Putrescine Increases Frost Tolerance and Effectively Mitigates Sweet Cherry (Prunus avium L.) Cracking: A Study of Four Different Growing Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Sweet Cherry Frost Tolerance Evaluation
2.3. Sweet Cherry Cracking Evaluation
2.4. Fruit Quality Parameters at Harvest
2.5. Statistical Analysis
3. Results and Discussion
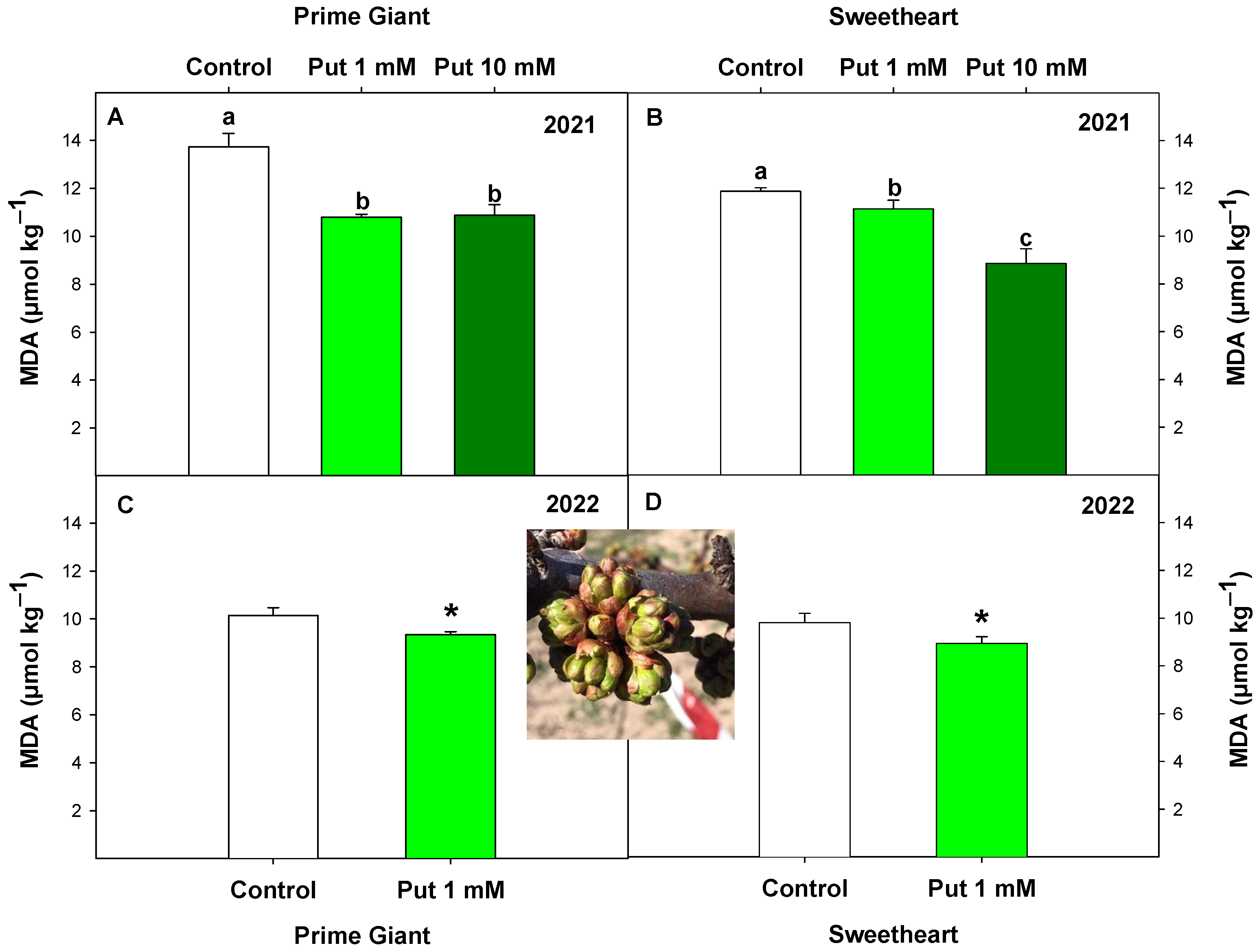
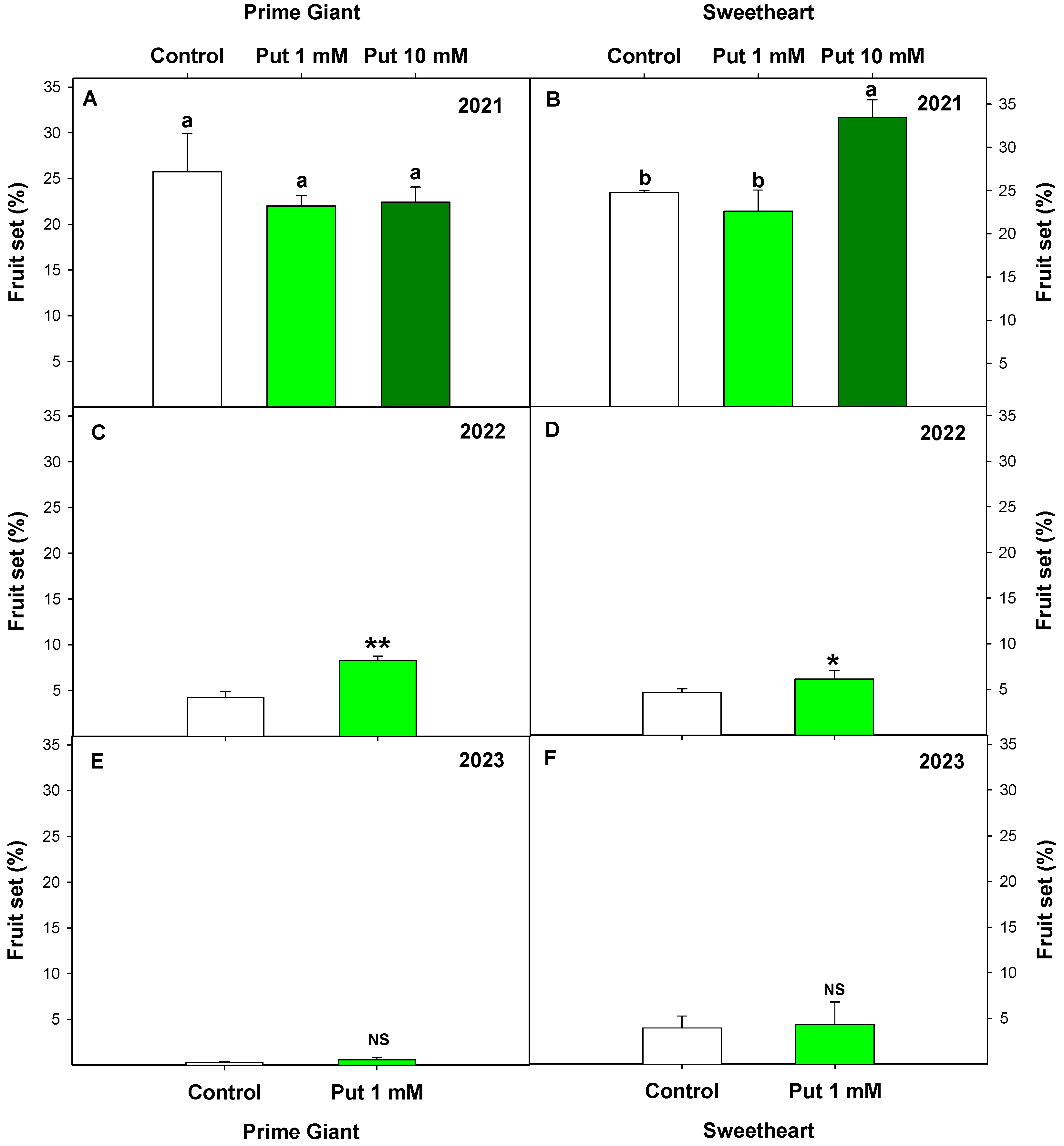
3.1. Effect of Exogenous Putrescine on Sweet Cherry Frost Tolerance: MDA, Ovary Integrity, and Fruit Set
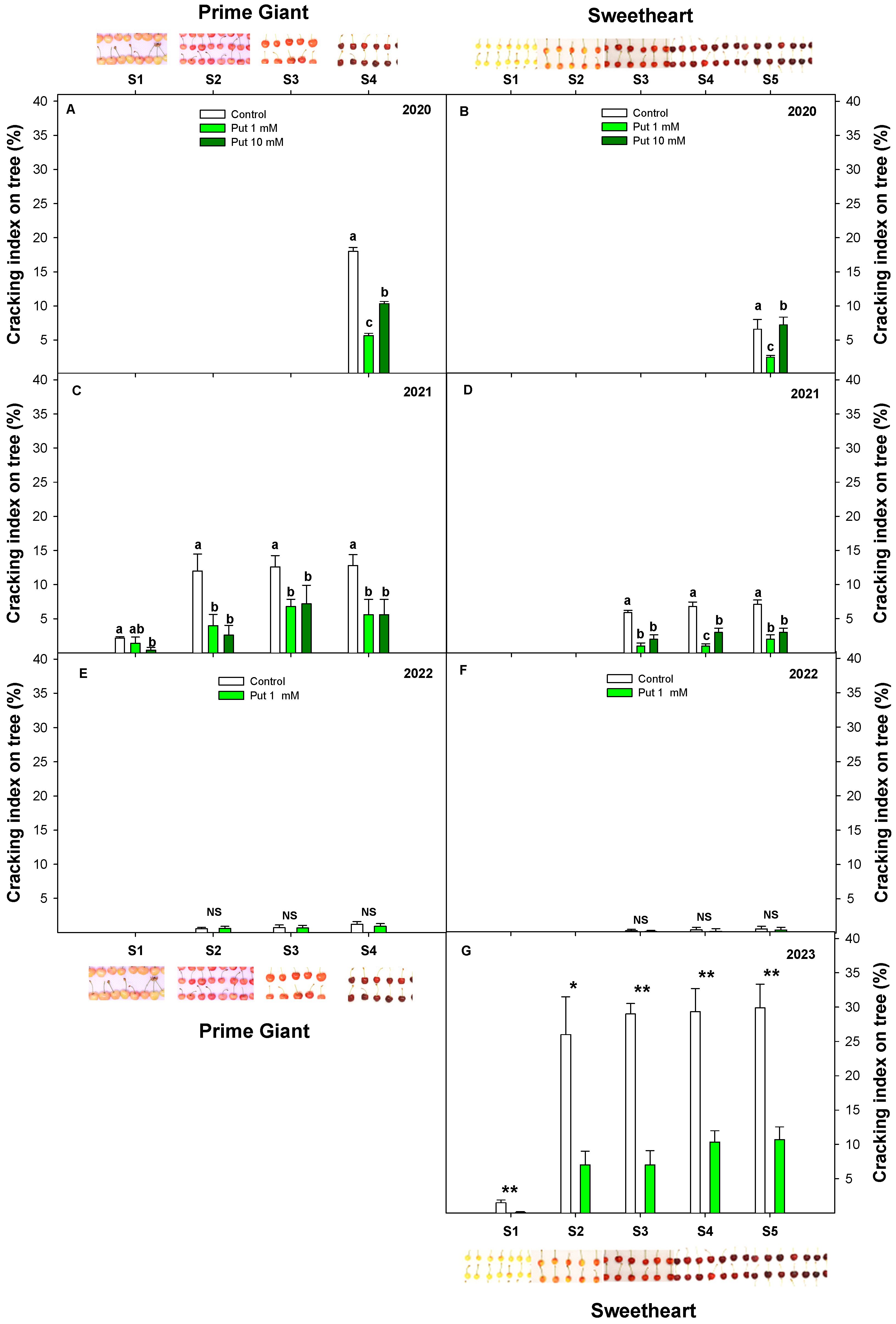
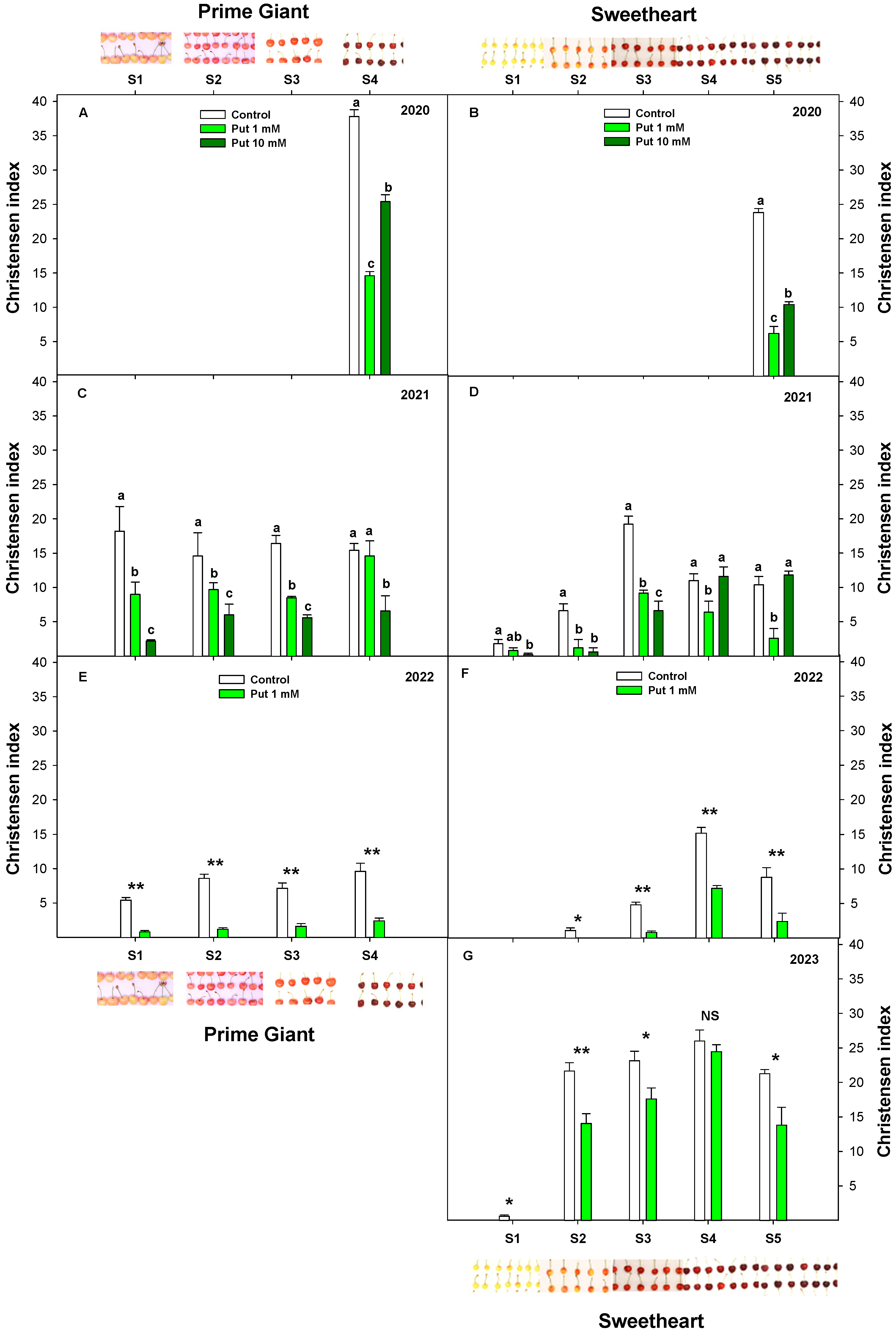
3.2. Effect of Exogenous Putrescine on Sweet Cherry Cracking during Ripening on Tree
3.3. Effect of the Preharvest Treatment with Putrescine on the Sweet Cherry Quality at Harvest
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernandez, E.; Mojahid, H.; Fadón, E.; Egea, J.A.; Mimoun, M.B.; Kodad, O.; El Yaacoubi, A.; Ghrab, M.; Egea, J.; Benmoussa, H.; et al. Climate change impacts on winter chill in Mediterranean temperate fruit orchards. Reg. Environ. Chang. 2023, 23, 7. [Google Scholar] [CrossRef]
- Kaya, O.; Kose, C. Sensitivity of some sweet cherry (Prunus avium L.) cultivars to late spring frosts during different phenological stages following bud burst. Theor. Appl. Climatol. 2022, 148, 1713–1725. [Google Scholar] [CrossRef]
- Huang, W.; Gao, Z.; Wang, L.; Zhang, Y.; Fu, X. Advances in research on cherry fruit cracking: Causes, prevention and control. Front. Plant Sci. 2019, 10, 986. [Google Scholar]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S. Water on the surface aggravates microscopic cracking of the sweet cherry fruit cuticle. J. Am. Soc. Hortic. Sci. 2006, 131, 192–200. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Lorente-Mento, J.M.; Carrión-Antolí, A.; Castillo, S.; Martínez-Romero, D.; Guillén, F. Cracking during development on the tree and at harvest sweet cherry (Prunus avium L.): The impact of methyl jasmonate on four different growing seasons. Agriculture 2023, 13, 1244. [Google Scholar] [CrossRef]
