Abstract
The cultivation of perennial flowering wild plant species like common tansy (Tanacetum vulgare L.) seems promising for increasing biodiversity friendliness in rather monotonous bioenergy cropping systems in Central Europe, particularly on marginal sites. However, it is still unclear for which types of marginal agricultural land common tansy would be suitable and where; as a result, low-risk indirect land-use change biomass production through common tansy could be considered. Therefore, the aim of this study was to gather initial insights into the suitability of common tansy for sandy sites by means of a 6 L-pot experiment. For this purpose, five replicates of three substrates were prepared: Luvisol topsoil (control) from a field site near the University of Hohenheim, Germany; and admixtures of 50 and 83.4weight(wt)% of sand to the control (M1, and M2), respectively. This resulted in varying sand contents of the substrates of 4.7 (control), 53.3 (M1), and 83.0wt% (M2). In autumn 2021, common tansy seeds were collected from mother plants bearing the breeder’s indentifier ‘Z.8TAV 85/78’. These plants were part of a long-term field trial initiated at Hohenheim in 2014, where common tansy was grown as part of a wild plant mixture. In June 2022, 0.5 g of the seeds were sown in each pot. The pots were placed in outdoor conditions, arranged in a randomized complete block design and watered evenly as required. At harvest in July 2023, significant differences between the substrates in terms of the above- (shoots) and belowground (roots) development of the common tansy seedlings were observed. In M1, common tansy provided notable biomass growth of 56.6% of the control, proving to be potentially suitable for low-input cultivation under sandy soil conditions. However, an even higher share of sand and low nutrient contents in M2 resulted in minor plant development (14.4% of the control). Hence, field trials on sandy soils of about 50wt% of sand in the texture under tailored fertilization and various climatic conditions are recommended.
1. Introduction
The cultivation of perennial biomass crops (PBCs) not only provides biomass, for example to replace fossil resources, but also ecosystem services such as climate regulation, erosion mitigation, feed and habitat for pollinators and wildlife, among numerous others [1]. Compared with annual crops, PBCs were found to enrich the soil with organic carbon (humus), contributing to soil health and climate protection in the long term [2,3,4]. Therefore, measures for soil carbon enrichment are of global concern due to the decline in organic carbon associated with intensive agriculture and climate change [3,4,5,6]. Hence, the cultivation of PBCs for bioenergy and bio-based purposes is considered an essential measure to help defossilizing economies worldwide in order to mitigate climate change and the expected associated devastating environmental and societal consequences [7]. While the material use of plant biomass feedstocks has been gaining more and more attention in recent years, the application for energy purposes remains particularly relevant in terms of independency and supply diversification [8,9,10,11].
However, in addition to climate change, the global decline in biodiversity has also been recognized as an increasingly serious problem for humans and the environment [12,13,14,15], which should be counteracted via PBC cultivation. In recent years, there have been initial calls in the EU for practicable and holistically more sustainable cultivation methods to foster biodiversity-friendly biomass production [16,17]. It is therefore no longer enough to promote agricultural biodiversity in the cultivation of PBCs through soil dormancy, but it should be plant species in particular that can provide additional habitat services for fauna in rural areas through the provision of nectar and pollen. This additional task of ensuring a certain degree of biodiversity friendliness must be considered a distinct challenge when cultivating PBCs. In this regard, only abandoned and/or marginal agricultural land should be chosen for PBC cultivation in order to avoid the food-versus-fuel trade-off and to ameliorate such soils via plant-derived carbon input. However, marginal agricultural land can be somewhat vulnerable to the potential negative impacts of agricultural use. Therefore, emphasis should be placed on the suitability of PBCs to the given agroecosystem in which the marginal agricultural land is embedded. The most obvious approach would, therefore, be the use of only native plant species and genotypes in order to minimize the imbalance of the native flora and fauna. While cultivating high-yielding PBCs like miscanthus (Miscanthus Andersson, native to eastern Asia [18,19,20]) and cup plant (Silphium perfoliatum L., native to eastern and central North America [21,22,23]) might be less disruptive to agroecosystems in their native regions, introducing them to non-native regions like Europe poses a higher risk of flora distortion. This increased risk stems from the absence of natural controls on their growth and potential invasiveness in these new environments [24].
