties Released during Different Decades" Agronomy 14, no. 1: 2. https://doi.org/10.3390/agronomy14010002