- Bound, S.A.; Close, D.C.; Jones, J.E.; Whiting, M.D. Improving fruit set of ‘Kordia’ and ‘Regina’ sweet cherry with AVG. Acta Hortic. 2014, 1042, 285–292. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D.; Sharifi, M. Horticultural practices in early spring to mitigate the adverse effect of low temperature on fruit set in ‘Lapins’ sweet cherry. Plants 2023, 12, 468. [Google Scholar] [CrossRef]
- Ureta-Ovalle, A.; Atenas, C.; Larraín, P. Application of an Ecklonia maxima seaweed product at two different timings can improve the fruit set and yield in ‘Bing’ sweet cherry trees. Acta Hortic. 2019, 1235, 319–326. [Google Scholar] [CrossRef]
- Alhamid, J.O.; Mo, C.; Zhang, X.; Wang, P.; Whiting, M.D.; Zhang, Q. Cellulose nanocrystals reduce cold damage to reproductive buds in fruit crops. Biosyst. Eng. 2018, 172, 124–133. [Google Scholar] [CrossRef]
- Zago, M.; Ropelato, E.; Gobber, M.; Franchini, S. Defence against late frost with frost protection candles. Laimburg J. 2005, 2, 58–61. [Google Scholar]
- Feldmane, D. Effects of irrigation and woodchip mulch on growth and habit of sour cherries. In Proceedings of the Annual 15th International Scientific Conference Research for Rural Development, Aguascalientes, Mexico, 18–21 June 2009; pp. 64–70. [Google Scholar]
- Salvadores, Y.; Bastías, R.M. Environmental factors and physiological responses of sweet cherry production under protective cover systems: A review. Chilean J. Agric. Res. 2023, 83, 484–498. [Google Scholar] [CrossRef]
- Pino, S.; Palma, M.; Sepúlveda, Á.; Sánchez-Contreras, J.; Moya, M.; Yuri, J.A. Effect of rain cover on tree physiology and fruit condition and quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ sweet cherry trees. Horticulturae 2023, 9, 109. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High tunnel cultivation of sweet cherry (Prunus avium L.): Physiological and production variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Matteo, M.; Zoffoli, J.P.; Ayala, M. Calcium sprays and crop load reduction increase fruit quality and postharvest storage in sweet cherry (Prunus avium L.). Agronomy 2022, 12, 829. [Google Scholar] [CrossRef]
