Phosphorus HotSpots in Crop Plants Production on the Farm—Mitigating Critical Factors
Abstract
:1. Introduction
2. Scientific Concept—Research Materials
3. Mechanisms of Phosphorus Uptake
- (1)
- Passive—flow of Pi anions in the soil solution towards the plant root surface;
- (2)
- Active—transfer of the Pi ions across the plasmalemma of the cortical root cell.
- (1)
- Pi concentration in the soil solution;
- (2)
- Root absorption surface;
- (3)
- Transport rate of Pi ions from the soil solution to the root surface;
- (4)
- The rate of Pi ions’ incorporation into the plant’s metabolic processes.
4. Response Strategies of Crop Plants to Pi Deficiency
5. Environmental Factors Controlling Phosphorus Uptake by Crop Plants
5.1. Temperature
5.2. Water—Drought
- (1)
- Cells: (i) water photolysis—water acts as a nutrient; (ii) a component of spatial structures of organic compounds, including proteins, carbohydrates, and fats; (iii) a component of the osmotic sap of cells;
- (2)
- Tissues and organs: (i) connects cells to form tissues and then organs; (ii) determines the turgor of conducting cells (xylem); (iii) a critical component of assimilates transported in the phloem;
- (3)
- Plant: controls (i) the optimum level of temperature through transpiration of water and thus the rate of metabolic processes, (ii) the movement of stomata during the circadian cycle, and (iii) CO2 uptake from the atmosphere.
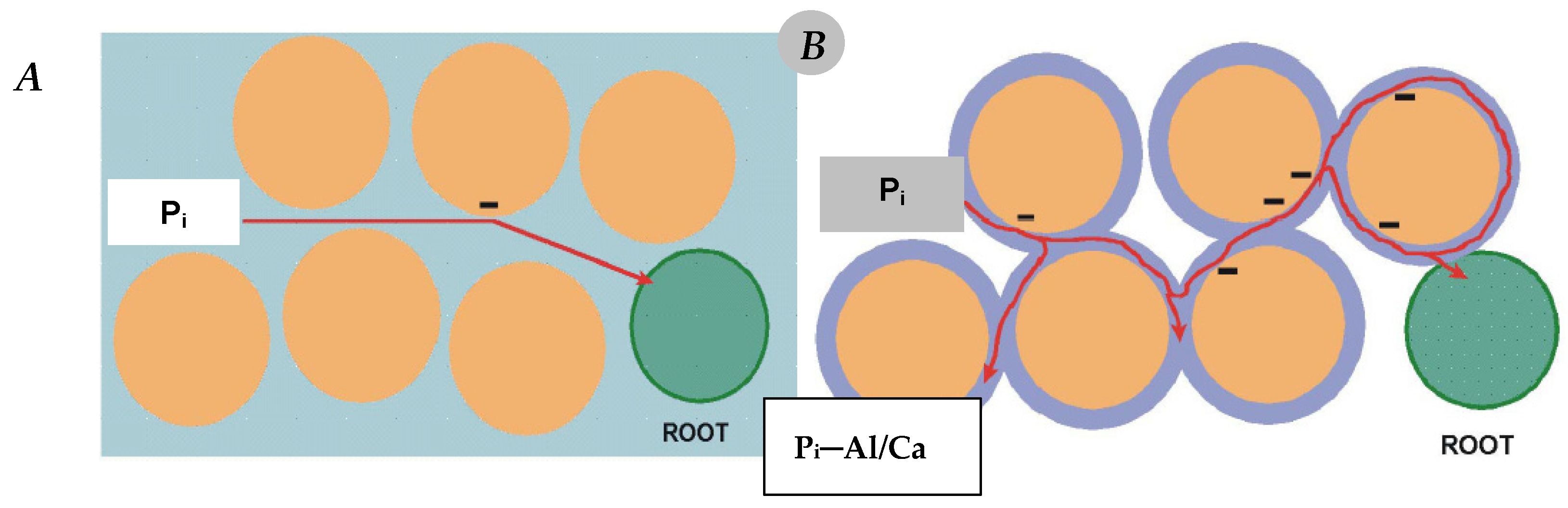
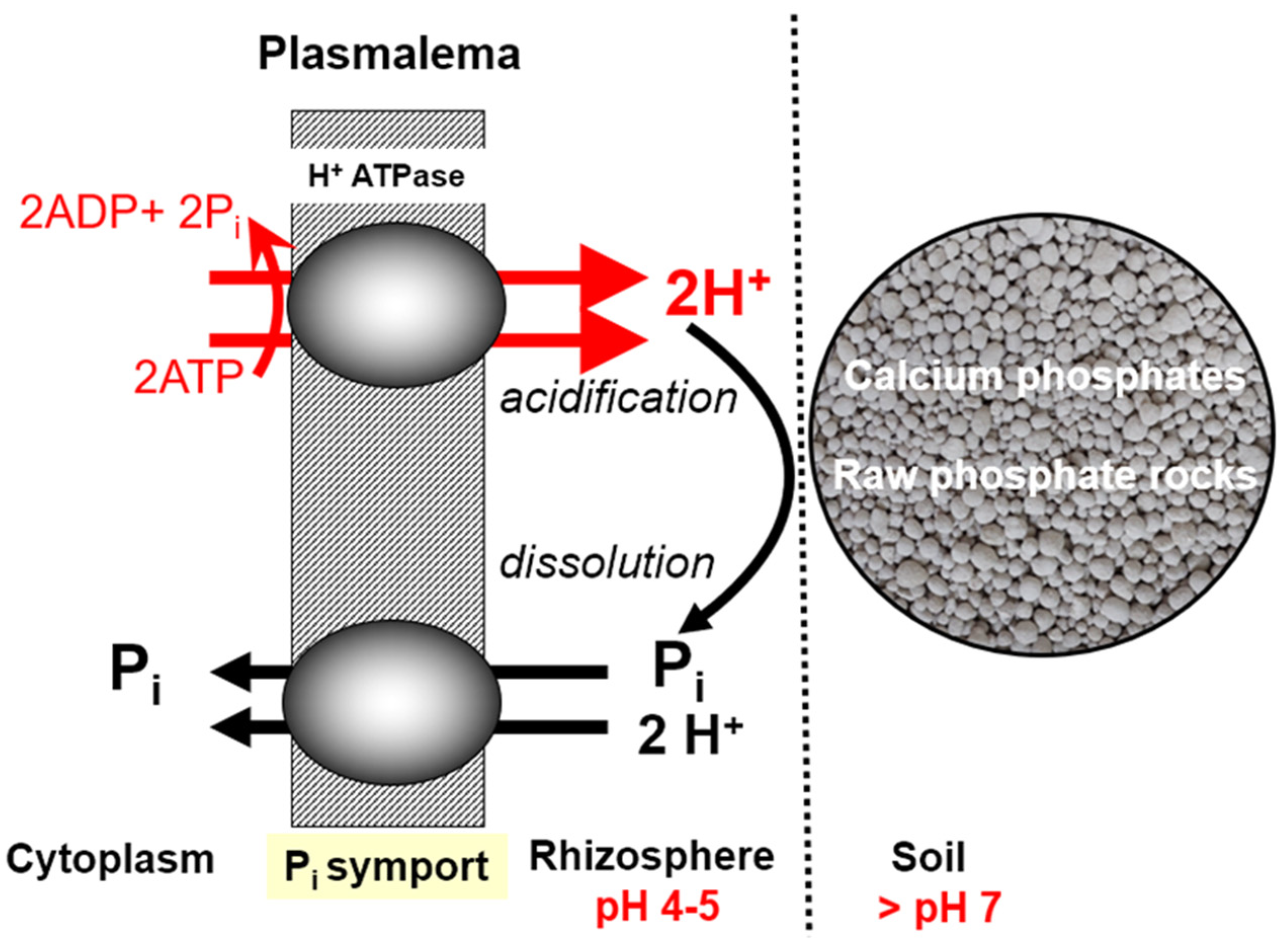
6. Soil Factors Controlling Phosphorus Uptake by Crop Plants
6.1. Soil Acidity—Disturbances in P Uptake
- (1)
- Strong impact on the growth dynamics and the size of the root system of the cultivated plant. A drop in soil pH below 5.0 (1 M KCl extraction solution) leads to its drastic reduction, which ultimately results in a loss of yield [110].