Thus, looking at Europe, where miscanthus and cup plant are quite well-established bioenergy crops [25,26,27], it seems reasonable that native plant species should be chosen, because they might be more beneficial for the local agroecosystems. While woody species native to Europe, such as willow or poplar, would be suitable for agroforestry approaches, the selection of (and knowledge about) native high-yielding and biodiversity-friendly herbaceous PBCs for cultivation on arable land is still very limited. In Germany, however, research activities increased during the last 15 years, with main emphasis on the cultivation of perennial wild plant species (WPM) for biogas production [28,29,30,31,32,33]. Nevertheless, many open questions remained in this field of research with regard to agricultural practices for optimizing biomass yield, biomass yield stability, usability and the integrability of WPM into farm processes and landscape structures [34,35].
Previous studies already revealed that some species of WPM, such as common tansy (Tanacetum vulgare L.), would be particularly suitable for further pilot studies to test their performance under sole cultivation due to its high yielding character and stable performance against adverse environmental conditions [36,37]. Common tansy is a perennial herbaceous wild plant native to Europe [38] and is an integral part of natural plant communities such as common tansy–tall oat-grass meadows (Tanaceto vulgaris–Arrhenatheretum elatioris) and tansy–mugwort communities (Tanaceto–Artemisietum vulgaris). Common tansy was traditionally and is currently still used for multiple purposes, such as (i) medicinal use like herbal treatments against lice infestation [39,40,41], and (ii) biobased plant protection measures, for example, as a biogenic insecticide against bird cherry-oat aphid (Rhopalosiphum padi L.), a major pest in cereal cultivation [42], and as a botanical antifeedant (repellent) against codling moth (Cydia pomonella L. (Tortricidae)), a major cosmopolitan pest of apples [43]. Each year, common tansy flowers from July to September and provides food for over twenty species of wild bees and numerous other insects and spiders. In its natural environment, common tansy is a medium nitrogen indicator (i.e., it requires a medium nitrogen supply) and can cope with a medium water supply [44].
Only sparse information is available on the biomass yield level and nutrient utilization efficiency of common tansy grown in monoculture. Furthermore, no information is available on whether it can be grown economically on marginal, sandy and nutrient-depleted soils. However, initial long-term field trials have already shown that the biomass yields of common tansy on good (i.e., non-marginal) soils can be significantly higher (>30%) in some years than those of silage maize (Zea mays L.), which was previously considered the reference species as a high-yielding energy crop [45]. An earlier study on different genotypes of common tansy grown for medicinal purposes on a gleyed melanic brunisol soil type showed a mean dry matter yield among five different chemotypes of 5.8 ± 1.3 Mg ha−1 [42]. According to Elbersen et al. (2017), the majority of marginal agricultural land most relevant in terms of area is characterized by a high share of sand [46,47]. Nitrogen and water supply are relatively low on sandy soils compared to favorable soils, also due to limitations in water-holding capacity and lack of organic carbon [48]. Both would be considered disadvantageous for common tansy with its mediocre requirements, but as it is a wild plant species with missing breeding lines, a certain tolerance against droughts and limited (or “low-input”) nutrient sources can be expected. To date, no information is available regarding whether common tansy would be suitable for producing biomass on sandy marginal soils.
The aim of this study was, therefore, to gain initial insights into the tolerance level of common tansy when grown on sandy soil compared with more favorable soil conditions (control). The associated hypothesis was that the sand content has a significant influence on the growth of common tansy. As a proof of concept, first insights were intended to provide recommendations as to whether and, if so, which further investigations of common tansy as a scalable and biodiversity-friendly PBC should be undertaken in the future.
2. Materials and Methods
2.1. Location and Climatic Conditions
The pot trial was carried out from June 2022 until July 2023 under outdoor conditions at the University of Hohenheim in southwest Germany (48°42′45.3″ N 9°12′21.7″ E). The local climate is characterized by an average annual mean temperature of 9.2 °C and annual precipitation sum of about 650 mm. Missing precipitation during plant establishment (e.g., in July 2022, see Figure 1) was compensated by manual watering to ensure a minimum soil moisture of about 50% of maximum water-holding capacity (WHCmax) for each treatment.