- Rombolà, A.; Quartieri, M.; Rodríguez-Declet, A.; Minnocci, A.; Sebastiani, L.; Sorrenti, G. Canopy-applied silicon is an effective strategy for reducing sweet cherry cracking. Hortic. Environ. Biotechnol. 2023, 64, 371–378. [Google Scholar] [CrossRef]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalves, B. Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. Procedia Environ. Sci. 2015, 29, 251–252. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Štampar, F. Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
- Correia, S.; Queirós, F.; Ferreira, H.; Morais, M.C.; Afonso, S.; Silva, A.P.; Gonçalves, B. Foliar application of calcium and growth regulators modulate sweet cherry (Prunus avium L.) tree performance. Plants 2020, 9, 410. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef]
- Kamiab, F.; Tavassolian, I.; Hosseinifarahi, M. Biologia futura: The role of polyamine in plant science. Biol. Futur. 2020, 71, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, N.; Egea, J.; Burgos, L.; Martinez-Romero, D.; Valero, D.; Serrano, M. The influence of polyamines on apricot ovary development and fruit set. Ann. Appl. Biol. 2006, 149, 27–33. [Google Scholar] [CrossRef]
- Valero, D.; Romero, M.D.; Serrano, M. The role of polyamines in the improvement of shelf life of fruit. Trends Food Sci. Technol. 2002, 13, 228–234. [Google Scholar] [CrossRef]
- Martinez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory effects of exogenously applied polyamines on postharvest physiology, antioxidant system and shelf life of fruits: A review. Int. J. Mol. Sci. 2017, 18, 1789. [Google Scholar] [CrossRef] [PubMed]
- Mirdehghan, S.H.; Rahimi, S. Pre-harvest application of polyamines enhances antioxidants and table grape (Vitis vinifera L.) quality during postharvest period. Food Chem. 2016, 196, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence, and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Malik, A.; Singh, Z. Pre-storage application of polyamines improves shelf-life and fruit quality of mango. J. Hortic. Sci. Biotechnol. 