- (2)
- a.
- adsorption by aluminum oxides,
- b.
- fixation by carbonates,
- c.
- forming highly inaccessible–insoluble P forms due to:
- i.
- adsorption on iron oxides,
- ii.
- binding by aluminum and iron cations.
- 1.
- Calcium hydroxide
- 2.
- Calcium carbonate
6.2. Soil Compaction
- (1)
- >30 µm; water content at soil matric potential above −10 kPa. This is the threshold value defining the field capacity (FC). At this level of water content in the soil, only pores (so-called non-capillary pores) are occupied by air. They are a natural route of transporting rainwater in the soil when the pores with a diameter less than 30 µm (so-called capillary pores) are saturated.
- (2)
- 30–0.2 µm; water content at soil matric potential is in the range of −10 kPa –1500 kPa. These pores contain water available to plants.
- (3)
- <0.2 µm; water content at soil matric potential below –1500 kPa is not available to plants.
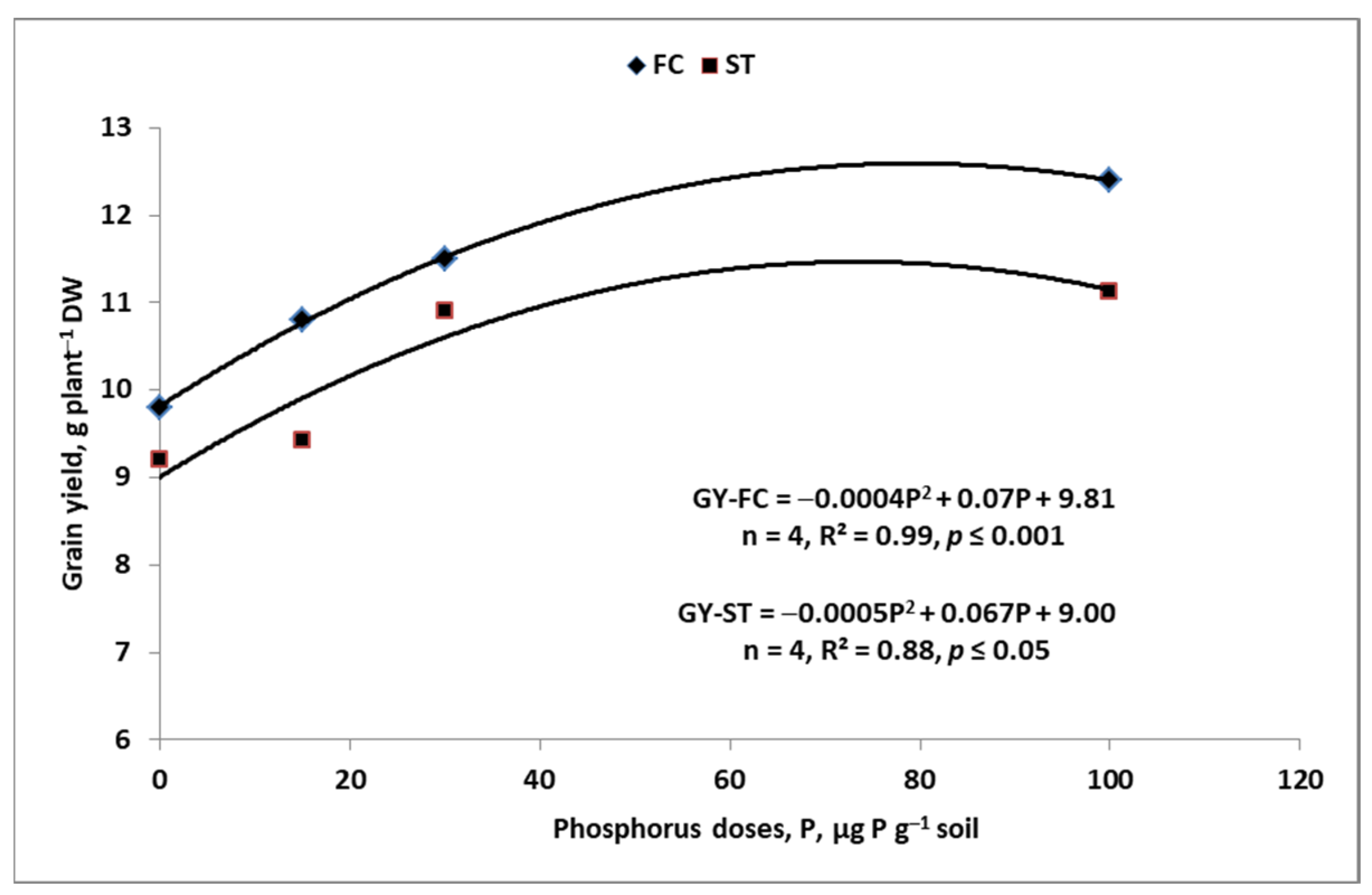
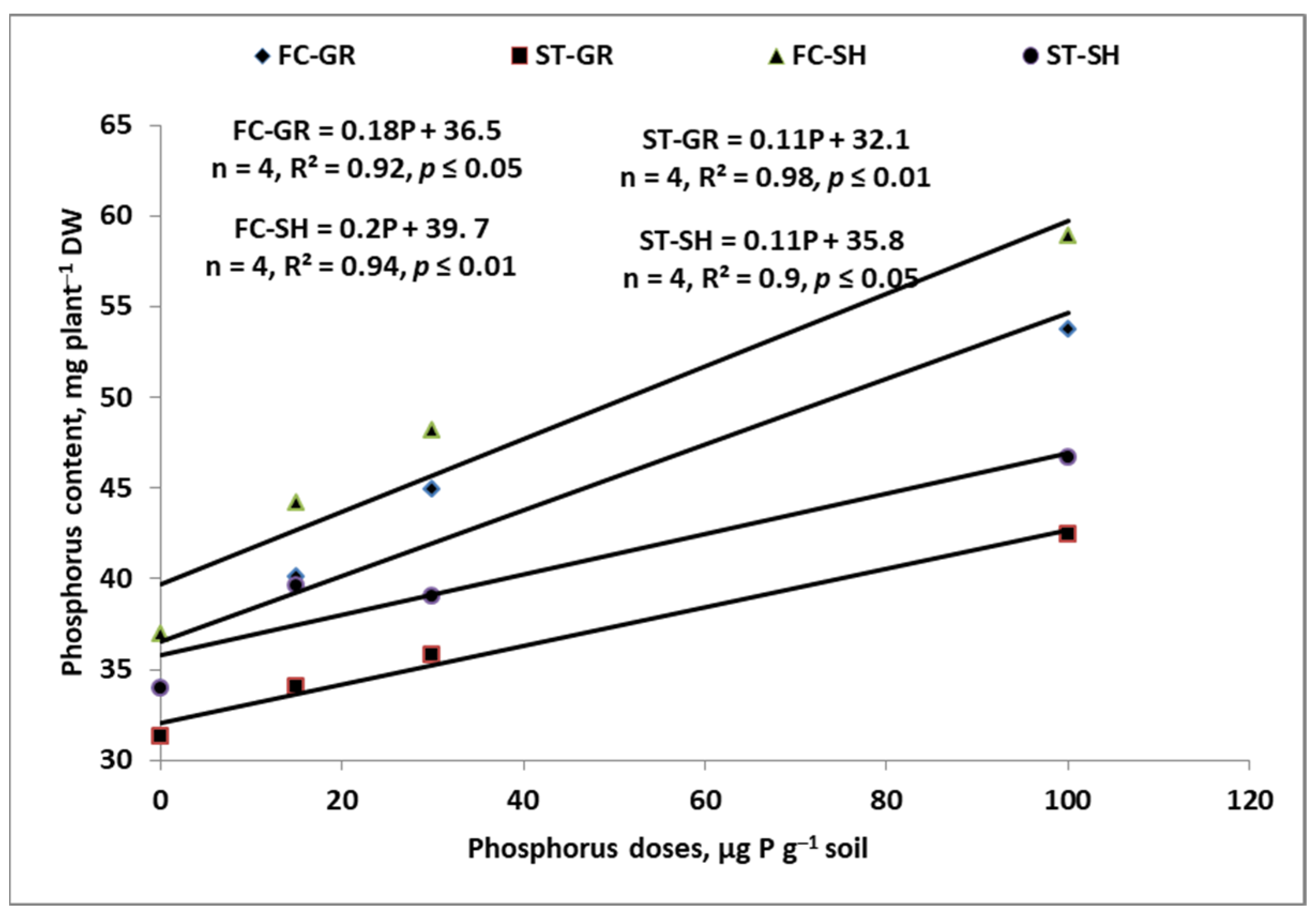
7. Sustainable Management of P Resources on the Farm
7.1. Soil
7.2. Mineral P Fertilizers
- Superphosphates: (i) single superphosphate (SSP), triple superphosphate (TSP);
- Ammonium phosphate: (i) mono-ammonium phosphate (MAP), (ii) di-ammonium phosphate (DAP);
- Nitro-phosphates (NP).