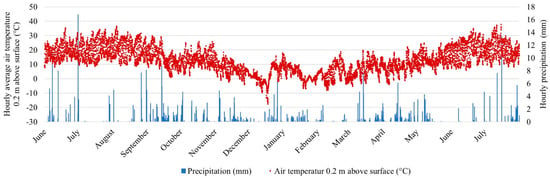
Figure 1.
Hourly average air temperature (0.2 m above soil) and hourly precipitation (mm) at the site of the pot trial from June 2022 to July 2023. The watering events are not included.
2.2. Setup of the Pot Trial, Soil Analyses, Seed Origin and Plant Management
For the pot experiment, three different soil variants were prepared, representing a marginality gradient as follows: (i) topsoil from a site near the University of Hohenheim, Stuttgart, Germany (control, Luvisol), (ii) a mixture of 50:50weight(wt)% pure sand (containing neither any plant-relevant nutrients nor organic carbon) and topsoil (M1), and (iii) a mixture of 83.4:16.6wt% pure sand and topsoil (M2) (Figure 2).
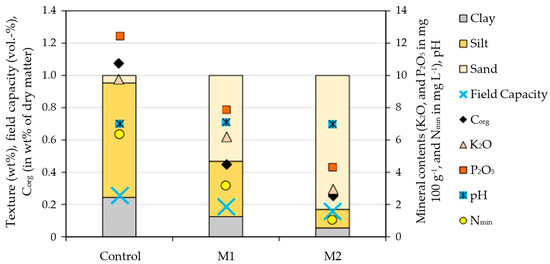
Figure 2.
Texture composition and biochemical properties of the substrate types prepared for and used in this study.
The sand content of the substrates thus varied as follows: 4.7 (control), 53.3 (M1), and 83.0wt% (M2). Figure 2 illustrates the share of sand, silt and clay, the field capacity, the contents of Corg, Nmin, P2O5, K2O, and the reactivity (pH) in each substrate variant. All soil analyses were carried out according to standard procedures of “Methods Book I” of the Association of German Agricultural Research and Research Institutes (VDLUFA) [49]. Then, five 6 L-standard pots were filled per variant with 6 kg of the respective substrate (Figure 3A,B).
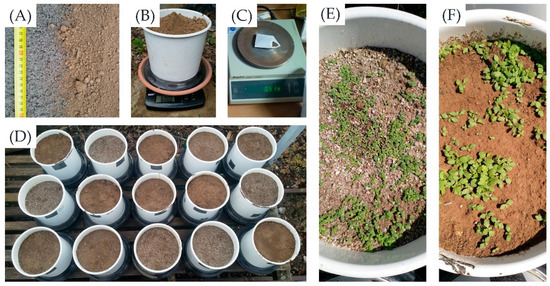
Figure 3.
Sand (left) and control substrate (right) in comparison (A), weighing of the soil substrates per pot (B), weighing of the common tansy seeds (0.5 g) per pot (314.2 cm²) (C), arrangement of the pots at the beginning of the pot trial in June 2022 (D), impression of common tansy seedlings, 14 days after sowing: in M1 (E), and control (F).
Per pot, 0.5 g of common tansy seeds were weight (Figure 3C) and sown by evenly placing the seeds on the soil surface. The seeds were collected in late summer 2021 from common tansy plants bearing the breeder’s indentifier ‘Z.8TAV 85/78’ growing in a field trial at the University of Hohenheim (Figure 4A–C). These plants were originally sown in 2014 and thrived there ever since, without any chemical–synthetical plant protection but with moderate annual N-fertilization of 50–90 kg N ha−1. The common tansy plants were part of a seed mixture intended to establish species-rich wild plant mixtures (‘BG90’, Saaten Zeller GmbH & Co. KG, Eichenbühl-Guggenberg, Germany). To collect the seeds, inflorescences (Figure 4C) were rubbed together and tapped over a box on a warm, dry day, allowing ripe seeds to fall out. While the seeds can be easily removed from the inflorescence at maturity (Figure 4C), they still need to be sieved (1 mm mesh) to separate them from the petals.

Figure 4.
Impression of the common tansy plants that were available for seed collection at the Hohenheim site: The common tansy plants harvested in March 2022 (A2) in comparison with miscanthus (Miscanthus x giganteus Greef et Deuter, (A1)) and Virginia fanpetals (Sida hermaphrodita L. var. Rusby, (A3)), which are conventional bioenergy crops used for combustion; one of the common tansy plants in the field in late summer 2024 (B); an inflorescence of common tansy shortly after the seeds have fully ripened (C). The scale in (A,B) shows 50 cm intervals.