2005, 80, 363–369. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z.; Abbasi, N.A. Pre-storage putrescine application suppresses ethylene biosynthesis and retards fruit softening during low temperature storage in ‘Angelino’ plum. Postharvest Biol. Technol. 2007, 46, 36–46. [Google Scholar] [CrossRef]
- Shaaban, F.K.M.; El-Hadidy, G.A.M.; Mahmoud, T.S.M. Effects of salicylic acid, putrescine and moringa leaf extract application on storability, quality attributes and bioactive compounds of plum cv. ‘Golden Japan’. Future Food J. 2020, 8, 1–14. [Google Scholar]
- Shanbehpour, F.; Rastegar, S.; Ghasemi, M. Effect of preharvest application of calcium chloride, putrescine, and salicylic acid on antioxidant system and biochemical changes of two Indian jujube genotypes. J. Food Biochem. 2020, 44, e13474. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jawandha, S.K.; Gill, P.S.; Singh, D. Preharvest putrescine application extends the shelf life and maintains the pear fruit quality. Int. J. Fruit Sci. 2022, 22, 514–524. [Google Scholar] [CrossRef]
- Singh, V.; Jawandha, S.K.; Gill, P.S.; Kaur, R. Pre-storage putrescine treatment alleviates internal browning of stored pear fruit by regulating enzyme activity. Erwerbs-Obstbau 2023, 65, 1291–1301. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage. Sci. Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Christensen, J.V. Rain-induced cracking of sweet cherries: Its causes and prevention. In Cherries: Crop Physiology, Production and Uses; Webster, A.D., Looney, N.E., Eds.; CAB International: Wallingford, UK, 2006; pp. 297–327. [Google Scholar]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin pre-harvest treatments leads to maintenance of sweet cherry quality during storage by increasing antioxidant systems. Front. Plant Sci. 2022, 13, 984. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Ren, L.; Zhang, J.; Reed, B.M.; Zhang, D.; Shen, X.-H. Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 2015, 70, 38–47. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Rezanejad, F.; Hajilou, J. Effect of putrescine and proline on proflies of GABA, antioxidant activities in leaves of three Citrus species in response to low temperature stress. J. Plant Biochem. Biotechnol. 2021, 30, 545–553. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M. Abiotic Stress and Reactive Oxygen Species: Generation. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef]
- Matzneller, P.; Götz, K.P.; Chmielewski, F.M. Spring frost vulnerability of sweet cherries under controlled conditions. Int. J. Biometeorol. 2016, 60, 123–130. [Google Scholar] [CrossRef]
- Beppu, K.; Kataoka, I. Studies on pistil doubling and fruit set of sweet cherry in warm climate. J. Jpn. Soc. Hortic. Sci. 2011, 80, 1–13. [Google Scholar] [CrossRef]
- Cline, J.A.; Meland, M.; Sekse, L.; Webster, A.D. Rain cracking of sweet cherries: II. Influence of rain covers and rootstocks on cracking and fruit quality. Acta Agric. Scand. 1995, 45, 224–230. [Google Scholar] [CrossRef]
- Imrak, B.; Sarier, A.; Kuden, A.; Kuden, A.B.; Comlekcioglu, S.; Tutuncu, M. Studies on shading system in sweet cherries (Prunus avium L.) to prevent double fruit formation under subtropical climatic conditions. Acta Hortic. 2014, 1059, 171–176. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Thinning Methods to Regulate Sweet Cherry Crops—A Review. Appl. Sci. 2022, 12, 1280. [Google Scholar] [CrossRef]
- Sabir, I.A.; Liu, X.; Jiu, S.; Whiting, M.; Zhang, C. Plant Growth regulators modify fruit set, fruit quality, and return bloom in sweet cherry. HortScience 2021, 56, 922–931. [Google Scholar] [CrossRef]
- Sayyad-Amin, P.; Davarynejad, G.; Abedi, B. Effects of Preharvest Application of Chemical Compounds on Pear (Pyrus communis cv. ‘Shekari’) Fruit Traits. Erwerbs-Obstbau 2022, 64, 657–662. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf Life 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Blažek, J.; Zelený, L.; Suran, P. Sweet cherry research world overview 2015–2017. Hortic. Sci. 2022, 49, 121–146. [Google Scholar] [CrossRef]