- 1.
- The liquid formulation of P fertilizer allows direct supplementation of the soil solution P pool; therefore, Pi ions are directly available to plant roots;
- 2.
- The proportion of orthophosphate to polyphosphate is 30:70; this relationship allows for covering:
- a.
- current crop needs;
- b.
- upcoming needs, i.e., those occurring during the most critical stages of plant growth;
- 3.
- Less fixation of polyphosphates than orthophosphates by soil particles.
7.3. Crop Residues, Manures—P Recycling
8. Challenges and Threats in Phosphorus Management in Plant Production
Author Contributions
Funding
Conflicts of Interest
References
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Year Book for 2009; FAO: Rome, Italy, 2009.
- Alexander, P.; Brown, C.; Ameth, A.; Finnigan, J. Human appropriation for land and food: The role of diet. Glob. Environ. Chang. 2016, 41, 88–98. [Google Scholar] [CrossRef]
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.N. Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elem. Sci. Anth. 2018, 5, 52. [Google Scholar] [CrossRef]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Machado Lins, S.R.; Vazquez, F.F.; Willing, E.; Spera, S.A.; Van Wey, L.L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Röös, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Stewart, W.M.; Roberts, T.L. Food security and the role of fertilizers in supporting it. Procedia Eng. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- Izumi, T.; Sakai, T. The global data set of historical yield of major crop 1981–2016. Sci. Data 2020, 7, 97. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the world’s phosphorus for a food secure future. Ann. Rev. Environ. Resour. 2014, 39, 161–168. [Google Scholar] [CrossRef]
- Marschner, P. Marchner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Ltd.: London, UK, 2012; p. 649. [Google Scholar]
- Pereira, A.M.; Coimbra, S. Advances in plant reproduction: From gametes to seeds. J. Exp. Bot. 2019, 15, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W.; Diatta, J.; Barłóg, P.; Biber, M.; Potarzycki, J.; Łukowiak, R.; Przygocka-Cyna, K.; Szczepaniak, W. Soil Fertility Clock—Crop Rotation as a Paradigm in Nitrogen Fertilizer Productivity Control. Plants 2022, 11, 2841. [Google Scholar] [CrossRef]
- Nicholls, J.W.; Chin, J.P.; Williams, T.A.; Lenton, T.M.; O’Flaherty McGrath, J.W. On the potential roles of phosphorus in the early evolution of energy metabolism. Front. Microbiol. 2023, 14, 1239189. [Google Scholar] [CrossRef] [PubMed]
- Lima Viana, J.; Moretti de Souza, J.L.; Hoshide, A.K.; de Oliveira, R.A.; de Abreu, D.C.; da Silva, W.M. Estimating sugarcane yield in subtropical climate using climatic variable and soil water storage. Sustainability 2023, 15, 4360. [Google Scholar] [CrossRef]
- Tanveer, A.; Ikram, R.M.; Ali, H.H. Crop rotation: Principles and practices. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 1–13. [Google Scholar]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Łukowiak, R.; Grzebisz, W.; Sassenrath, G.F. New insights into phosphorus management in—A crop rotation approach. Sci. Tot. Environ. 2016, 542, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Paluchowski, W.J. Methodological or theoretical framework controversy—Qualitative versus quantitative research. Rocz. Psychol. 2020, XIII, 7–22, (In Polish with English Summary). [Google Scholar]
- Olfs, H.-W.; Blankenau, K.; Brentrup, F.; Jasper, J.; Link, A.; Lammel, J. Soil- and plant-based nitrogen-fertilizer recommendations in arable farming. J. Plant Nutr. Soil Sci. 2005, 168, 414–431. [Google Scholar] [CrossRef]
- Grzebisz, W.; Łukowiak, R.; Sassenrath, G. Virtual nitrogen as a tool for assessment of nitrogen at the field scale. Field Crops Res. 2018, 218, 182–184. [Google Scholar] [CrossRef]
- Krouk, G.; Kiba, T. Nitrogen and phosphorus interactions in plants: From agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Ann. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Lu, H.; Mao, C. Phosphate uptake and transport in plants: An elaborate regulatory system. Plant Cell Physiol. 2021, 62, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; Wiley and Sons: New York, NY, USA, 1995; p. 432. [Google Scholar]
- McKay Fletecher, D.M.; Ruiz, S.; Dias, T.; Petroselli, C.; Roose, T. Linking root structure to functionality: The impact of root system architecture to citrate enhanced phosphate uptake. New Phytol. 2020, 227, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.H.; Wiren, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef]
- Lynch, J.L. Root phenotypes for improved capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.E. Nutrients: Bioavailability and plant uptake. In Managing Global Resources and Universal Processes; Fath, B.D., Jorgensen, S.E., Cole, M., Eds.; CRC Press: London, UK, 2020; pp. 501–509. [Google Scholar]
- Van Noordwijk, M.; Brouwer, G. Review of quantitative root length data in agriculture. In Plant Roots and Their Environment, 1st ed.; McMichael, B., Persson, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 515–525. [Google Scholar]
- Haberle, J.; Svoboda, P. Calculation of available water supply in crop zone and the water balance. Plant Soil Environ. 2015, 45, 285–298. [Google Scholar]
- De Sousa Nunes, R.; Gones de Sousa, D.M.; Goedert, W.J.; Zancanaro de Oliveira, L.E.; Pinheiro, T.D. Vrops; yield and roots response to soil phosphorus distribution resulting from long-term soil and phosphate fertilization management strategies. Front. Agron. 2021, 3, 757100. [Google Scholar] [CrossRef]
- McKay, A.D.; Barber, S.A. Effect of soil moisture and phosphate level on root hair growth on corn roots. Plant Soil 1985, 86, 321–331. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Camberato, J.J.; Murrel, S.T.; Vyn, T.J. Maize nutrient accumulation and partitioning in response to plant density and nitrogen rate. I. Macronutrients. Agron. J. 2013, 10593, 7830. [Google Scholar] [CrossRef]
- Julia, C.; Wissuva, M.; Kretzschmar, T.; Jeong, K.; Rose, T. Phosphorus uptake, partitioning and redistribution during grain filling in rice. An. Bot. 2016, 118, 1151–1162. [Google Scholar] [CrossRef]
- Subedi, K.; Ma, B. Nitrogen uptake and partitioning in stay-green and leafy maize hybrids. Crops Sci. 2005, 45, 740–747. [Google Scholar] [CrossRef]
- Guan, P. Dancing with hormones: A current perspective of nitrate signaling and regulation in Arabidopsis. Front. Plant Sci. 2017, 8, 1697. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture. J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.-O.; White, S. The story pf phosphorus global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Grzebisz, W.; Szczepaniak, W.; Barłóg, P.; Przygocka-Cyna, K.; Potarzycki, J. Phosphorus sources for winter oilseed rape (Brassica napus L.) during reproductive growth—Magnesium sulfate management impact on P use efficiency. Arch. Agron. Soil Sci. 2018, 64, 1646–1662. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hasan, M.M.; Texeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell. Mol. Biol. Lett. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Bhadouria, J.; Giri, J. Purple acid phosphatases: Roles in phosphate utilization and new emerging functions. Plant Cell Rep. 2022, 41, 33–51. [Google Scholar] [CrossRef]
- Ciereszko, I.; Żebrowska, E.; Ruminowicz, M. Acid phosphatases and growth of barley (Hordeum vulgare L.) cultivars under diverse phosphorus nutrition. Acta Physiol. Plant. 2011, 33, 2355–2368. [Google Scholar] [CrossRef]
- Shen, Q.; Wen, Z.; Dong, Y.; Li, H.; Miao, Y.; Shin, J. The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB Plants 2018, 10, ply054. [Google Scholar] [CrossRef]
- López-Arrendondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance. Ann. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Selvi, K.B.; Paul, J.J.A.; Vijaya, V.; Saraswathi, K. Analyzing the efficacy of phosphate solubilizing microorganisms by enrichment culture techniques. Bioch. Mol. Biol. J. 2017, 3, 1–7. [Google Scholar]
- Kumar, A.; Kumar, A.; Patel, H. Role of microbes in phosphorus availability and acquisition by plants. Inter. J. Curr. Microb. Appl. Sci. 2018, 7, 1344–1347. [Google Scholar] [CrossRef]
- Sane, S.A.; Mehta, S.K. Isolation and evaluation of rock phosphate solubilizing fungi as potential biofertilizer. J. Fertil Pestic. 2015, 6, 156–160. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Arbuscular mycorrhizal networks: Process and functions: A review. Agron. Sustain. Dev. 2010, 30, 581–599. [Google Scholar] [CrossRef]
- Vance, C.P. Plants without arbuscular mycorrhizae. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer Science + Business Media BV: Berlin/Heidelberg, Germany, 2008; pp. 117–142. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Stajich, J.E. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Zobel, M. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inoculate. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hort. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 2016, 39, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Shane, M.W.; Lambers, H. Cluster roots: A curiosity in context. Plant Soil 2005, 27, 101–125. [Google Scholar] [CrossRef]
- Abdolzadeh, A.; Wang, X.; Veneklaas, E.J.; Lambers, H. Effects of phosphorus supply on growth, phosphate concentration and cluster-root formation in three Lupinus species. Ann. Bot. 2010, 105, 365–374. [Google Scholar] [CrossRef]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef]
- White, C.; Sylvester-Bradley, R.; Berry, P.M. Root length densities of UK wheat and oilseed rape crops with implications for water capture and yield. J. Exp. Bot. 2015, 66, 2293–2303. [Google Scholar] [CrossRef]
- Remus, R.; Pandey, D.; Lüttschwager, D. What regulates the rhizodeposition of winter oilseed rape during growth. Plant Soil 2022, 478, 283–310. [Google Scholar] [CrossRef]
- Duan, X.; Jin, K.; Ding, G.; Wang, C.; Cai, H.; Wang, S.; White, P.J.; Xu, F.; Shi, L. The impact of different morphological and biochemical traits on phosphorus acquisition and seed yield of Brassica napus. Field Crops Res. 2020, 258, 107960. [Google Scholar] [CrossRef]
- Ziadi, N.; Whalen, J.K.; Messiga, A.J.; Morel, C. Assessment and modelling of soil available phosphorus in sustainable cropping systems. Adv. Agron. 2013, 122, 85–126. [Google Scholar]
- McMichael, B.L.; Burke, J.J. Soil temperature and root growth. HortScience 1998, 33, 947–951. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Bland, W.L. Soil temperature and root growth. Soil Sci. 1992, 154, 290–299. [Google Scholar] [CrossRef]
- Fonseca de Lima, C.F.; Klein-Vehn, J.; De Smet, I.; Feraru, E. Getting to the root belowground high temperature responses in plants. J. Exp. Bot. 2021, 72, 7404–7413. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.I.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Xu, H.; Hassan, M.A.; Sun, D.; Wu, Z.; Jiang, G.; Liu, B.; Ni, Q.; Yang, W.; Fang, H.; Li, J.; et al. Effects of low temperature stress on source-sink organs in wheat and phosphorus mitigation strategies. Front. Plant Sci. 2022, 13, 807844. [Google Scholar] [CrossRef]
- Tiwari, M.; Kumar, R.; Subramanian, S.; Doherty, C.J.; Krishna Jagadish, S.V. Auxin-cytokinin interplay shapes root functionality under low-temperatures stress. Trends Plant Sci. 2023, 28, 447–459. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.-X.; Wang, W.-S.; Gong, W.; Liu, W.-C.; Chen, H.-G.; Xu, H.-H.; Lu, Y.-T. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 2015, 56, 727–736. [Google Scholar] [CrossRef]
- Sadok, W.; Krishna Jagadish, S.V. The hidden cost of nighttime warming on yields. Trends Plant Sci. 2020, 25, 644–651. [Google Scholar] [CrossRef]
- Calleja-Cabrera, J.; Boter, M.; Onate-Sanchez, L.; Pernas, M. Root growth adaptation to climate change in crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Ben-Noach, I.; Friedman, S.P. Review and evaluation of root respiration and of natural and agricultural processes of soil respiration. Vadose Zone J. 2018, 17, 1–47. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Phosphorus nutrition affects temperature response of soybean growth and canopy photosynthesis. Front. Plant Sci. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Rabbinge, R. The ecological background of food production. In Crop Production and Sustainable Agriculture; Rabbinge, R., Ed.; John Wiley and Sons: New York, NY, USA, 1993; pp. 2–29. [Google Scholar]
- Ievinsh, G. Water content of plant tissues: So simple that almost forgotten. Plants 2023, 12, 1238. [Google Scholar] [CrossRef]
- Chavarria, G.; Pessoa des Santos, H. Plant water relations: Absorption, transport and control mechanisms. In Advances in Selected Plant Physiology Aspects; Montanaro, G., Ed.; Intech Europe: Rijeka, Croatia, 2012; pp. 105–133. [Google Scholar]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef] [PubMed]
- Smucker, A.J.M.; Aiken, R.M. Dynamic root responses to water deficits. Soil Sci. 1992, 154, 281–289. [Google Scholar] [CrossRef]
- Fromm, H. Root plasticity in the pursuit of water. Plants 2019, 8, 236. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, Y.; Sun, Z.; Zheng, J.; Bai, W.; Zhang, Y.; Liu, Y.; Feng, L.; Feng, C.; Zhang, Z.; et al. Morphological plasticity of root growth under mild water stress increases water use efficiency without reducing yield on maize. Biogeosciences 2017, 14, 3851–3858. [Google Scholar] [CrossRef]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-Z.; Li, G.; Yan, G.; Liu, H.; Turner, N.C. Morphological features and biomass partitioning of lucerne plants (Medicago sativa L.) subjected to water stress. Agronomy 2020, 10, 322. [Google Scholar] [CrossRef]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Al-Kaisi, M.; Broner, I. Crop Water Use and Growth Stages; CSA, No. 4.715; Colorado State University Extension: Fort Collins, CO, USA, 2009; p. 4. [Google Scholar]
- Passioura, J. Increasing crop productivity when water is scarce—From breeding to field management. Agric. Water Manag. 2006, 80, 176–196. [Google Scholar] [CrossRef]
- Kang, S.; Gu, B.; Du, T.; Zhang, J. Crop coefficient and ration of transpiration to evapotranspiration of winter wheat and maize in a semi-humid region. Agric. Water Manag. 2003, 59, 239–254. [Google Scholar] [CrossRef]
- Sah, R.P.; Chacraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar]
- Rodriguez, D.; Goudriaan, J. Effects of phosphorus and drought stress an dry matter and phosphorus allocation in wheat. J. Plant Nutr. 1995, 189, 2501–2517. [Google Scholar] [CrossRef]
- Dixon, M.; Simonne, E.; Obreza, T.; Liu, G. Crop response to low phosphorus bioavailability with focus on tomato. Agronomy 2020, 10, 617. [Google Scholar] [CrossRef]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef]
- Najewski, A.; Madajska, K.; Skrzypek, A. Preliminary results of cultivar yield in post-registration experiments. In Winter Cereals; COBORU: Słupia Wielka, Poland, 2023; p. 18. [Google Scholar]
- Meki, M.N.; Osorio, J.M.; Steglich, E.M.; Kiniry, J.R. Drought-induced nitrogen and phosphorus carryover nutrients in corn/sybean rotations in the Upper Mississippi River Basin. Sustainability 2022, 14, 15108. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Chander, S.; Barcelo, J.; Poschenrieder, C. Aluminum stress in plants. In Recent Advances in Plant Stress Physiology; Yadav, P., Kumar, S., Jain, V., Eds.; Daya Publishing House: New Delhi, India, 2016; pp. 265–282. [Google Scholar]