Given a net area of 314.16 cm2 per pot, this corresponded to a sowing rate of 159.2 kg ha−1. Then, the pots were arranged in a randomized complete block design on a wooden trolley about 50 cm above the ground and left outdoors for the entire duration of the experiment (Figure 3D). This means that the pots were arranged in five blocks, each including all three substrates but in randomized order (Figure 3D). Even though the pots were watered evenly in very dry conditions, the plants experienced naturally induced drought stress occasionally during the pot trial due to missing automated watering.
2.3. Harvest and Biomass Analyses
At harvest on 18 July 2023, all plants were removed from the pots (Figure 5A). Both root and shoot biomass was separated and measured (Figure 5B). To determine differences per soil treatment and intraspecies differences in root growth and proliferation, the separated roots were also measured in length prior drying and further analysis (Figure 5C). To determine the dry matter biomass yield of roots and shoots per pot, the samples were dried at 60 °C for 48 h until constant weight. For the elemental analyses, all biomass samples were prepared and analyzed according to the VDLUFA standard methods (Methods book III) [49]. In addition, the ash content was determined for root and shoot biomass samples from the substrate variants control and M1. Therefore, the ash content could only be determined for the biomass of common tansy grown in the control and M1 substrates. This was because not enough biomass had grown in M2 for the determination of the ash content to be taken into account here as well.

Figure 5.
Arrangement of the pots in July 2023 (A), setup of the root washing place (B), impression of root length comparison between different substrates (C): control (left), M2 (right).
2.4. Statistical Analysis
All data were analyzed using the PROC MIXED procedure of the SAS® Proprietary Software 9.4 TS level 1M7 (SAS Institute Inc., Cary, NC, USA) using Equation (1):
where is the fixed effect for the ith substrate (Control, M1, M2), is the intercept, and is the error of observation with substrate-specific variance. The Kenward–Roger method was used to approximate both standard errors and degrees of freedom [50]. The letter-based representation of all pairwise comparisons was carried out using an SAS macro by Piepho [51].
3. Results and Discussion
3.1. Morphological and Phenological Plant Development
The common tansy plants showed a clear response to the increased sand content in the soil in terms of reduced growth height and biomass yield (Figure 6).
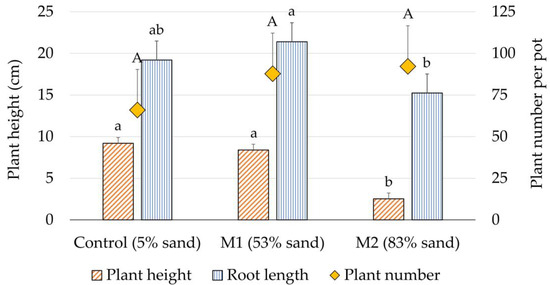
Figure 6.
Results of the statistical analyses of the effects of the substrate (control, M1, and M2) on average plant height, average root length, and average plant density (plant number pot−1). The positive error bars show the standard errors, different letters denote significant (p < 0.05) differences between the substrates within each trait (plant height, root length, and plant number, respectively).
This could be explained by the fact that the plants experienced pot volume-related nutrient and water deficiencies in the potted substrates [52]. These factors were even more pronounced with increasing sand content in the substrate (M1 and M2, Figure 2). Water and nutrient availability were not consistently monitored in all treatments. Hence, we only refer to the different nutrient contents in the substrates at the beginning of the experiment. The water shortages were visible but were also not monitored. Therefore, this could be a potential confounding factor within the experiment and should be investigated more thoroughly in future large-scale field trials. Nevertheless, under high (M1) and very high (M2) sand content conditions, the plants were inhibited in growth, which was reflected in the lower growth height and overall reduced biomass production (Figure 6 and Figure 7). In contrast, however, there were no significant differences between the substrates in terms of the total number of plants (stand density) (Figure 3E,F and Figure 6). This can initially be explained by the fact that equal numbers of seeds were sown in all substrates (Figure 3). The sowing density (0.5 g per pot, i.e., 314 cm2 equaling 15 g m−2) was higher compared with the study by Dragland et al. [42] (2.0 g per tray, i.e., assumed to be 2400 cm2, equaling about 8.3 g m−2), because it was assumed that the simulated marginality conditions in the pot trial would require a higher seed density than under normal conditions. Furthermore, Dragland et al. transferred the seedlings into plug trays before planting them to the field at a planting density of 8 plants m−2 [42]. Therefore, a direct comparison of the yields between the results of this study and the study by Dragland et al. is not reasonable.