- Quero-García, J.; Letourmy, P.; Campoy, J.A.; Branchereau, C.; Malchev, S.; Barreneche, T.; Dirlewanger, E. Multi-year analyses on three populations reveal the first stable QTLs for tolerance to rain-induced fruit cracking in sweet cherry (Prunus avium L.). Hortic. Res. 2021, 8, 136. [Google Scholar] [CrossRef]
- Winkler, A.; Blumenberg, I.; Schürmann, L.; Knoche, M. Rain cracking in sweet cherries is caused by surface wetness, not by water uptake. Sci. Hortic. 2020, 269, 10940023. [Google Scholar] [CrossRef]
- Faizy, A.H.; Ozturk, B.; Aglar, E.; Yıldız, K. Role of methyl jasmonate application regime on fruit quality and bioactive compounds of sweet cherry at harvest and during cold storage. J. Food Process. 2021, 45, e15882. [Google Scholar] [CrossRef]
- Brijwal, M.; Dimri, D.C.; Mishra, D.S. Effect of foliar application of Ga3, putrescine and boric acid on yield and physical characteristics of litchi cultivars. Ecol. Environ. Conserv. 2017, 23, 7–12. [Google Scholar]
- Torrigiani, P.; Bregoli, A.M.; Ziosi, V.; Scaranagli, S.; Ciriaci, T.; Rasori, A.; Biondi, S.; Costa, G. Pre-harvest polyamine and aminoethoxyvinylglycine (AVG) applications modulate fruit ripening in Stark Red Gold nectarines (Prunus persica L. Batsch). Postharvest Biol. Technol. 2004, 33, 293–308. [Google Scholar] [CrossRef]
- Marzouk, H.A.; Kassem, H.A. Improving yield, quality, and shelf life of Thompson seedless grapevine by preharvest foliar applications. Sci. Hortic. 2011, 130, 425–430. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Demirsoy, L.; Bilgener, S. The effects of preharvest calcium hydroxide applications on cracking in ‘0900 Ziraat’, ‘Lambert’ and ‘Van’ sweet cherries. Acta Hortic. 1998, 468, 657–662. [Google Scholar] [CrossRef]
- Pereira, S.; Silva, V.; Bacelar, E.; Guedes, F.; Silva, A.P.; Ribeiro, C.; Gonçalves, B. Cracking in sweet cherry cultivars Early Bigi and Lapins: Correlation with quality attributes. Plants 2020, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, M.; Knoche, M. Mechanical properties of skins of sweet cherry fruit of differing susceptibilities to cracking. J. Am. Soc. Hortic. Sci. 2016, 141, 162–168. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; p. 138. [Google Scholar]