- Prakash, J.; Agrawal, S.B.; Agrawal, M. Global trends of acidity in rainfall and its impact on plants and soil. J. Soil Sci. Plant Nutr. 2023, 23, 398–419. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Lee, S.H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current status and opportunities. Int. Mol. Sci. 2018, 19, 3083. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanism of aluminum toxicity and resistance in plants. Ann. Rev. Plant Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. Aluminum toxicity in plants: A review. Agronomie 2003, 21, 3–21. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and fertilization strategies mitigating soil factors constraining efficiency of nitrogen in plant Production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley & Sons Ltd.: New York, NY, USA, 1979; p. 449. [Google Scholar]
- Gessa, C.E.; Mimmo, T.; Deiana, S.; Marzadori, C. Effect of aluminum and pH on the mobility of phosphate through a soil-root interface model. Plant Soil 2005, 272, 301–311. [Google Scholar] [CrossRef]
- Von Wandruszka, R. Phosphorus retention in calcareous soils and the effect of organic matter on its mobility. Geochemic. Trans. 2006, 7, 1–8. [Google Scholar] [CrossRef]
- Baccari, B.; Krouma, A. Rhizosphere acidification determines phosphorus availability in calcareous soil and influences faba bean (Vicia faba L.) tolerance to P deficiency. Sustainability 2022, 15, 6203. [Google Scholar] [CrossRef]
- Russell, R.S. The plant Root Systems: Their Functions and Interaction with Soil; MC Graw-Hill Book Company: Berkshire, UK, 1977; p. 298. [Google Scholar]
- Hamblin, A. The influence of soil structure on water movement, crop growth, and water uptake. Adv. Agron. 1985, 39, 95–157. [Google Scholar]
- Colombi, T.; Torres, L.C.; Walter, A.; Keller, T. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth—A vicious circle. Sci. Total Environ. 2018, 626, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Zalewski, D.; Hryńczuk, B. Influence of the soil penetration resistance, bulk density and moisture on some components of winter wheat yield. Int. Agrophysics. 2004, 18, 91–96. [Google Scholar]
- Soil Science Society of America. Glossary of Soil Science Terms; Soil Science Society of America: Madison, WI, USA, 2008. [Google Scholar]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 2019, 70, 6019–6034. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.W.; Kaspar, T.C. Soil compaction and root growth: A review. Agron J. 1994, 86, 759–766. [Google Scholar] [CrossRef]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A review on the effect of soil compaction and its management for sustainable crop production. J. Biosys. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil Tillage Res. 2012, 121, 74–81. [Google Scholar] [CrossRef]
- Claupen, W. Nitrogen fertilization and chemical plant protection in a long-term field experiment and the yield response laws of Liebig. Liescher. Wollny and Mitscherlich. J. Agron. Crop Sci. 1993, 171, 102–113. [Google Scholar]
- Chields, P.E. Irelands early chemical industry. Chem. Action 1998, 55, 10–20. [Google Scholar]
- He, X.; Augusto, L.; Goll, D.S.; Ringeval, B.; Wang, Y.; Helfenstein, J.; Huang, Y.; Yu, K.; Wang, Z.; Yang, Y.; et al. Global patterns and drivers of soil total phosphorus concentration. Earth Syst. Sci. Data 2021, 13, 5831–5846. [Google Scholar] [CrossRef]
- Conijn, J.G.; Bindraban, P.S.; Schroder, J.J.; Jongschaap, R.E.E. Can our global food system meet food demand within planetary boundaries? Agric. Ecosys. Environ. 2018, 252, 244–256. [Google Scholar] [CrossRef]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact by 2050. Glob. Food Secur. 2020, 26, 100426. [Google Scholar] [CrossRef]
- Arenberg, M.R.; Arai, Y. Chapter three—Uncertainties in soil physicochemical factors controlling phosphorus mineralization and immobilization processes. Adv. Agron. 2019, 154, 153–200. [Google Scholar]
- Kabala, C.; Galka, B.; Labaz, B.; Anjos, L.; de Souza Cavassani, R. Towards more simple and coherent chemical criteria in a classification of anthropogenic soils: A comparison of phosphorus tests for diagnostic horizons and properties. Geoderma 2018, 320, 1–11. [Google Scholar] [CrossRef]
- Bon van der, E.J.T.; Mclaren, T.I.; Doolette, A.L.; Magid, J.; Frossard, E.; Oberson, A.; Jensen, L.S. Influence of long-term phosphorus fertilization history on the availability and chemical nature of soil phosphorus. Goederma 2019, 355, 113909. [Google Scholar] [CrossRef]
- Fixen, P.E.; Grove, J.H. testing soils for phosphorus. In Soil Testing and Plant Analysis, 3rd ed.; SSSA Book Series; Westerman, R.L., Ed.; John Wiley: Hoboken, NJ, USA, 1990; Volume 3, pp. 141–180. [Google Scholar]
