The Impact of Shale Oil Residue on the Growth and Physiological Characteristics of Corn Seedlings under Saline Soil Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Index Determination and Methods
2.3.1. Growth Index Determination
2.3.2. Physiological Index Measurement
2.4. Statistical Analysis
3. Results
3.1. Growth Characteristics
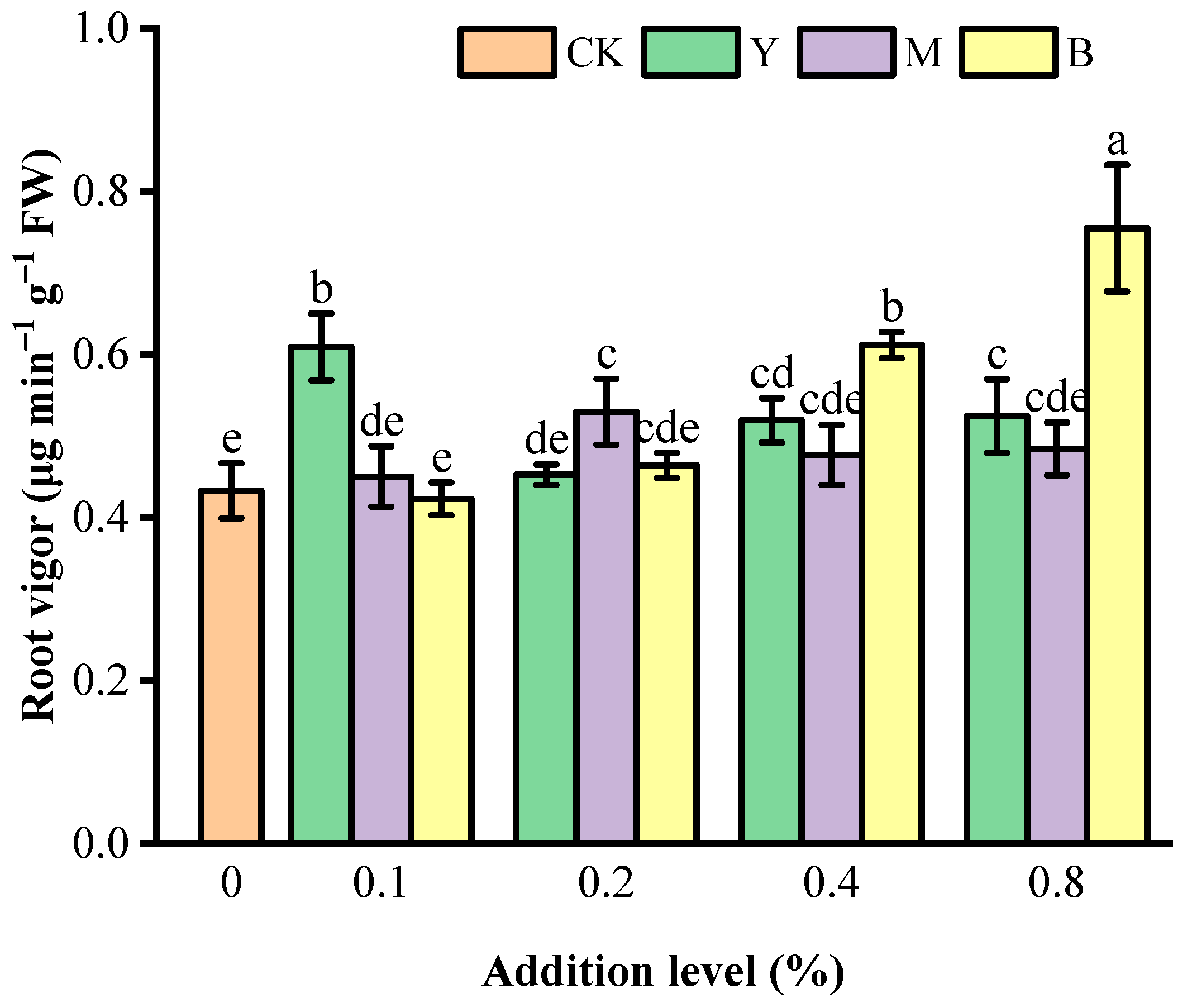
3.2. Root Vitality
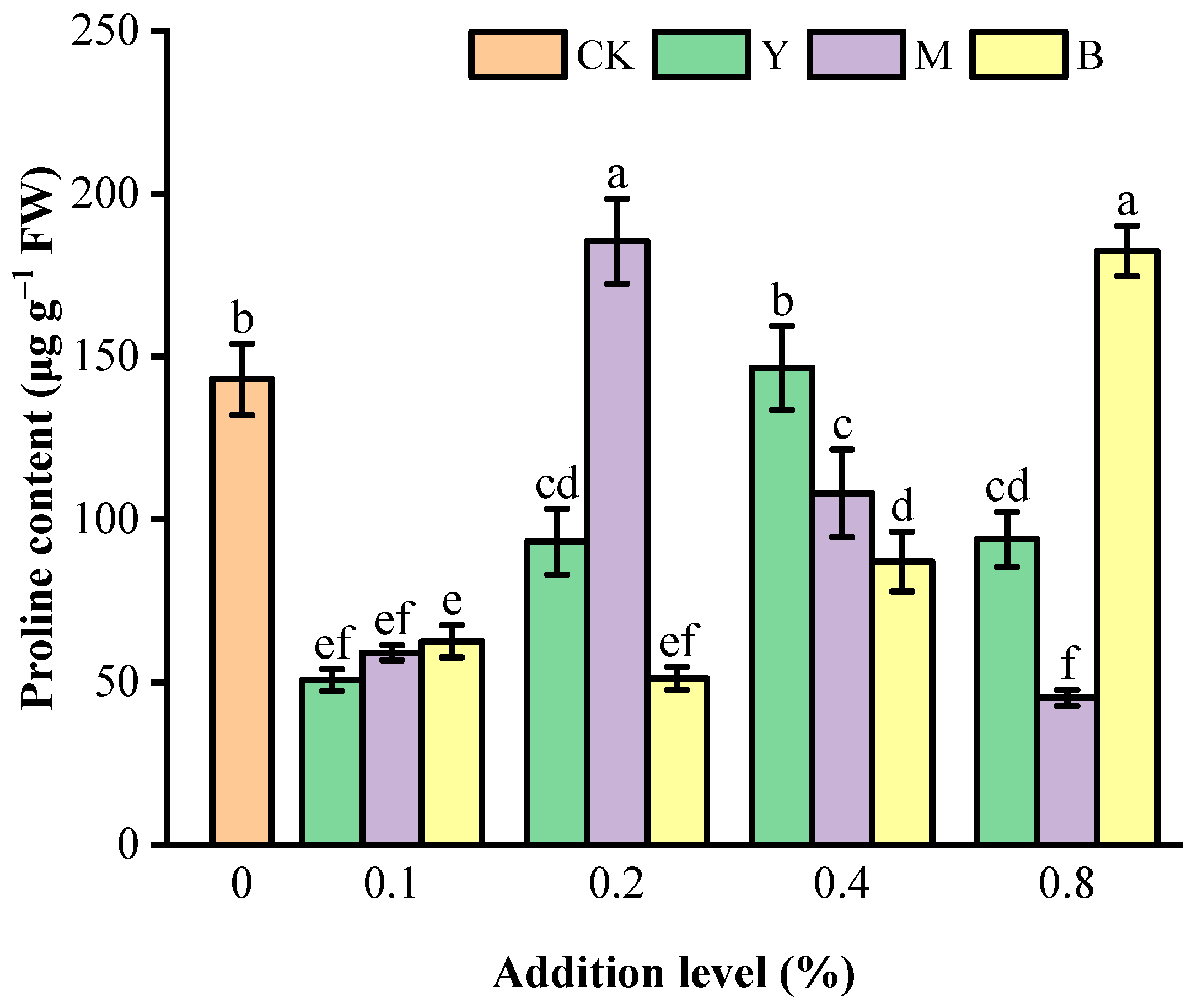
3.3. Proline Content
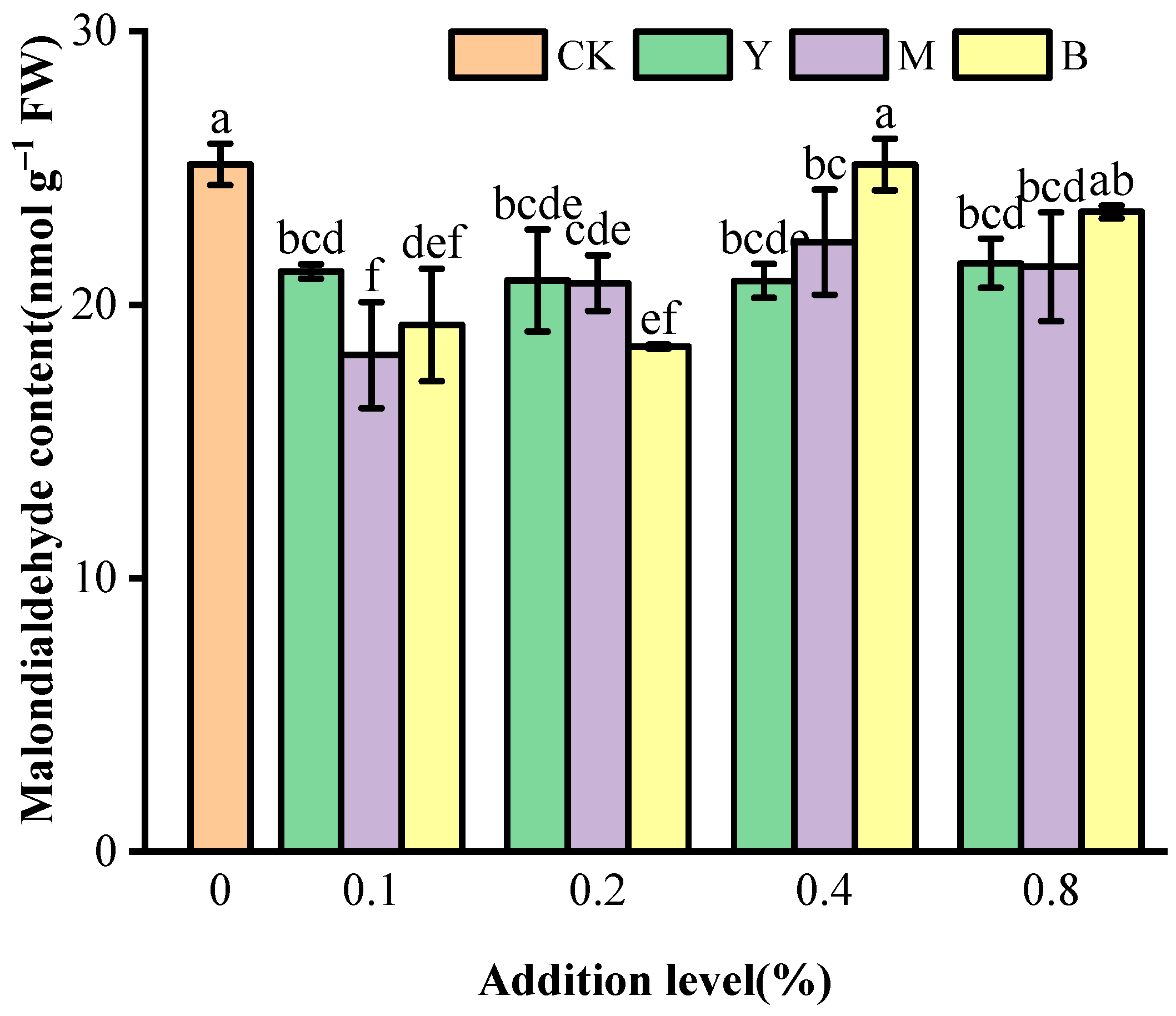
3.4. Malondialdehyde Content
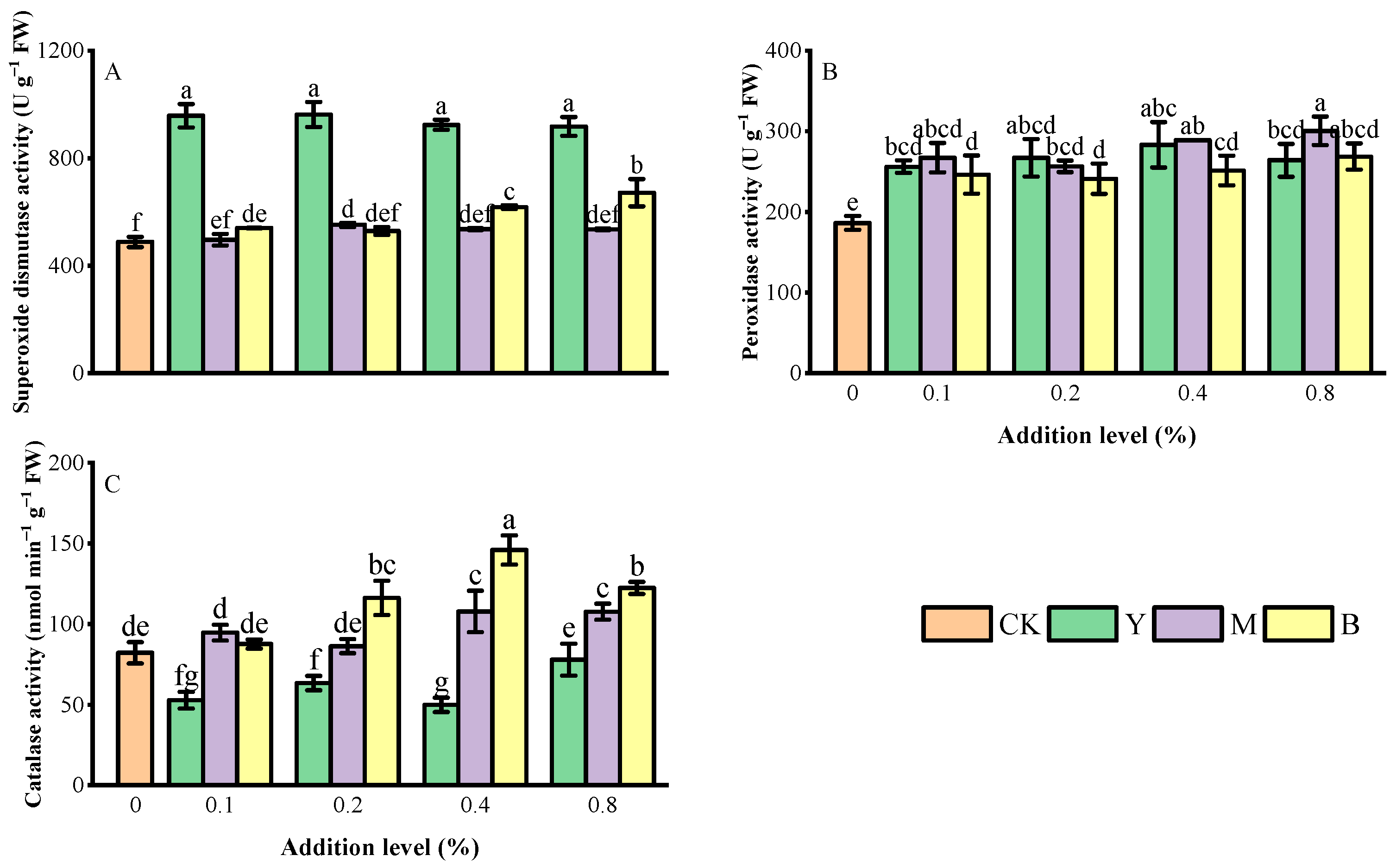
3.5. Antioxidant Enzyme Activity
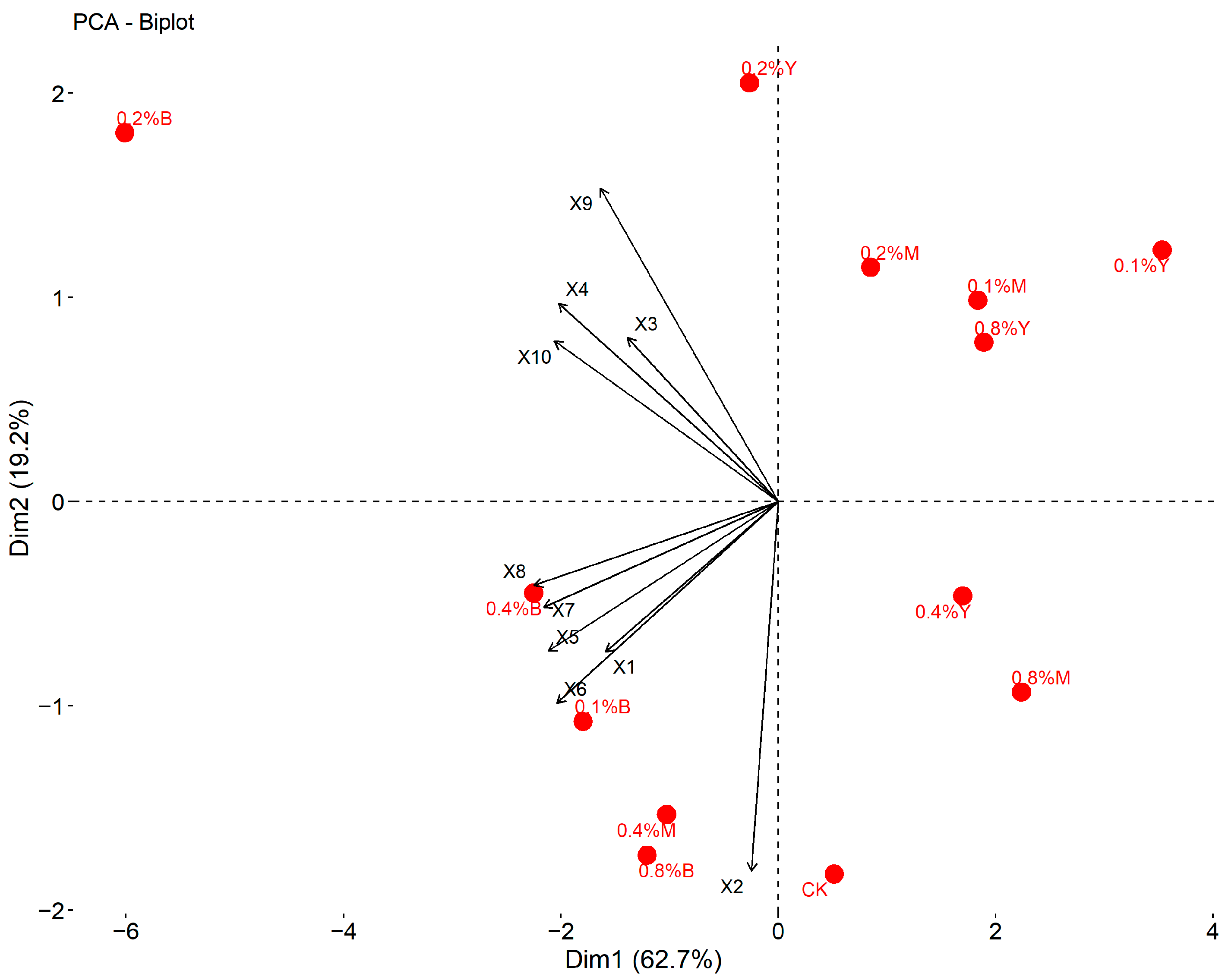
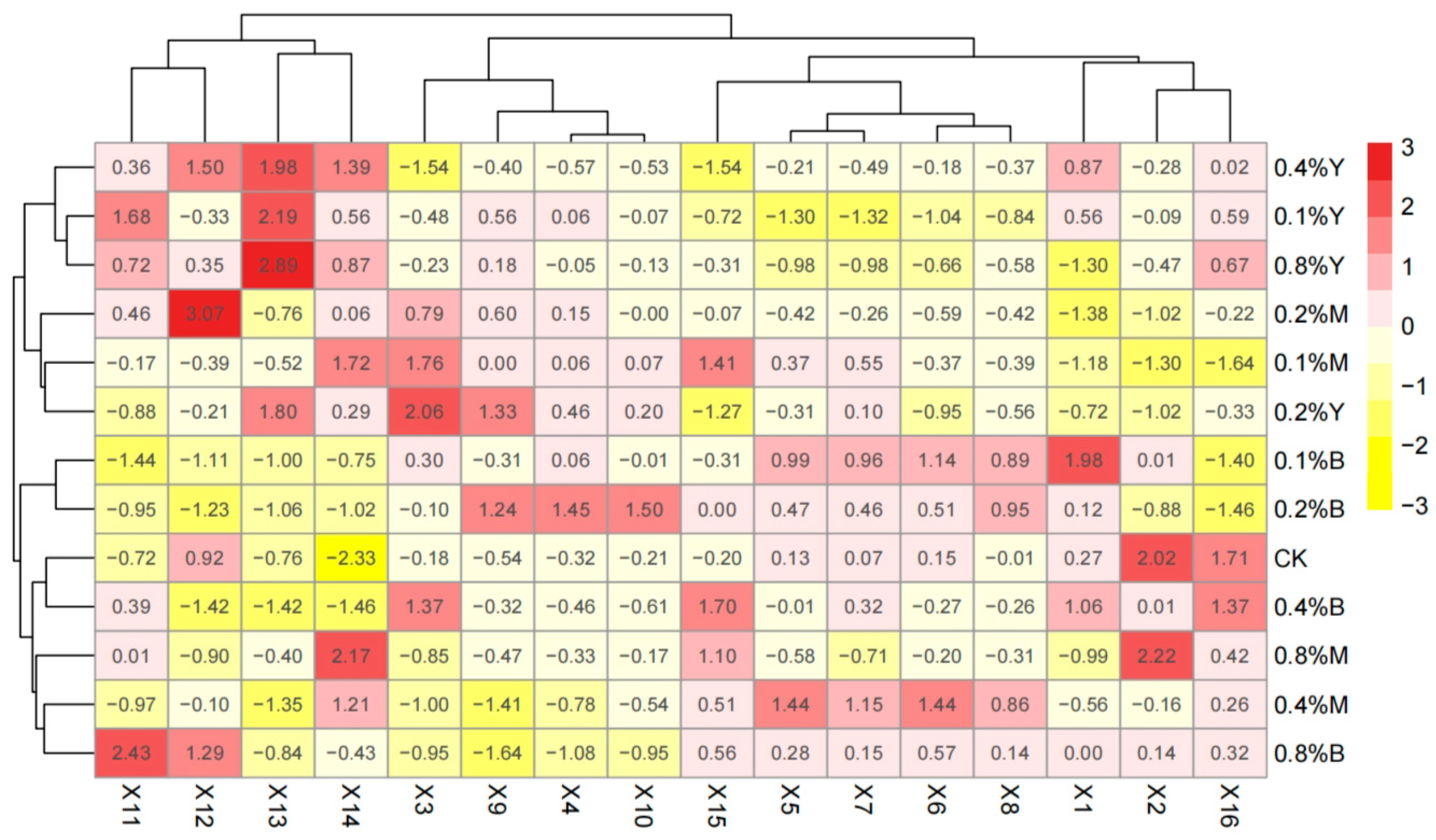
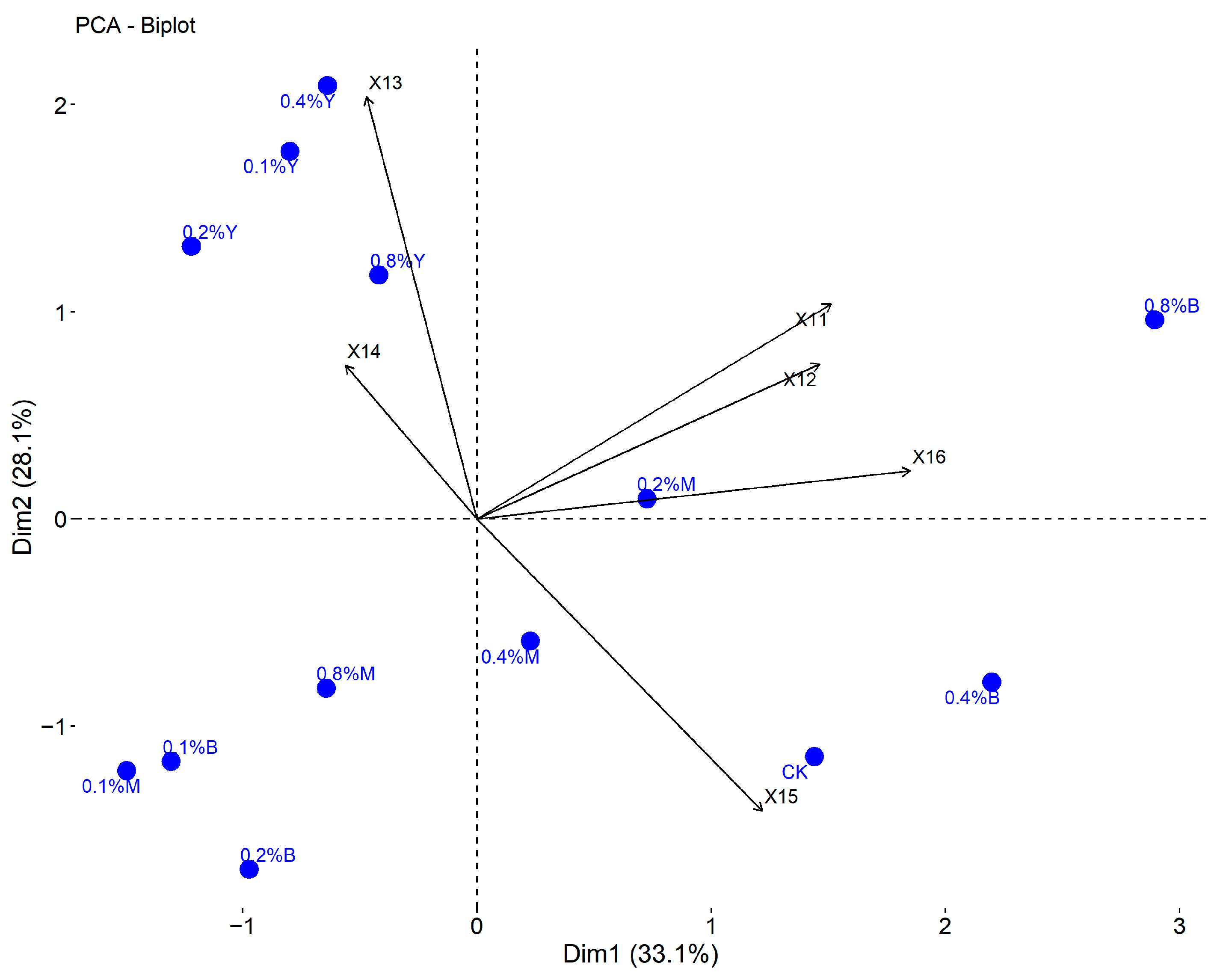