- Du, H.; Liu, D.; Liu, G.; Liu, H.; Kurtenbach, R. Polyamines conjugated to the bio-membranes and membrane conformations are involved in the melatonin-mediated resistance of harvested plum fruit to cold stress. Postharvest Biol. Technol. 2023, 204, 112480. [Google Scholar] [CrossRef]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. Red Delicious. Sci. Hortic. 2018, 240, 57–64. [Google Scholar] [CrossRef]
- Beigbeder, A.; Vavadakis, M.; Navakoudis, E.; Kotzabasis, K. Influence of polyamine inhibitors on light-independent and light-dependent chlorophyll biosynthesis and on the photosynthetic rate. J. Photochem. Photobiol. B 1995, 28, 235–242. [Google Scholar] [CrossRef]
- Serrano, M.; Zapata, P.J.; Martínez-Romero, D.; Díaz-Mula, H.M.; Valero, D. Polyamines as an ecofriendly postharvest tool to maintain fruit quality. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.W., Ed.; Academic Press-Elsevier: London, UK, 2016; pp. 219–242. [Google Scholar]
- Singh, V.; Jawandha, S.K.; Gill, P.P.S.; Gill, M.S. Suppression of fruit softening and extension of shelf life of pear by putrescine application. Sci. Hortic. 2019, 256, 108623. [Google Scholar] [CrossRef]

| Fruit Firmness (N mm−1) | |||
| Cultivar | Year | Control | Put 1 mM |
| ‘Prime Giant’ | 2020 | 1.82 ± 0.09 | 1.88 ± 0.09 NS |
| ‘Prime Giant’ | 2021 | 1.51 ± 0.07 | 1.66 ± 0.09 * |
| ‘Prime Giant’ | 2022 | 1.18 ± 0.05 | 1.20 ± 0.06 NS |
| ‘Sweetheart’ | 2020 | 2.08 ± 0.10 | 2.09 ± 0.10 NS |
| ‘Sweetheart’ | 2021 | 1.78 ± 0.09 | 2.03 ± 0.04 * |
| ‘Sweetheart’ | 2022 | 1.69 ± 0.07 | 1.80 ± 0.09 NS |
| ‘Sweetheart’ | 2023 | 1.89 ± 0.05 | 2.20 ± 0.09 * |
| Fruit color (CIE h°) | |||
| Cultivar | Year | Control | Put 1 mM |
| ‘Prime Giant’ | 2020 | 21.15 ± 0.80 | 23.29 ± 0.97 * |
| ‘Prime Giant’ | 2021 | 13.36 ± 0.30 | 15.66 ± 0.34 ** |
| ‘Prime Giant’ | 2022 | 23.37 ± 0.31 | 22.91 ± 0.43 NS |
| ‘Sweetheart’ | 2020 | 17.57 ± 0.58 | 18.63 ± 0.91 NS |
| ‘Sweetheart’ | 2021 | 20.65 ± 0.19 | 20.37 ± 0.16 NS |
| ‘Sweetheart’ | 2022 | 19.27 ± 0.25 | 18.77 ± 0.35 NS |