- Potarzycki, J. Forms of phosphorus in long-lasting soils fertilization with mineral and organic fertilizers. In Science. Industry. Technology. Marketing. International Scientific-Research Seminar; Górecki, H., Dobrzański, Z., Kafarski, P., Eds.; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Czech Republic, 2000; pp. 69–76. [Google Scholar]
- Kęsik, K. Application of the Mehlich 3 method in the fertilizer advisory system. Stud. I Rap. IUNG-PIB 2016, 48, 95–104, (In Polish with English Summary). [Google Scholar]
- Mallarino, A.P.; Borges, R. Phosphorus and potassium distribution in soil following long-term deep-band fertilization in different tillage systems. Soil Soc. Soc. Am. J. 2006, 70, 702–707. [Google Scholar] [CrossRef]
- Kautz, T.; Amelung, W.; Ewert, F.; Gaiser, T.; Horn, R.; Jahn, R.; Javaux, M.; Kemna, A.; Kuzyakova, Y.; Munch, J.-C.; et al. Nutrient acquisition from arable subsoils in temperate climates: A review. Soil Biol. Biochem. 2013, 57, 1003–1022. [Google Scholar] [CrossRef]
- Bauke, S.L.; von Sperber, C.; Tamburini, F.; Gocke, M.I.; Honermeier, B.; Schweitzer, K.; Baumecker, M.; Don, A.; Sandhage-Hofmann, A.; Amelung, W. Subsoil phosphorus is affected by fertilization regime in long-term agricultural experimental trials. Europ. J. Soil Sci. 2018, 69, 103–112. [Google Scholar] [CrossRef]
- Spinoni, J.; Naumann, G.; Voght, J.V. Pan-European seasonal trends and recent changes of drought frequency and severity. Glob. Planet. Chang. 2017, 148, 113–130. [Google Scholar] [CrossRef]
- Minea, I.; Iosub, M.; Boicu, D. Multi-scale approach for different type of drought in temperate climatic conditions. Nat. Hazards 2022, 110, 1153–1177. [Google Scholar] [CrossRef]
- Barłóg, P.; Łukowiak, R.; Grzebisz, W. Predicting the content of soil mineral nitrogen based on the content of calcium chloride-extractable nutrients. J. Plant Nutr. Soil Sci. 2017, 180, 624–635. [Google Scholar] [CrossRef]
- Samreen, S.; Kausar, S. Phosphorus fertilizer: The original and commercial sources. In Phosphorus; Zhang, T., Ed.; Intech Europe: Rijeka, Croatia, 2019; pp. 81–94. [Google Scholar]
- Mortvedt, J.; Murphy, L.; Follet, R. Fertilizer Technology and Application. Meister Pu. Co.: Madison, WI, USA, 1999; p. 199. [Google Scholar]
- Potarzycki, J.; Grzebisz, W. Dynamics of water-soluble phosphorus release from soils fertilized in the past with slurry and mineral fertilizers (incubation experiment). Pr. Nauk. Akad. Ekon. We Wrocławiu 2001, 888, 190–196, (In Polish with English summary). [Google Scholar]
- Fernández, V.; Sotiropoulus, T.; Brown, P. Foliar Fertilization: Scientific Principles and Field Practices, 1st ed.; IFA: Paris, France, 2013; p. 145. [Google Scholar]
- Singh, J.; Singh, M.; Jain, A.; Bhardway, S.; Singh, A.; Singh, D.K.; Bhushan, B.; Dubey, S.K. An introduction of plant nutrients and foliar fertilization: A review. In Precision Farming: A New Approach; Ram, T., Kumar Lohan, S., Singh, R., Singh, P., Eds.; Astral Inter. Pvt. Ltd.: New Delhi, India, 2014; pp. 258–320. [Google Scholar]
- Wójcik, P. Uptake of mineral nutrients from foliar fertilization (review). J. Fruit Ornam. Plant Res. 2004, 12, 201–208, (In Polish with English summary). [Google Scholar]
- Grzebisz, W. Part I. Oil crops, root and tuber crops, legumes. In Technology of Crop Plant Fertilization—Yielding Physiology; PWRiL: Poznań, Poland, 2009; p. 414. (In Polish) [Google Scholar]
- Almeida, R.F.; Queiroz, I.D.S.; Mikhael, J.E.R.; Oliveira, R.C.; Borges, E.N. Enriched animal manure as a source of phosphorus in agriculture. Inter. J. Rec. Org. Waste Agric. 2019, 8 (Suppl. S1), S203–S210. [Google Scholar] [CrossRef]
- Andriamananjara, A.; Rakotoson, T.; Razafimbelo, T.; Rabeharisoza, L.; Razafimanantsoa, M.-P.; Masse, D. Farmyard manure improves phosphorus use efficiency in weathered P deficient soil. Nutr. Cycl. Agroecosyst. 2019, 115, 407–425. [Google Scholar] [CrossRef]
- Pasket, A.; Zhang, H.; Raun, W.; Deng, S. Recovery of phosphorus in soil amended with manure for 119 years. Agronomy 2020, 10, 1947. [Google Scholar] [CrossRef]
- Stevenson, F.; Cole, M.A. Cycles of Soil, 2nd ed.; J. Wiley & Sons Inc.: New York, NY, USA, 1999; p. 427. [Google Scholar]
- Steiner, G.; Geissler, B.; Watson, I.; Mew, N.C. Efficiency developments in phosphate rock mining over the last three decades. Resour. Conserv. Recycl. 2015, 105, 235–245. [Google Scholar] [CrossRef]
- Nogalska, A.; Załuszniewska, A. The effect of meat and bone meal applied without or with mineral nitrogen on macronutrient content and uptake by winter oilseed rape. J. Elem. 2010, 25, 905–915. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, A.A.M.; Rahid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewater through struvite crystallization—A review. Arabian J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Tymchuk, I.; Shkvirko, O.; Sakalova, H.; Malovany, M.; Dabizhuk, T.; Shevchuck, O.; Matviichuk, O.; Vasylinych, T. Wastewater as a source of nutrients for crops growth and development. J. Ecol. Eng. 2020, 21, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage sludge ash—A promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- EU. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (Text with EEA relevance). Off. J. Eur. Union 2019, L170, 1–114. [Google Scholar]