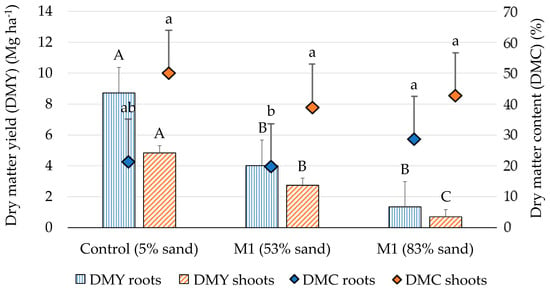
Figure 7.
Results obtained from the statistical analyses of the effects of the substrate (control, M1, and M2) on the dry matter yield (bars) and dry matter content (marks) in the belowground (“roots”) and aboveground (“shoots”) parts of common tansy. The positive error bars show the standard errors, different letters per parameter per planting fraction show significant differences (p < 0.05).
In the present study, substrates did not significantly affect the total number of plants (Figure 6). This is probably due to the high robustness and intraspecific competitiveness, or tolerance of the common tansy genotype used here. However, the calculated plant density of 2100 to 2934 plants m−2 is considerably higher than plant densities of common tansy grown in its natural environment, accounting for 37.3 ± 11.1 plants m−2 [53], though this number may likely fluctuate site-specifically. For field cultivation, the plant density should therefore be adjusted for site-specific conditions in order to reduce the intraspecific competition between the common tansy plants. Reducing this intraspecific competition might be crucial, because it might help lowering the mutual hindrance of the plants during growth. Additionally, it may result in a smaller number of plants to grow more productively and to complete their life cycle.
Further, common tansy will only be able to provide the additional ecosystem services such as nectar and pollen for pollinators if the plant also flowers. In the M1 and M2 substrate variants, not a single individual plant flowered and reached maturity. This observation was in contrast to the control, in which some individual plants reached the flowering stage. This indicated that the phenological development of common tansy was also slowed down due to stress induced by an increasing sand content in the substrate and the associated limiting water and nutrient supply conditions, among volume limitations [52].
3.2. Biomass Yield and Quality Parameters
At the end of this pot trial, calculated dry matter yields of 0.7 to 4.8 Mg ha−1 were recorded (Figure 6). This is in line with Dragland et al. [42], who compared the biomass productivity of five different common tansy genotypes for medicinal purposes [42]. However, the biomass yield of common tansy can be much higher in natural surroundings following Ciesielczuk et al. [53], who reported an average dry matter yield of 9.2 ± 2.7 Mg ha−1.
Whilst a dry matter yield level of about 9 Mg ha−1 is still low compared with high-yielding PBCs such as miscanthus and cup plant [20,23], Ciesielczuk et al. pointed out that there was no irrigation, fertilization or other types of plant protection measures applied [53]. Therefore, the growth of common tansy in the natural habitats is likely to be limited by growth parameters such as water and/or nutrient availability and competition with other plants. Consequently, it can be expected that the dry matter yield potential of common tansy grown for bioenergy or biobased material purposes could be much higher (about 15 Mg ha−1) under optimized agricultural management as was for example previously reported by Cossel and Lewandowski [45], who found common tansy to reach 22 Mg ha−1 in certain years on favorable soil without fertilization. However, there is still limited knowledge on improving the cultivation of perennial wild plant species such as common tansy towards a higher and more stable dry matter yield level [34], especially under marginal conditions such as sandy soil.
Furthermore, extrapolating dry matter yields from pot studies to hectares should be viewed with great caution, as the soil volume, water and nutrient supply conditions for the plants in the field are very different from those in pots. For example, the rooting depth is usually much greater under field conditions, and in the absence of rain events, the root zone does not dry out as quickly under field conditions as in the pot trial. One of the most important recommendations that can be derived from the results of this study is, therefore, to investigate the true yield potential of common tansy in field trials on sandy soils, which can therefore be described as marginal agricultural land. The question of water-use efficiency would be of particular importance here, as the “low-input” concept of biomass crop cultivation generally requires moderate fertilization but no (continuous) irrigation [47,54]. The precipitation gradient should therefore be integrated into the field trial as a second factor in addition to a gradient in the sand content. Further, we recommend including additional control elements, such as fertilizer application and a comparison with other well-known perennial bioenergy crops, such as miscanthus, cup plant and Virginia fanpetals (Sida hermaphrodita L. var. Rusby), to better contextualize the performance of common tansy. In doing so, particular attention should be paid not only to yield and suitability for bioenergy production, but also to the holistic ecosystem performance potential of the cultivation systems, which, in addition to providing biomass, also includes, for example, habitat functions, erosion protection, climate regulation and landscape aesthetics.
The common tansy plants showed significant responses to the quality of the three substrates investigated here, both above and below ground (Figure 7), which is why the hypothesis can be accepted. This was shown by the fact that the dry matter yield tended to decrease with decreasing soil quality, i.e., increasing the share of sand (“Control” > “M1” > “M2”). Similar observations were reported by Rebele [40], who also found a significant difference of dry matter yield between a favorable (control) and a sandy soil (sand content > 90%).
There were significant differences in shoot biomass between all substrates, while in root biomass, there was only a significant difference between the “Control” and the two limiting substrates (“M1” and “M2”). However, there was a recognizable tendency of a further decreasing root biomass from “M1” to “M2” (Figure 7). The fact that there was no significant difference between “M1” and “M2” in root biomass compared to shoot biomass could be due to a stress reaction of the plants. This means that the fewer nutrients and less water available to the plants in the substrate (from “M1” to “M2”), the more energy and assimilates the plant will invest in the root biomass in order to extract available resources from the substrate and to continue growing.
With regard to biomass composition, it was indicated that the combustion quality of common tansy was much lower for all substrates compared with earlier observations by Von Cossel et al. [55,56]. This was due to the relatively high ash contents of >12% (Table 1), which exceeded the thresholds given for use in industrial and residential applications [57].

Table 1.
Results obtained from statistical analyses of the effects of substrate (Control, M1 and M2) on the contents of ash, nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), and calcium (Ca) in the above-ground (“shoots”) and below-ground (“roots”) parts of common tansy. Estimates, standard errors and significant differences (p < 0.05, for different lower-case letters) within plant fractions are shown.
This could be explained by more stressful growth conditions of common tansy in the pot trial leading to smaller plant height, earlier maturation, a higher share of minerals in the biomass, and thus higher ash contents compared with observations by Piatkowska et al. [58], who found an average ash content of approx. 8.8 ± 0.8% of dry matter for common tansy grown in a natural habitat northwest of Poland. These values in ash content are much higher than those presented in earlier studies investigating common tansy for combustion, accounting for 2.4 to 4.7wt% of dry matter [55,59]. Such differences in mineral and thus ash content in the biomasses might be related to local conditions with regard to water, soil and nutrient, and general abiotic and biotic conditions, and therefore higher biomass yields. Higher biomass yields of common tansy could be associated with a dilution effect of the mineral content in the biomass. This is also reflected in significantly lower values for P, K, Mg, and Ca in common tansy biomass in the reference study compared with the results of this study, accounting for 0.47 mg g−1, 2.75 mg g−1, 0.75 mg g−1, and 6.27 mg g−1, respectively [55].
While N values in the cited study are given with 0.38 N in % of dry matter (corresponding to 3.8 mg g−1), it can be assumed that the common tansy biomass used in the previous study was grown under favorable soil conditions with sufficient N supply and resulting high biomass yields [55]. This, in turn, supports the assumption of the “diluting effects” of the other elements P, K, Mg, and Ca in the biomass, which would be beneficial when the biomass is meant for combustion purposes or in general energy applications. These results suggest that a sufficient or adequate supply of nutrients is essential, irrespective of the substrate and level of marginality if the common tansy biomass is to be used for energy purposes (e.g., combustion) or possibly other material applications (e.g., fibers).