| ‘Sweetheart’ | 2023 | 19.22 ± 0.47 | 18.48 ± 0.56 NS |
| Total soluble solids (g 100 g−1) | |||
| Cultivar | Year | Control | Put 1 mM |
| ‘Prime Giant’ | 2020 | 18.45 ± 0.12 | 18.18 ± 0.34 NS |
| ‘Prime Giant’ | 2021 | 17.76 ± 0.18 | 18.20 ± 0.54 NS |
| ‘Prime Giant’ | 2022 | 22.75 ± 0.41 | 23.01 ± 0.59 NS |
| ‘Sweetheart’ | 2020 | 22.42 ± 0.07 | 21.80 ± 0.23 * |
| ‘Sweetheart’ | 2021 | 18.90 ± 0.16 | 19.26 ± 0.29 NS |
| ‘Sweetheart’ | 2022 | 22.65 ± 0.44 | 23.07 ± 0.11 NS |
| ‘Sweetheart’ | 2023 | 21.56 ± 0.20 | 21.08 ± 0.04 * |
| Titratable acidity (g 100 g−1) | |||
| Cultivar | Year | Control | Put 1 mM |
| ‘Prime Giant’ | 2020 | 1.00 ± 0.04 | 1.07 ± 0.06 NS |
| ‘Prime Giant’ | 2021 | 1.09 ± 0.01 | 1.10 ± 0.01 NS |
| ‘Prime Giant’ | 2022 | 1.58 ± 0.01 | 1.53 ± 0.02 NS |
| ‘Sweetheart’ | 2020 | 1.22 ± 0.02 | 1.36 ± 0.02 * |
| ‘Sweetheart’ | 2021 | 1.33 ± 0.02 | 1.43 ± 0.02 * |
| ‘Sweetheart’ | 2022 | 1.44 ± 0.03 | 1.60 ± 0.08 * |
| ‘Sweetheart’ | 2023 | 1.98 ± 0.08 | 1.89 ± 0.09 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aracil, M.C.; Valverde, J.M.; Beltrà, A.; Carrión-Antolí, A.; Lorente-Mento, J.M.; Nicolás-Almansa, M.; Guillén, F. Putrescine Increases Frost Tolerance and Effectively Mitigates Sweet Cherry (Prunus avium L.) Cracking: A Study of Four Different Growing Cycles. Agronomy 2024, 14, 23. https://doi.org/10.3390/agronomy14010023
Ruiz-Aracil MC, Valverde JM, Beltrà A, Carrión-Antolí A, Lorente-Mento JM, Nicolás-Almansa M, Guillén F. Putrescine Increases Frost Tolerance and Effectively Mitigates Sweet Cherry (Prunus avium L.) Cracking: A Study of Four Different Growing Cycles. Agronomy. 2024; 14(1):23. https://doi.org/10.3390/agronomy14010023
Chicago/Turabian StyleRuiz-Aracil, María Celeste, Juan Miguel Valverde, Aleixandre Beltrà, Alberto Carrión-Antolí, José Manuel Lorente-Mento, Marta Nicolás-Almansa, and Fabián Guillén. 2024. "Putrescine Increases Frost Tolerance and Effectively Mitigates Sweet Cherry (Prunus avium L.) Cracking: A Study of Four Different Growing Cycles" Agronomy 14, no. 1: 23. https://doi.org/10.3390/agronomy14010023