- MARD-PL. Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 regarding the implementation of certain provisions of the Act on fertilizers and fertilization. J. Laws 2008, 119, 765. [Google Scholar]
- Vogel, T.; Nells, M.; Eichler-Lobermann, B. Phosphorus effects recycled products from municipal wastewater on crop in a field experiment. Plant Soil Environ. 2017, 63, 475–482. [Google Scholar] [CrossRef]
- Oenema, O.; Chardon, W.; Ehlert, P.; van Dijk, K.; Dchoumana, O.; Rulkens, W. Phosphorus Fertilisers from by-Products and Wastes; IFS Proceedings No. 177; International Fertilizer Society: Staffordshire, UK, 2012; 56p. [Google Scholar]
- Hertzberger, A.J.; Cusik, R.D.; Margenot, A.J. A Review and meta-analysis of the agricultural potential of struvite as phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]


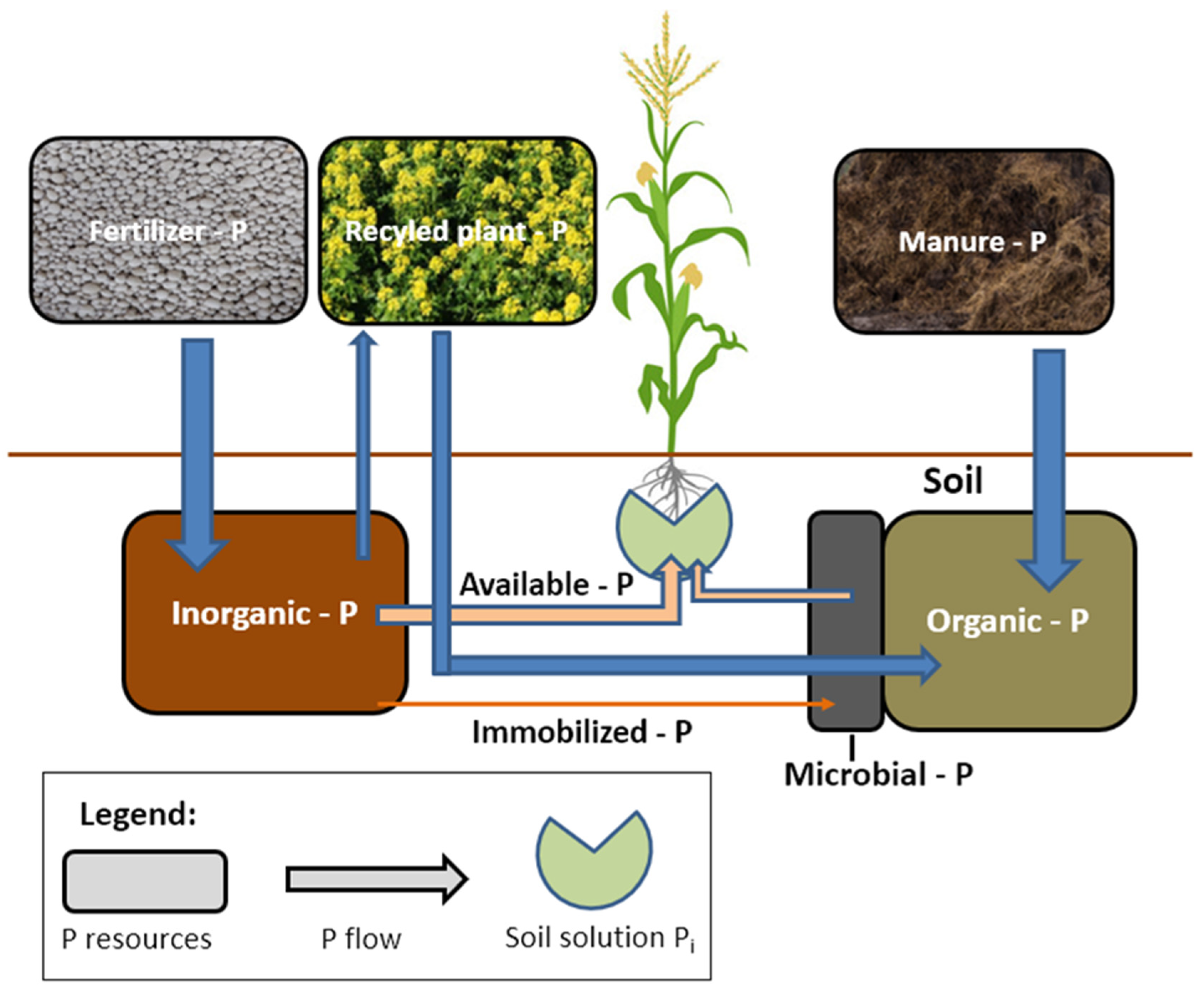
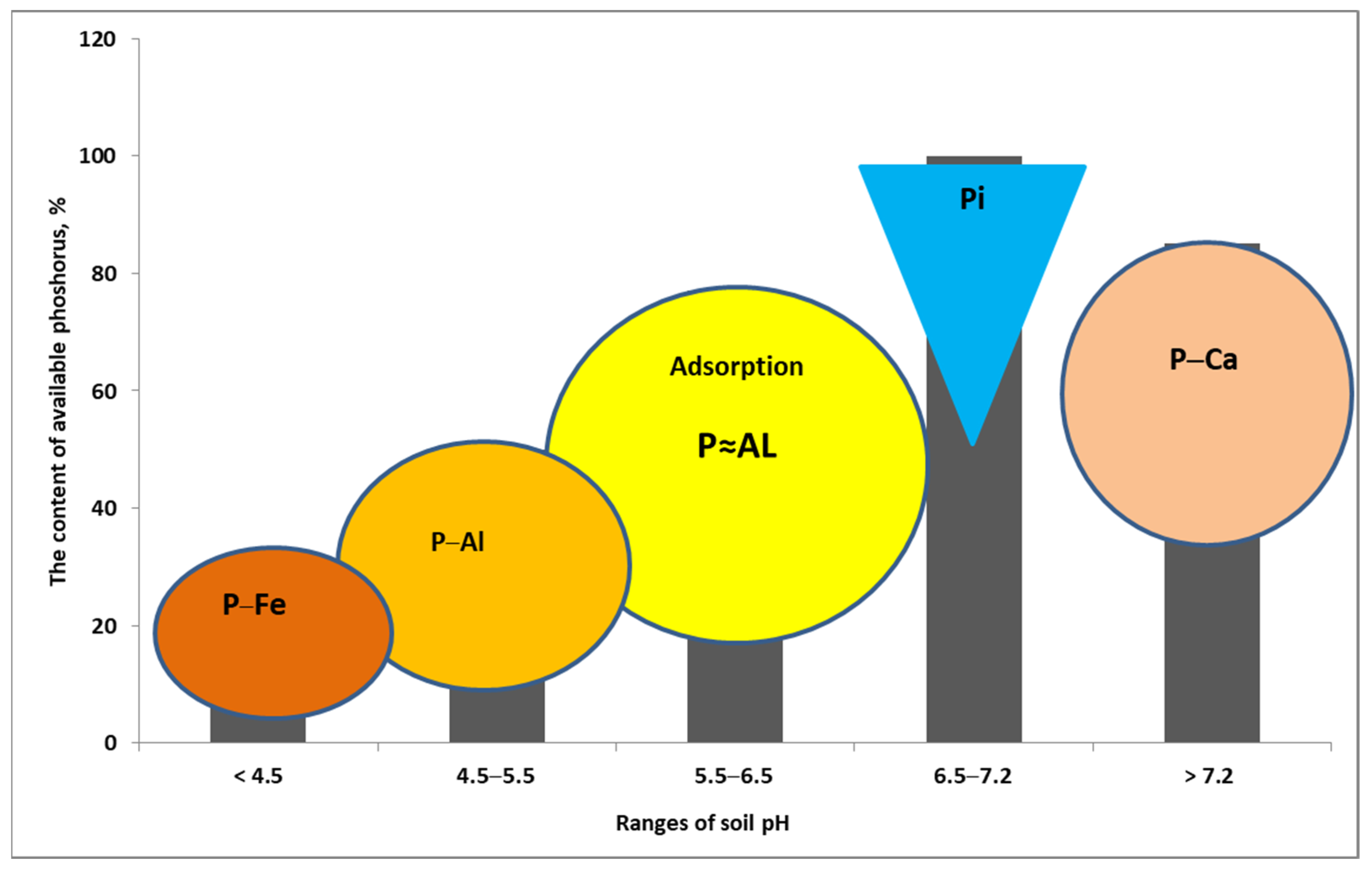
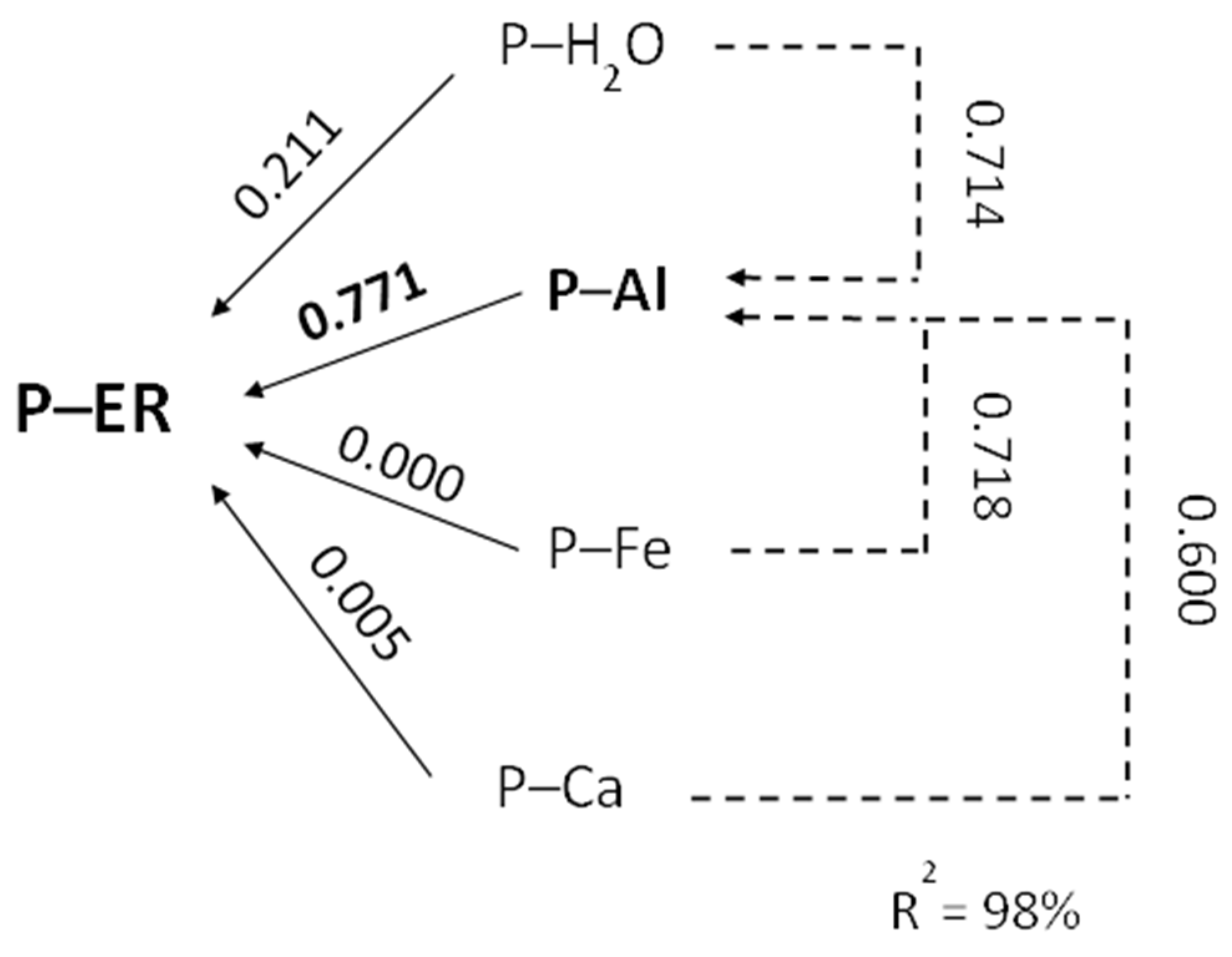



| Crop Plant | Density of Roots in the Soil | Effective Rooting Depth |
|---|---|---|
| cm cm−3 | cm | |
| Potato | 1–2 | 40–50 |
| Sugar beet | 1–2 | 150 |
| Pulses, pea | 0.2–2 | 40–60 |
| Maize | 3–4 | 90–130 |
| Winter wheat | 4–5 (8) | 80–100 |
| Spring barley | 4–5 | 50–80 |
| Grasses | 3–20 | 100 |
| Winter oilseed rape | 4–5 | 60–80 |
| PSM Isolates | Organic Acids | References |
|---|---|---|
| Pseudomonas | Citric acid, succinic acid, fumaric acid, gluconic acid, 2-ketogluconic acids | [48,49] |
| Bacillus sp. | Citric acid, malic acid, succinic acid, fumaric acid, tartaric acid, gluconic acid | [48] |
| Proteus sp. | Citric acid, succinic acid, fumaric acid, gluconic acid | [48] |
| Azospirillum sp. | Citric acid, succinic acid, fumaric acid, gluconic acid | [48] |
| Aspergillus | Citric acid, gluconic acid, oxalic acid, succinic acid, malic acid, glycolic acid | [50] |
| Penicillium sp. | Gluconic acid, glycolic acid, succinic acid, malic acid, oxalic acid, citric acid | [50] |
| Erwinia herbicola | Gluconic acid, 2-ketogluconic acid | [49] |
| Soil pH Range | Soil Fertility Classes | ||||
|---|---|---|---|---|---|
| Very Low | Low | Medium | High | Very High | |
| Egner–Riehm Doppel lactate method | |||||
| - | <50 | 56–100 | 101–150 | 151–200 | >200 |
| Mehlich 3 method | |||||
| Very acid (<pH 4.5) | <115 | 115–252 | 254–426 | 428–600 | >600 |
| Acid (pH (pH 4.6–5.5) | <112 | 112–236 | 238–362 | 364–493 | >493 |
| Slightly acid (pH 5.6–6.5) | <108 | 108–227 | 229–348 | 351–474 | >474 |
| Neutral (pH 6.6–7.2) | <62 | 62–124 | 126–172 | 174–227 | >227 |
| Alkaline (pH > 7.2) | <62 | 62–124 | 126–172 | 174–227 | >227 |
| Name | Chemical Formula | P content, % P2O5 | Water Solubility, % | Soil Acidifying Effect |
|---|---|---|---|---|
| Solid | ||||
| Single superphosphate, SSP | Ca(H2PO4)2 · H2O + CaSO4 · 2H2O | 16–21 | 85 | Neutral |
| Triple superphosphate, (TSP) | Ca(H2PO4)2 · 2H2O | 41–50 | 85 | Neutral |
| Monoammonium phosphate (MAP) | NH4H2PO4, 11%N | 51 | 82 | Slightly |
| Diammonium phosphate (DAP) | (NH4)2HPO4, 18% N | 46 | 92 | Moderate |
| Partially acidulated phosphate rocks (PAPRs) | Ca(H2PO4)2 · H2O + CaSO4 ·2H2O × Ca3PO4 | 23–26 | Variable | Non-acidifying |
| Nitrophosphates, NP | 14–29%N | 22–35 | ≥50 | Moderate |
| Liquid/foliar fertilizer | Solution reaction | |||
| Ammonium polyphosphate, APP | NH4P2O7; 10; 11% N | 34, 37 | 100 | Slightly acid |
| Dipotassium phosphate | K2 HPO4 | 40 | 100 | Alkaline |
| Monosodium phosphate | NaH2 HPO4 | 53 | 87 | Acid |
| Phosphoric acid | H3PO4 | 54 | 100 | Acid |
| Manure | Dry Matter, % | pH | Norg, % | C:N | P, % | C:P |
|---|---|---|---|---|---|---|
| Solid manures | ||||||
| Dairy | 20–25 | 6.5–7.0 | 2.0–3.0 | 13–20 | 1.0–1.50 | 25–40 |
| Pig | 20–25 | 7.0–7.5 | 2.0–3.5 | 11–20 | 1.0–2.0 | 20–40 |
| Chicken | 40–60 | 6.0–8.0 | 3.0–5.0 | 8–14 | 0.4–0.8 | 50–100 |
| Liquid manures and digestate | ||||||
| Cattle slurry | 8–10 | 7.0–7.5 | 2.5–3.5 | 11–15 | 0.8–1.5 | 25–50 |
| Pig slurry | 4–6 | 7.0–8.0 | 3.0–5.0 | 8–12 | 2.0–3.0 | 15–20 |
| Digestate | 5–8 | 7.0–8.0 | 4.0–6.0 | 7–10 | 0.6–1.5 | 25–65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzebisz, W.; Niewiadomska, A.; Potarzycki, J.; Andrzejewska, A. Phosphorus HotSpots in Crop Plants Production on the Farm—Mitigating Critical Factors. Agronomy 2024, 14, 200. https://doi.org/10.3390/agronomy14010200
Grzebisz W, Niewiadomska A, Potarzycki J, Andrzejewska A. Phosphorus HotSpots in Crop Plants Production on the Farm—Mitigating Critical Factors. Agronomy. 2024; 14(1):200. https://doi.org/10.3390/agronomy14010200
Chicago/Turabian StyleGrzebisz, Witold, Alicja Niewiadomska, Jarosław Potarzycki, and Agnieszka Andrzejewska. 2024. "Phosphorus HotSpots in Crop Plants Production on the Farm—Mitigating Critical Factors" Agronomy 14, no. 1: 200. https://doi.org/10.3390/agronomy14010200
APA StyleGrzebisz, W., Niewiadomska, A., Potarzycki, J., & Andrzejewska, A. (2024). Phosphorus HotSpots in Crop Plants Production on the Farm—Mitigating Critical Factors. Agronomy, 14(1), 200. https://doi.org/10.3390/agronomy14010200