4. Conclusions and Outlook
The results of the pot trial revealed significant effects of the varied sand content in the substrate on the above- and belowground phenological development of the common tansy seedlings. However, common tansy provided 180 g m−2 aboveground matter in the soil with 53.4% sand (M1), which is more than half the increment in the soil control (320 g m−2), despite lower nutrient availability and lower field capacity in M1. These promising results suggest future field trials with common tansy on marginal sandy soils (i.e., soils with about 50wt% sand) under different climatic conditions, compensating soil-specific nutrient deficiencies with organic fertilizers such as digestate from biogas production and solid manure. Given that common tansy is a light germinator, and its seeds are extremely light and fine, we recommend pre-planting young plants for future field cultivation. These seedlings could then be transplanted into the field using conventional planting equipment. This approach opens avenues for further field research on optimal planting techniques, planting and row spacing, as well as effective weed management practices. Depending on the degree of marginality of the soil and the planting density, a sufficient supply of nutrients/fertilizers is crucial. This will not only significantly influence the biomass yield, but also the biomass quality for subsequent applications or uses of the biomass.
Another research question that emerged from the first insights of this study was as follows: What is the optimal plant geometry of common tansy in terms of yield performance and yield stability, and how could it be adapted to site-specific conditions? Thus, future research should also focus on how common tansy reacts to factors such as row width, spacing in the row and plant density with varying soil quality. As the seeds are particularly small, it could also be useful to pill the seeds in order to achieve a targeted arrangement and seed density when establishing common tansy stands. However, as common tansy is a light germinator, this could place special demands on the pelleting material.
Overall, this experiment provided first insights to and derived recommendations for further research on the growth suitability of common tansy on marginal sites characterized by high sand contents in order to contribute towards a more biodiversity-friendly biomass production for the bioeconomy in Central Europe.
Author Contributions
Conceptualization, M.v.C., J.K., Y.I. and N.D.J.; Data curation, M.v.C. and T.T.; Formal analysis, M.v.C., T.T. and N.D.J.; Funding acquisition, M.v.C.; Investigation, M.v.C., J.K., E.B., T.T. and G.G.; Methodology, M.v.C., J.K., Y.I., E.B., T.T., G.G. and N.D.J.; Project administration, M.v.C.; Resources, M.v.C.; Supervision, M.v.C.; Visualization, M.v.C.; Writing—original draft, M.v.C. and N.D.J.; Writing—review and editing, M.v.C., J.K., Y.I., E.B., G.G. and N.D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received co-funding from the Federal Ministry of Education and Research (01PL16003) and the University of Hohenheim.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
Special thanks go to Bastian Winkler, and the technical staff at the University of Hohenheim for their support in carrying out the pot experiments. Preliminary results of this study were presented as an oral presentation at the 32nd European Biomass Conference and Exhibition (EUBCE), Marseille, France, on 26 June 2024 (Session code 6CO.4.4). The authors are very grateful to the organizers of the EUBCE for this honorable opportunity.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brander, L.M.; Groot, R.; Guisado Goñi, V.; van ’t Hoff, V.; Schägner, P.; Solomonides, S.; McVittie, A.; Eppink, F.; Sposato, M.; Do, L.; et al. Ecosystem Services Valuation Database (ESVD). Available online: https://www.esvd.net/ (accessed on 18 July 2024).
- Bai, Y.; Cotrufo, M.F. Grassland Soil Carbon Sequestration: Current Understanding, Challenges, and Solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lærke, P.E.; Jørgensen, U. Land Conversion from Annual to Perennial Crops: A Win-Win Strategy for Biomass Yield and Soil Organic Carbon and Total Nitrogen Sequestration. Agric. Ecosyst. Environ. 2022, 330, 107907. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Hastings, A.; Amaducci, S. Soil Organic Carbon Significantly Increases When Perennial Biomass Plantations Are Reverted Back to Annual Arable Crops. Agronomy 2023, 13, 447. [Google Scholar] [CrossRef]
- Das, S.; Teuffer, K.; Stoof, C.R.; Walter, M.F.; Walter, M.T.; Steenhuis, T.S.; Richards, B.K. Perennial Grass Bioenergy Cropping on Wet Marginal Land: Impacts on Soil Properties, Soil Organic Carbon, and Biomass During Initial Establishment. Bioenerg. Res. 2018, 11, 262–276. [Google Scholar] [CrossRef]
- Frank, S.; Schmid, E.; