Choice and No-Choice Feeding Assays of Cotton Fleahoppers (Pseudatomoscelis seriatus) on Cotton Expressing the Mpp51Aa2 Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Insect Sources
2.2. Choice Feeding Assay
2.3. No-Choice Feeding Assay
2.4. Statistical Analyses
3. Results
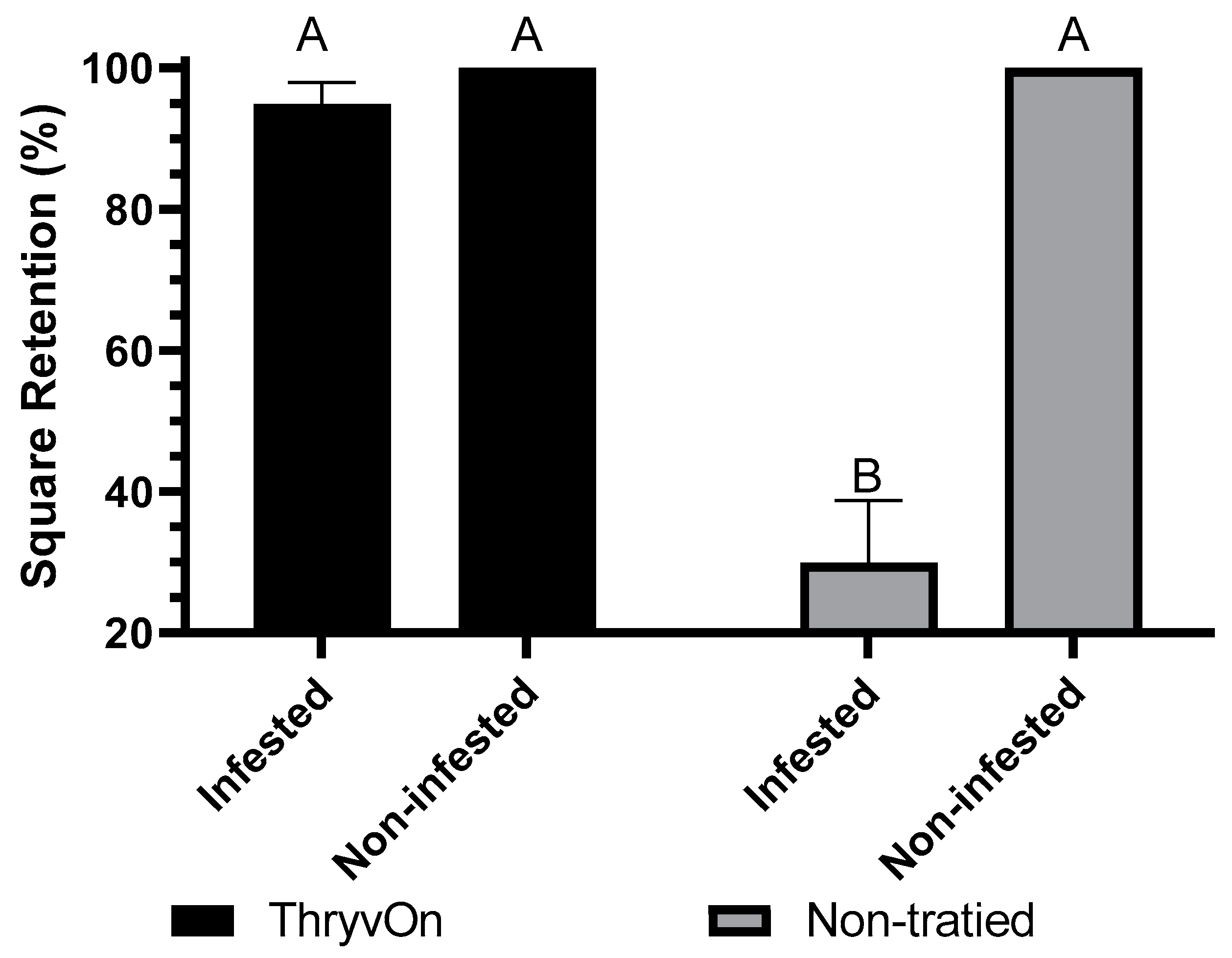
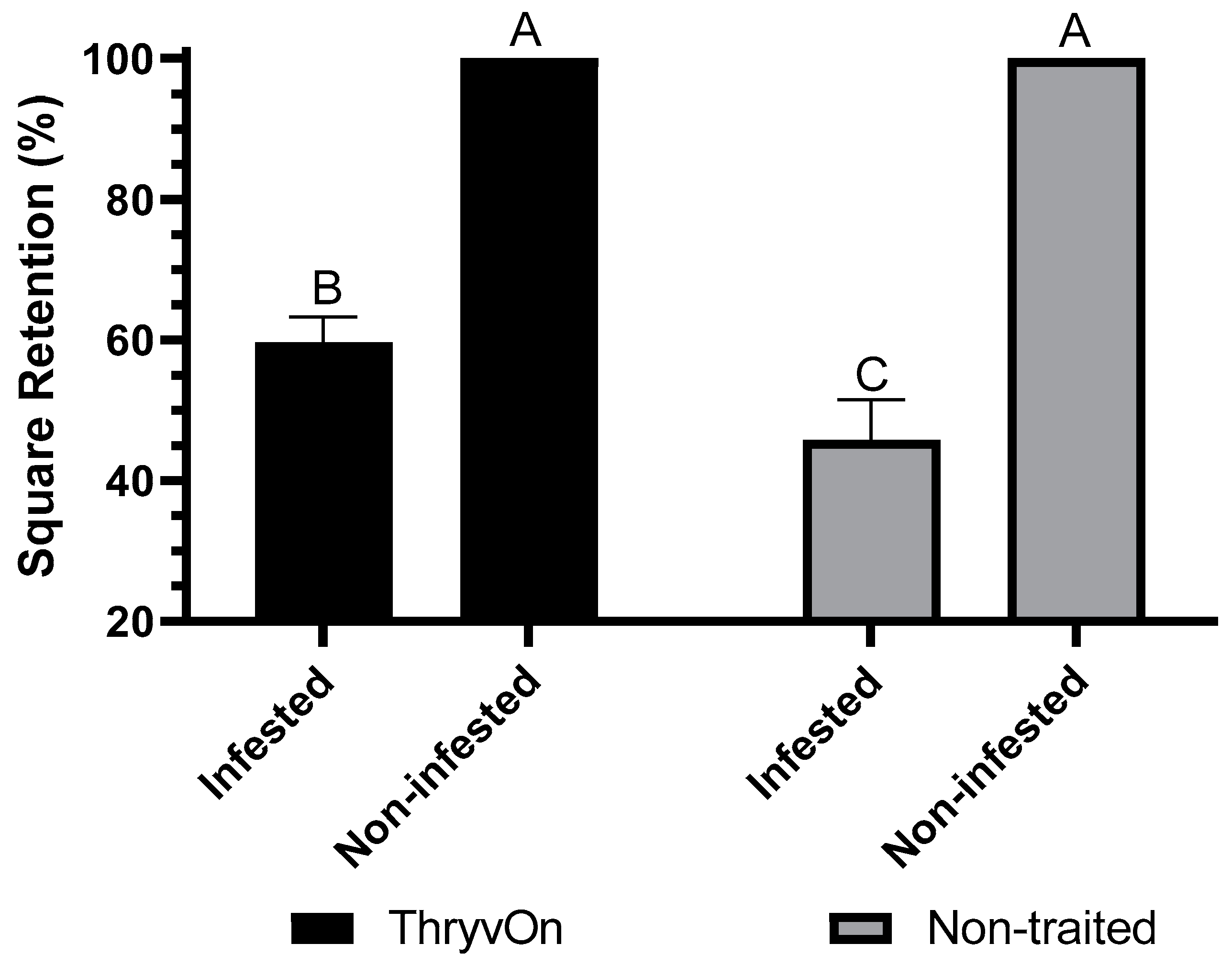
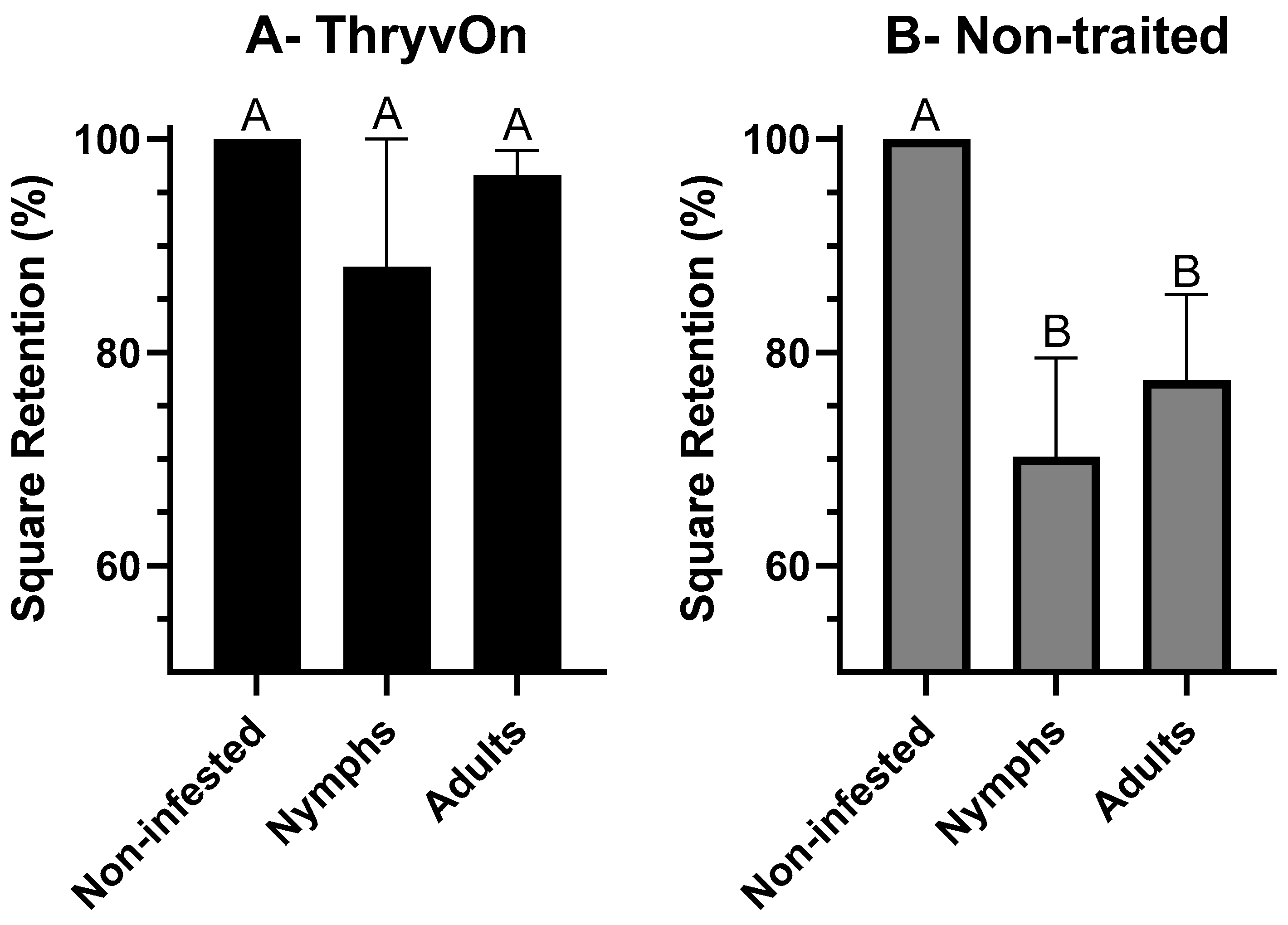
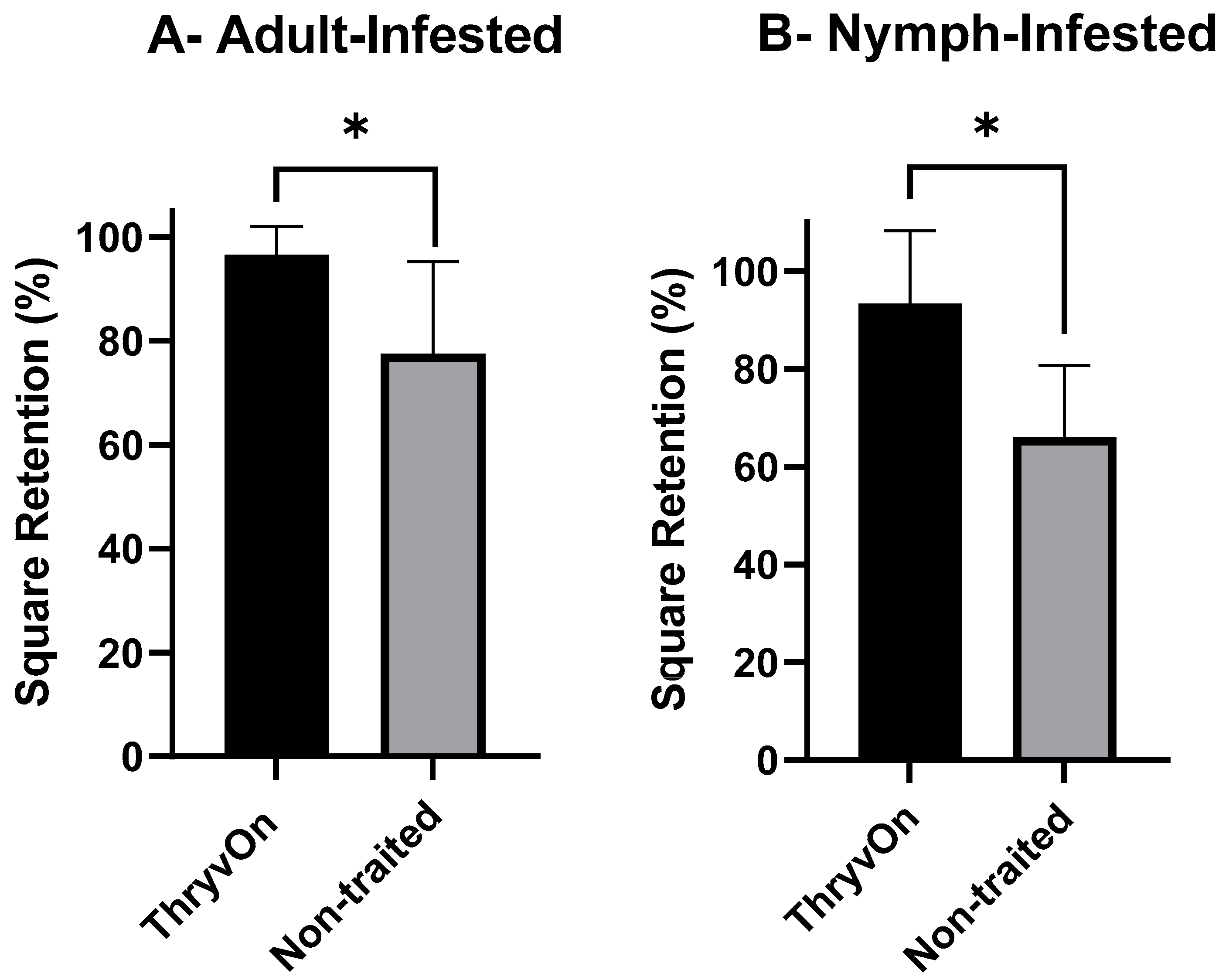
3.1. Choice Feeding Assay
3.2. No-Choice Feeding Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, D.R.; Threet, M.; Huff, K. 2022 Cotton Insect Losses Estimates. In Proceedings of the Beltwide Cotton Conference, New Orleans, LA, USA, 10–12 January 2023. [Google Scholar]
- Williams, M.R. Cotton Insect Losses 2012. In Proceedings of the Beltwide Cotton Conference, San Antonio, TX, USA, 4–7 January 2013; pp. 546–586. [Google Scholar]
- González, J.C.S.; Kerns, D.L.; Head, G.P.; Yang, F. Status of Cry1Ac and Cry2Ab2 resistance in field populations of Helicoverpa zea in Texas, USA. Insect Sci. 2021, 29, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.A.; Guo, Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.D.; Knutson, A.; Allen, E.; Biles, S.; Kerns, D.L.; Jungman, M.J. Managing Cotton Insects in the Southern, Eastern and Blackland Areas of Texas; Texas A&M University: College Station, TX, USA, 2008. [Google Scholar]
- Vyavhare, S.S.; Reed, B. Control of Cotton Fleahopper in Cotton Using Foliar Applied Insecticides, 2019. Arthropod Manag. Tests 2020, 45, tsaa090. [Google Scholar] [CrossRef]
- McLoud, L.A.; Hague, S.; Knutson, A.; Wayne Smith, C.; Brewer, M. Cotton Square Morphology Offers New Insights into Host Plant Resistance to Cotton Fleahopper (Hemiptera: Miridae) in Upland Cotton. J. Econ. Entomol. 2015, 109, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Knutson, A.; Isaacs, S.; Campos, C.; Campos, M.; Smith, C. Resistance to Cotton Fleahopper Feeding in Primitive and Converted Race Stocks of Cotton, Gossypium hirsutum. J. Cotton Sci. 2014, 18, 385–392. [Google Scholar]
- Walker, J.K.; Niles, G.A.; Gannaway, J.R.; Robinson, J.V.; Cowan, C.B.; Lukefahr, M.J. Cotton Fleahopper Damage to Cotton Genotypes. J. Econ. Entomol. 1974, 67, 537–542. [Google Scholar] [CrossRef]
- Celorio-Mancera, M.d.l.P.; Carl Greve, L.; Teuber, L.R.; Labavitch, J.M. Identification of endo-and exo-polygalacturonase activity in Lygus hesperus (Knight) salivary glands. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2009, 70, 122–135. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zeng, F.; Oppert, B. Molecular cloning of trypsin-like cDNAs and comparison of proteinase activities in the salivary glands and gut of the tarnished plant bug Lygus lineolaris (Heteroptera: Miridae). Insect Biochem. Mol. Biol. 2003, 33, 889–899. [Google Scholar] [CrossRef]
- Brett, C.H. The Cotton Flea Hopper in Oklahoma; Oklahoma Agricultural Experiment Station: Stillwater OK, USA, 1946. [Google Scholar]
- Shackel, K.A.; de la Paz Celorio-Mancera, M.; Ahmadi, H.; Greve, L.C.; Teuber, L.R.; Backus, E.A.; Labavitch, J.M. Micro-injection of Lygus salivary gland proteins to simulate feeding damage in alfalfa and cotton flowers. Arch. Insect. Biochem. Physiol. 2005, 58, 69–83. [Google Scholar] [CrossRef]
- Esquivel, J.F.; Esquivel, S.V. Identification of Cotton Fleahopper (Hemiptera: Miridae) Host Plants in Central Texas and Compendium of Reported Hosts in the United States. Environ. Entomol. 2009, 38, 766–780. [Google Scholar] [CrossRef]
- Martin, W.R., Jr.; Morgan, P.W.; Sterling, W.L.; Meola, R.W. Stimulation of Ethylene Production in Cotton by Salivary Enzymes of the Cotton Fleahopper (Heteroptera: Miridae). Environ. Entomol. 1988, 17, 930–935. [Google Scholar] [CrossRef]
- Brandt, S.L.; Coudron, T.A.; Habibi, J.; Brown, G.R.; Ilagan, O.M.; Wagner, R.M.; Wright, M.K.; Backus, E.A.; Huesing, J.E. Interaction of two Bacillus thuringiensis delta-endotoxins with the digestive system of Lygus hesperus. Curr. Microbiol. 2004, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gowda, A.; Rydel, T.J.; Wollacott, A.M.; Brown, R.S.; Akbar, W.; Clark, T.L.; Flasinski, S.; Nageotte, J.R.; Read, A.C.; Shi, X.; et al. A transgenic approach for controlling Lygus in cotton. Nat. Commun. 2016, 7, 12213. [Google Scholar] [CrossRef] [PubMed]
- Akbar, W.; Gowda, A.; Ahrens, J.E.; Stelzer, J.W.; Brown, R.S.; Bollman, S.L.; Greenplate, J.T.; Gore, J.; Catchot, A.L.; Lorenz, G.; et al. First transgenic trait for control of plant bugs and thrips in cotton. Pest Manag. Sci. 2019, 75, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Jerga, A.; Chen, D.; Zhang, C.; Fu, J.; Kouadio, J.-L.K.; Wang, Y.; Duff, S.M.; Howard, J.E.; Rydel, T.J.; Evdokimov, A.G. Mechanistic insights into the first Lygus-active β-pore forming protein. Arch. Biochem. Biophys. 2016, 600, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Sukuru, U.R.; Penn, S.R.; Meyer, S.E.; Subbarao, S.; Shi, X.; Flasinski, S.; Heck, G.R.; Brown, R.S.; Clark, T.L. Cotton Plants Expressing a Hemipteran-Active Bacillus thuringiensis Crystal Protein Impact the Development and Survival of Lygus hesperus (Hemiptera: Miridae) Nymphs. J. Econ. Entomol. 2012, 105, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.H.; Stewart, S.D. Field Study Investigating Cry51Aa2.834_16 in Cotton for Control of Thrips (Thysanoptera: Thripidae) and Tarnished Plant Bugs (Hemiptera: Miridae). J. Econ. Entomol. 2018, 111, 2717–2726. [Google Scholar] [CrossRef]
- Corbin, J.C.; Towles, T.B.; Crow, W.D.; Catchot, A.L.; Cook, D.R.; Dodds, D.M.; Gore, J. Evaluation of Current Tarnished Plant Bug (Hemiptera: Miridae) Thresholds in Transgenic MON 88702 Cotton Expressing the Bt Cry51Aa2.834_16 Trait. J. Econ. Entomol. 2020, 113, 1816–1822. [Google Scholar] [CrossRef]
- Whitfield, A. Evaluation of Thresholds, Control, and Behavioral Responses of Tobacco Thrips, Frankliniella fusca (Hitch), and Tarnished Plant Bugs, Lygus lineolaris (Beauvoris), in ThryvOn Cotton; University of Arkansas: Fayetteville, AR, USA, 2023. [Google Scholar]
- Miles, P.W. The Saliva of Hemiptera. In Advances in Insect Physiology; Treherne, J.E., Berridge, M.J., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, MA, USA, 1972; Volume 9, pp. 183–255. [Google Scholar]
- Bachman, P.M.; Ahmad, A.; Ahrens, J.E.; Akbar, W.; Baum, J.A.; Brown, S.; Clark, T.L.; Fridley, J.M.; Gowda, A.; Greenplate, J.T.; et al. Characterization of the Activity Spectrum of MON 88702 and the Plant-Incorporated Protectant Cry51Aa2.834_16. PLoS ONE 2017, 12, e0169409. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-Evolved Resistance to Bt Maize by Western Corn Rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef]
- Raszick, T.J.; Suh, C.P.-C.; Dickens, C.M.; Sword, G.A. Genome-wide markers reveal temporal instability of local population genetic structure in the cotton fleahopper, Pseudatomoscelis seriatus (Hemiptera: Miridae). Pest Manag. Sci. 2020, 76, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kerns, D.L.; Brown, S.; Kurtz, R.; Dennehy, T.; Braxton, B.; Head, G.; Huang, F. Performance and cross-crop resistance of Cry1F-maize selected Spodoptera frugiperda on transgenic Bt cotton: Implications for resistance management. Sci. Rep. 2016, 6, 28059. [Google Scholar] [CrossRef] [PubMed]
- Mekala, D.K. Screening Upland Cotton for Resistance to Cotton Fleahopper (Heteroptera: Miridae); Texas A&M University: College Station, TX, USA, 2004. [Google Scholar]
- Ritchie, G.L.; Bednarz, C.W.; Jost, P.H.; Brown, S.M. Cotton Growth and Development; University of Georgia: Athens, GA, USA, 2007. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 1925, 18, 265–267. [Google Scholar] [CrossRef]
- GraphPad Prism. GraphPad Prism for Windows 64-Bit, 9.3.0; GraphPad: Boston, MA, USA, 2021. [Google Scholar]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Knutson, A.E.; Mekala, K.D.; Smith, C.W.; Campos, C. Tolerance to Feeding Damage by Cotton Fleahopper (Hemiptera: Miridae) Among Genotypes Representing Adapted Germplasm Pools of United States Upland Cotton. J. Econ. Entomol. 2013, 106, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Arthur, B.P.; Suh, C.P.; McKnight, B.M.; Parajulee, M.N.; Yang, F.; Kerns, D.L. Field Evaluation of Cotton Expressing Mpp51Aa2 as a Management Tool for Cotton Fleahoppers, Pseudatomoscelis seriatus (Reuter). Toxins 2023, 15, 644. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.H.; Musser, F.M.; Jacobson, A.L.; Chitturi, A.; Catchot, B.; Stewart, S.D. Behavioral Responses of Thrips (Thysanoptera: Thripidae) and Tarnished Plant Bug (Hemiptera: Miridae) to a New Bt Toxin, Cry51Aa2.834_16 in Cotton. J. Econ. Entomol. 2019, 112, 1695–1704. [Google Scholar] [CrossRef]
- Asiimwe, P.; Brown, C.R.; Ellsworth, P.C.; Reisig, D.D.; Bertho, L.; Jiang, C.; Schapaugh, A.; Head, G.; Burzio, L. Transgenic cotton expressing Mpp51Aa2 does not adversely impact beneficial non-target hemiptera in the field. Crop Prot. 2023, 173, 106384. [Google Scholar] [CrossRef]
- Beerwinkle, K.R.; Marshall, H.F. Cotton Fleahopper (heteroptera:Miridae) Responses to Volatiles from Selected Host Plants . J. Cotton Sci. 1999, 3, 153–159. [Google Scholar]
- Cooper, W.R.; Spurgeon, D.W. Feeding injury to cotton caused by Lygus hesperus (Hemiptera: Miridae) nymphs and prereproductive adults. Environ. Entomol 2013, 42, 967–972. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthur, B.P.; Suh, C.P.-C.; McKnight, B.M.; Parajulee, M.N.; Yang, F.; Kerns, D.L. Choice and No-Choice Feeding Assays of Cotton Fleahoppers (Pseudatomoscelis seriatus) on Cotton Expressing the Mpp51Aa2 Protein. Agronomy 2024, 14, 84. https://doi.org/10.3390/agronomy14010084
Arthur BP, Suh CP-C, McKnight BM, Parajulee MN, Yang F, Kerns DL. Choice and No-Choice Feeding Assays of Cotton Fleahoppers (Pseudatomoscelis seriatus) on Cotton Expressing the Mpp51Aa2 Protein. Agronomy. 2024; 14(1):84. https://doi.org/10.3390/agronomy14010084
Chicago/Turabian StyleArthur, Brady P., Charles P.-C. Suh, Benjamin M. McKnight, Megha N. Parajulee, Fei Yang, and David L. Kerns. 2024. "Choice and No-Choice Feeding Assays of Cotton Fleahoppers (Pseudatomoscelis seriatus) on Cotton Expressing the Mpp51Aa2 Protein" Agronomy 14, no. 1: 84. https://doi.org/10.3390/agronomy14010084
APA StyleArthur, B. P., Suh, C. P. -C., McKnight, B. M., Parajulee, M. N., Yang, F., & Kerns, D. L. (2024). Choice and No-Choice Feeding Assays of Cotton Fleahoppers (Pseudatomoscelis seriatus) on Cotton Expressing the Mpp51Aa2 Protein. Agronomy, 14(1), 84. https://doi.org/10.3390/agronomy14010084