3.6. Comprehensive Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.L.; Zhou, G.S.; Younas, M.U.; Suliman, M.S.E.; Liu, J.; Zhu, Y.M.; Salih, E.G.I. Integrated approaches for increasing plant yield under salt stress. Front. Plant Sci. 2023, 14, 1215343. [Google Scholar] [CrossRef]
- Zhang, L. Analysis and countermeasure research on saline-alkali land change in Xinjiang irrigation area in recent 20 years. Water Resour. Dev. Manag. 2020, 6, 72–76. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhou, X.Y.; Song, H.F.; Zhang, M.; Jiang, C.F. Advances in deciphering salt tolerance mechanism in maize. Crop J. 2023, 11, 1001–1010. [Google Scholar] [CrossRef]
- Li, X.; Shi, X.S.; Lu, M.Y.; Zhao, Y.Z.; Guo, R.B.; Peng, H. Improved nitrogen conservation capacity during composting of dairy manure amended with oil shale semi-coke as the porous bulking agent. J. Hazard. Mater. 2020, 388, 121742. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, X.M.; Han, X.X.; Tong, J.H. Investigation of Chinese oil shale resources comprehensive utilization performance. Energy 2012, 42, 224–232. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Chen, L.H.; Aisha, N. The development and utilization and broad prospects of Xinjiang shale oil. Mod. Che. Res. 2022, 8, 121–123. [Google Scholar]
- Jing, W.H.; Liu, H.; Zhang, P.; Xu, J.L. An experimental study on agricul tural utilization of shale oil industrial solid waste. J. Beijing Norm. Univ. (Nat. Sci.) 2001, 2, 260–265. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.-B.A.-E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Kul, R.; Arjumend, T.; Ekinci, M.; Yildirim, E.; Turan, M.; Argin, S. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 2021, 67, 693–706. [Google Scholar] [CrossRef]
- Keya, S.S.; Mostofa, M.G.; Rahman, M.M.; Das, A.K.; Sultana, S.; Ghosh, P.K.; Anik, T.R.; Ahsan, S.; Rahman, M.A.; Jahan, N. Salicylic Acid Application Improves Photosynthetic Performance and Biochemical Responses to Mitigate Saline Stress in Cotton. J. Plant Growth Regul. 2023, 42, 1–14. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Khojah, E.; Samra, B.N.; Fujita, M.; Nahar, K. Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in jute (Corchorus olitorius L.). Antioxidants 2021, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, X.S.; Lu, M.Y.; Guo, R.B.; Feng, Q. Improved fertilization efficiency of manure compost obtained by using oil shale semicoke as the bulking agent. J. Environ. Manag. 2022, 324, 116309. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.C. Research Progress of Agricultural Utilization of Oil Shale and Waste Residue. J. Liaoning Agric. Coll. 2014, 16, 12–14. [Google Scholar] [CrossRef]
- Huang, J.; Xia, H.P.; Kong, G.H. Impact of oil shale waste slag on plant growth and benefits of limestone amendment. Chin. J. Ecol. 2007, 01, 9–15. [Google Scholar] [CrossRef]
- Zhou, J.M.; Niu, X.C. Sources and Utilization of Oil Shale Residue. J. Guangdong Univ. Petrochem. Technol. 2013, 23, 11–14. [Google Scholar]
- Nicolini, J.; Pereira, B.F.; Pillon, C.N.; Machado, V.G.; Lopes, W.A.; de Andrade, J.B.; Mangrich, A.S. Characterization of Brazilian oil shale byproducts planned for use as soil conditioners for food and agro-energy production. J. Anal. Appl. Pyrolysis 2011, 90, 112–117. [Google Scholar] [CrossRef]
- GB15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. China MoEPotPsRo: Beijing, China, 2018.
- Wang, Y.; Li, L.Z.; Guo, W.Z.; Wen, J.L.; Li, Y.K.; Fan, F.C.; Wu, Y.S.; Li, H.P. Effects of Humic Acid on the Growth, Physiology and Water Use Efriciency of Zucchini Seedlings. Chin. Sci. Bull. 2022, 38, 47–53. [Google Scholar] [CrossRef]
- Wang, J.T.; Du, G.F.; Tian, J.S.; Zhang, Y.L.; Jiang, C.D.; Zhang, W.F. Effect of irrigation methods on root growth, root-shoot ratio and yield components of cotton by regulating the growth redundancy of root and shoot. Agric. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Chen, G.D.; Wang, L.; Fabrice, M.R.; Tian, Y.A.; Qi, K.J.; Chen, Q.; Cao, P.; Wang, P.; Zhang, S.L.; Wu, J.Y. Physiological and nutritional responses of pear seedlings to nitrate concentrations. Front. Plant Sci. 2018, 9, 1679. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Hasanuzzaman, M.; Khan, M.I.R.; Nahar, K.; Fujita, M. β-Aminobutyric Acid Pretreatment Confers Salt Stress Tolerance in Brassica napus L. by Modulating Reactive Oxygen Species Metabolism and Methylglyoxal Detoxification. Plants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, M.L.; Mao, P.S.; Jiang, Y.W. Effects of gamma-aminobutyric acid on seed germination, ion balance, and metabolic activity in perennial ryegrass under salinity stress. J. Plant Growth Regul. 2022, 41, 1835–1844. [Google Scholar] [CrossRef]
- Ullah, A.; Tariq, A.; Zeng, F.; Noor, J.; Sardans, J.; Asghar, M.A.; Zhang, Z.; Peñuelas, J. Application of GABA (γ-aminobutyric acid) to improve saline stress tolerance of chufa (Cyperus esculentus L. var. sativus Boeck) plants by regulating their antioxidant potential and nitrogen assimilation. S. Afr. J. Bot. 2023, 157, 540–552. [Google Scholar] [CrossRef]
- Soothar, M.K.; Mounkaila Hamani, A.K.; Kumar Sootahar, M.; Sun, J.S.; Yang, G.; Bhatti, S.M.; Traore, A. Assessment of acidic biochar on the growth, physiology and nutrients uptake of maize (Zea mays L.) seedlings under salinity stress. Sustainability 2021, 13, 3150. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Ma, Y.R.; Qi, T.; Feng, Y.Z.; Meng, A.J.; Wang, X.Y. Effects induced by inputting biochar into the saliferous gray desert soil on the soil moisture movement. Xinjiang Agric. Sci. 2017, 32, 182–188. [Google Scholar]
- Javeed, H.M.R.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Rehman, A.U.; Faheem, M.; Mubeen, M.; Iqbal, M.M.; Rahman, M.H.U. Lipoic acid combined with melatonin mitigates oxidative stress and promotes root formation and growth in salt-stressed canola seedlings (Brassica napus L.). Molecules 2021, 26, 3147. [Google Scholar] [CrossRef]
- Aibdin, U.Z.; Nafees, M.; Rizwan, M.; Ahmad, S.; Ali, S.; Obaid, W.A.; Alsubeie, M.S.; Darwish, D.B.E.; Abeed, A.H. Combined effect of Zinc lysine and biochar on growth and physiology of wheat (Triticum aestivum L.) to alleviate salinity stress. Front. Plant Sci. 2022, 13, 1017282. [Google Scholar] [CrossRef]
- Cheng, B.Z.; Zhou, L.; Liang, L.L.; Cao, Y.Q.; Zeng, W.H.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Nie, G.; Liu, W. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, X.Y.; Yu, G.G.; Zhao, Y.; Li, J.Y.; Luo, X.Z. Study on the Improvement Effect of Low-grade Oil Shale on Sandy Soil. China Resour. Compr. Util. 2021, 39, 26–28. [Google Scholar] [CrossRef]
- Li, X.J.; Yu, B.J.; Cui, Y.Q.; Yin, Y.F. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul. 2017, 83, 441–454. [Google Scholar] [CrossRef]
- Zhang, J.H.; Bai, Z.G.; Huang, J.; Hussain, S.; Zhao, F.T.; Zhu, C.Q.; Zhu, L.F.; Cao, X.C.; Jin, Q.Y. Biochar alleviated the salt stress of induced saline paddy soil and improved the biochemical characteristics of rice seedlings differing in salt tolerance. Soil. Tillage Res. 2019, 195, 104372. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Deng, Q.; Duan, H.L.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Wei, J.H.; Guo, L.L.; Fang, H.; Liu, X.J.; Liang, K.H.; Niu, W.Q.; Liu, F.L.; Siddique, K.H. Effects of two biochar types on mitigating drought and salt stress in tomato seedlings. Agronomy 2023, 13, 1039. [Google Scholar] [CrossRef]
- Islam, A.T.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Effect of salicylic acid seed priming on morpho-physiological responses and yield of baby corn under salt stress. Sci. Hortic. 2022, 304, 111304. [Google Scholar] [CrossRef]
- Kaya, C.; Aydemir, S.; Akram, N.A.; Ashraf, M. Epibrassinolide application regulates some key physio-biochemical attributes as well as oxidative defense system in maize plants grown under saline stress. J. Plant Growth Regul. 2018, 37, 1244–1257. [Google Scholar] [CrossRef]
- Wang, M.Q.; Gong, S.C.; Fu, L.X.; Hu, G.H.; Li, G.L.; Hu, S.X.; Yang, J.F. The involvement of antioxidant enzyme system, nitrogen metabolism and osmoregulatory substances in alleviating salt stress in inbred maize lines and hormone regulation mechanisms. Plants 2022, 11, 1547. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M. Impact of biochar in mitigating the negative effect of drought stress on cabbage seedlings. J. Soil Sci. Plant Nutr. 2021, 21, 2297–2309. [Google Scholar] [CrossRef]
- Jabborova, D.; Abdrakhmanov, T.; Jabbarov, Z.; Abdullaev, S.; Azimov, A.; Mohamed, I.; AlHarbi, M.; Abu-Elsaoud, A.; Elkelish, A. Biochar improves the growth and physiological traits of alfalfa, amaranth and maize grown under salt stress. PeerJ 2023, 11, e15684. [Google Scholar] [CrossRef]
- Qi, Q.; Ma, S.R.; Xu, W.D. Advances in the effects of salt stress on plant growth and physiological mechanisms of salt tolerance. Mol. Plant Breed. 2020, 18, 2741–2746. [Google Scholar] [CrossRef]
- Ren, J.H.; Ye, J.; Yin, L.N.; Li, G.X.; Deng, X.P.; Wang, S.W. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Hai, X.; Mi, J.Z.; Zhao, B.P.; Zhang, B.R.; Zhao, Z.; Liu, J.H. Foliar application of spermidine reduced the negative effects of salt stress on oat seedlings. Front. Plant Sci. 2022, 13, 846280. [Google Scholar] [CrossRef] [PubMed]
| Materials | Unit | Cultivation Substrate | Oil Shale Waste Residue | ||
|---|---|---|---|---|---|
| Saline–Alkali Soil | Fine Concentrate Ore | Fine Ore | Semi-Coke | ||
| pH | - | 8.55 | 8.26 | 7.89 | 8.43 |
| Water-soluble salts | g·kg−1 | 9.4 | 4.2 | 7.5 | 8.2 |
| Total nitrogen | g·kg−1 | 0.4 | 2.57 | 0.93 | 2.73 |
| Organic matter | % | 4.31 | 28.08 | 6.9 | 13.26 |
| Hydrolysable nitrogen | mg·kg−1 | 22.5 | 55.5 | 55.5 | 69.1 |
| Available phosphorus | mg·kg−1 | 7.9 | 44.3 | 42.8 | 52.9 |
| Quick-acting potassium | mg·kg−1 | 114 | 72 | 85 | 105 |
| Arsenic | mg·kg−1 | <0.002 | <0.002 | <0.002 | <0.002 |
| Mercury | mg·kg−1 | <0.002 | 0.044 | 0.028 | 0.041 |
| Chromium | mg·kg−1 | <0.04 | 0.7 | 0.6 | 0.7 |
| Lead | mg·kg−1 | 9 | 38 | 36 | 30 |
| Cadmium | mg·kg−1 | <0.01 | <0.01 | <0.01 | <0.01 |
| Nickel | mg·kg−1 | 12 | 66 | 68 | 66 |
| Addition Levels | Treatment | Height (cm) | Stem Width (mm) | Fresh Weight (g/plant) | Dry Weight (g/plant) | Root/Shoot Ratio | Seedling Vigor Index | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Underground | Aboveground | Total | Underground | Aboveground | Total | ||||||
| 0 | CK | 41.78 ± 0.59 a | 4.85 ± 0.16 a | 0.80 ± 0.18 ab | 6.68 ± 0.42 ab | 7.48 ± 0.60 abc | 0.10 ± 0.02 b | 0.98 ± 0.04 ab | 1.07 ± 0.05 b | 0.10 ± 0.02 a | 0.23 ± 0.02 b |
| 0.1% | Y | 41.22 ± 1.84 a | 4.33 ± 0.23 bc | 0.62 ± 0.24 b | 4.57 ± 1.03 b | 5.19 ± 1.14 c | 0.09 ± 0.03 b | 0.72 ± 0.11 b | 0.81 ± 0.10 b | 0.13 ± 0.05 a | 0.19 ± 0.03 b |
| M | 39.06 ± 2.56 a | 4.23 ± 0.46 bc | 0.93 ± 0.62 ab | 6.21 ± 2.41 ab | 7.14 ± 2.71 abc | 0.09 ± 0.07 b | 0.85 ± 0.28 ab | 0.94 ± 0.32 b | 0.10 ± 0.09 a | 0.20 ± 0.11 b | |
| B | 44.59 ± 4.03 a | 4.49 ± 0.36 abc | 0.94 ± 0.14 ab | 7.58 ± 0.25 a | 8.52 ± 0.27 ab | 0.14 ± 0.02 ab | 1.11 ± 0.06 a | 1.25 ± 0.05 ab | 0.13 ± 0.02 a | 0.28 ± 0.04 b | |
| 0.2% | Y | 40.39 ± 2.78 a | 4.32 ± 0.20 bc | 1.24 ± 0.46 a | 6.44 ± 1.76 ab | 7.67 ± 2.07 abc | 0.16 ± 0.06 ab | 0.86 ± 0.24 ab | 1.02 ± 0.29 b | 0.18 ± 0.04 a | 0.30 ± 0.10 b |
| M | 39.56 ± 4.06 a | 4.33 ± 0.27 bc | 0.94 ± 0.11 ab | 6.24 ± 0.92 ab | 7.18 ± 1.02 abc | 0.13 ± 0.01 b | 0.90 ± 0.12 ab | 1.02 ± 0.12 b | 0.14 ± 0.02 a | 0.26 ± 0.02 b | |
| B | 43.52 ± 2.78 a | 4.39 ± 0.29 abc | 1.02 ± 0.40 ab | 8.37 ± 1.59 a | 9.39 ± 1.67 a | 0.34 ± 0.38 a | 1.21 ± 0.26 a | 1.55 ± 0.59 a | 0.26 ± 0.26 a | 0.65 ± 0.69 a | |
| 0.4% | Y | 42.31 ± 4.64 a | 4.38 ± 0.11 abc | 0.55 ± 0.23 b | 6.21 ± 1.96 ab | 6.77 ± 1.82 abc | 0.08 ± 0.03 b | 0.91 ± 0.33 ab | 0.99 ± 0.32 b | 0.11 ± 0.07 a | 0.19 ± 0.03 b |
| M | 41.17 ± 1.32 a | 4.49 ± 0.18 abc | 0.77 ± 0.26 ab | 7.96 ± 1.67 a | 8.74 ± 1.71 ab | 0.11 ± 0.03 b | 1.15 ± 0.23 a | 1.26 ± 0.22 ab | 0.10 ± 0.04 a | 0.26 ± 0.03 b | |
| B | 44.34 ± 2.54 a | 4.61 ± 0.08 abc | 1.23 ± 0.27 a | 7.45 ± 1.06 a | 8.68 ± 0.79 ab | 0.16 ± 0.04 ab | 1.05 ± 0.13 ab | 1.21 ± 0.09 ab | 0.16 ± 0.07 a | 0.31 ± 0.05 b | |
| 0.8% | Y | 39.44 ± 1.58 a | 4.36 ± 0.13 bc | 0.77 ± 0.11 ab | 5.75 ± 1.17 ab | 6.52 ± 1.28 bc | 0.11 ± 0.02 b | 0.87 ± 0.13 ab | 0.98 ± 0.15 b | 0.12 ± 0.01 a | 0.23 ± 0.03 b |
| M | 39.17 ± 3.47 a | 4.75 ± 0.09 ab | 0.64 ± 0.15 b | 5.77 ± 0.91 ab | 6.41 ± 0.99 bc | 0.09 ± 0.01 b | 0.89 ± 0.12 ab | 0.98 ± 0.12 b | 0.10 ± 0.02 a | 0.22 ± 0.01 b | |
| B | 42.76 ± 2.12 a | 4.61 ± 0.28 abc | 0.85 ± 0.10 ab | 7.61 ± 0.90 a | 8.46 ± 0.97 ab | 0.12 ± 0.02 b | 1.13 ± 0.17 a | 1.25 ± 0.18 ab | 0.11 ± 0.02 a | 0.27 ± 0.04 b | |
| Indicators | Type | Addition Level | Type × Addition Level | ||||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| X1 | Height | 6.436 | ** | 0.89 | ns | 0.126 | ns |
| X2 | Stem width | 1.566 | ns | 1.931 | ns | 0.652 | ns |
| X3 | Underground fresh weight | 1.913 | ns | 1.849 | ns | 1.518 | ns |
| X4 | Underground dry weight | 2.212 | ns | 1.796 | ns | 0.526 | ns |
| X5 | Aboveground fresh weight | 6.557 | ** | 1.276 | ns | 0.805 | ns |
| X6 | Aboveground dry weight | 6.945 | ** | 0.885 | ns | 0.796 | ns |
| X7 | Total fresh weight | 7.146 | ** | 1.52 | ns | 0.786 | ns |
| X8 | Total dry weight | 7.21 | ** | 1.165 | ns | 0.786 | ns |
| X9 | Root/Shoot ratio | 1.067 | ns | 2.012 | ns | 0.31 | ns |
| X10 | Seedling vigor index | 2.273 | ns | 1.583 | ns | 0.626 | ns |
| X11 | Root vitality | 13.48 | *** | 14.508 | *** | 22.34 | *** |
| X12 | Proline | 0.639 | ns | 81.593 | *** | 134.448 | *** |
| X13 | Superoxide dismutase | 820.503 | *** | 4.205 | * | 9.575 | *** |
| X14 | Peroxidase | 6.738 | ** | 4.015 | * | 1.186 | ns |
| X15 | Catalase | 201.306 | *** | 23.447 | *** | 13.511 | *** |
| X16 | Malondialdehyde | 1.395 | ns | 12.374 | *** | 5.326 | ** |
| Components | Initial Eigenvalues | Total Extraction Sums of Squares Loadings | ||||
|---|---|---|---|---|---|---|
| Total | Variance Percentage | Accumulated % | Total | Variance Percentage | Accumulated % | |
| 1 | 6.993 | 43.707 | 43.707 | 6.993 | 43.707 | 43.707 |
| 2 | 3.041 | 19.009 | 62.716 | 3.041 | 19.009 | 62.716 |
| 3 | 1.536 | 9.603 | 72.319 | 1.536 | 9.603 | 72.319 |
| 4 | 1.36 | 8.499 | 80.818 | 1.36 | 8.499 | 80.818 |
| 5 | 0.94 | 5.873 | 86.691 | |||
| 6 | 0.883 | 5.518 | 92.209 | |||
| 7 | 0.595 | 3.719 | 95.928 | |||
| 8 | 0.423 | 2.647 | 98.575 | |||
| 9 | 0.126 | 0.787 | 99.362 | |||
| 10 | 0.075 | 0.469 | 99.832 | |||
| 11 | 0.019 | 0.118 | 99.949 | |||
| 12 | 0.008 | 0.051 | 100 | |||
| 13 | 1.21 × 10−16 | 7.57 × 10−16 | 100 | |||
| 14 | −8.16 × 10−17 | −5.10 × 10−16 | 100 | |||
| 15 | −3.56 × 10−16 | −2.23 × 10−15 | 100 | |||
| 16 | −7.47 × 10−16 | −4.67 × 10−15 | 100 | |||
| Variables | Loading Matrix | Eigenvectors | ||||||
|---|---|---|---|---|---|---|---|---|
| Principal Components 1 | Principal Components 2 | Principal Components 3 | Principal Components 4 | Principal Components 1 | Principal Components 2 | Principal Components 3 | Principal Components 4 | |
| X1 | 0.667 | 0.203 | 0.282 | −0.148 | 0.252 | 0.116 | 0.228 | −0.127 |
| X2 | 0.197 | 0.777 | −0.252 | −0.270 | 0.074 | 0.446 | −0.203 | −0.232 |
| X3 | 0.592 | −0.200 | 0.216 | −0.178 | 0.224 | −0.115 | 0.174 | −0.153 |
| X4 | 0.830 | −0.468 | 0.149 | −0.095 | 0.314 | −0.268 | 0.120 | −0.081 |
| X5 | 0.927 | 0.180 | −0.069 | 0.167 | 0.351 | 0.103 | −0.056 | 0.143 |
| X6 | 0.900 | 0.263 | −0.082 | 0.203 | 0.340 | 0.151 | −0.066 | 0.174 |
| X7 | 0.943 | 0.126 | −0.024 | 0.119 | 0.357 | 0.072 | −0.019 | 0.102 |
| X8 | 0.969 | 0.037 | −0.010 | 0.121 | 0.366 | 0.021 | −0.008 | 0.104 |
| X9 | 0.635 | −0.633 | 0.307 | −0.183 | 0.240 | −0.363 | 0.248 | −0.157 |
| X10 | 0.854 | −0.410 | 0.089 | −0.065 | 0.323 | −0.235 | 0.072 | −0.056 |
| X11 | −0.061 | 0.441 | 0.704 | 0.363 | −0.023 | 0.253 | 0.568 | 0.311 |
| X12 | −0.047 | 0.554 | 0.392 | 0.009 | −0.018 | 0.318 | 0.316 | 0.008 |
| X13 | −0.523 | −0.302 | 0.682 | −0.010 | −0.198 | −0.173 | 0.550 | −0.009 |
| X14 | −0.285 | −0.150 | −0.017 | 0.886 | −0.108 | −0.086 | −0.014 | 0.760 |
| X15 | 0.693 | 0.386 | −0.111 | 0.302 | 0.262 | 0.221 | −0.090 | 0.259 |
| X16 | −0.005 | 0.828 | 0.288 | −0.286 | −0.002 | 0.475 | 0.232 | −0.245 |
| Treatment | F1 | F2 | F3 | F4 | F | Rank |
|---|---|---|---|---|---|---|
| CK | −0.002 | 2.599 | −1.006 | −3.068 | 13.589 | 6 |
| 0.1%Y | −4.033 | −1.147 | 1.193 | −0.611 | −191.796 | 13 |
| 0.1%M | −1.544 | −1.449 | −1.685 | 0.645 | −105.749 | 12 |
| 0.1%B | 1.898 | −0.345 | −1.154 | −0.298 | 62.808 | 5 |
| 0.2%Y | −0.397 | −2.179 | 1.156 | −0.657 | −53.252 | 8 |
| 0.2%M | −0.788 | −0.163 | 0.315 | 0.002 | −34.479 | 7 |
| 0.2%B | 6.025 | −2.887 | 0.058 | −0.217 | 207.149 | 1 |
| 0.4%Y | −2.359 | −0.045 | 0.807 | 0.531 | −91.686 | 10 |
| 0.4%M | 1.210 | 1.146 | −1.106 | 1.502 | 76.794 | 4 |
| 0.4%B | 2.707 | 1.654 | 1.135 | −0.227 | 158.741 | 2 |
| 0.8%Y | −2.204 | −0.683 | 0.541 | 0.014 | −103.986 | 11 |
| 0.8%M | −1.879 | 0.639 | −1.985 | 0.935 | −81.094 | 9 |
| 0.8%B | 1.365 | 2.859 | 1.730 | 1.449 | 142.961 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Dong, Z.; Meng, A.; Qi, T.; Ge, C. The Impact of Shale Oil Residue on the Growth and Physiological Characteristics of Corn Seedlings under Saline Soil Conditions. Agronomy 2024, 14, 44. https://doi.org/10.3390/agronomy14010044
Huang J, Dong Z, Meng A, Qi T, Ge C. The Impact of Shale Oil Residue on the Growth and Physiological Characteristics of Corn Seedlings under Saline Soil Conditions. Agronomy. 2024; 14(1):44. https://doi.org/10.3390/agronomy14010044
Chicago/Turabian StyleHuang, Jian, Zhiduo Dong, Ajing Meng, Tong Qi, and Chunhui Ge. 2024. "The Impact of Shale Oil Residue on the Growth and Physiological Characteristics of Corn Seedlings under Saline Soil Conditions" Agronomy 14, no. 1: 44. https://doi.org/10.3390/agronomy14010044





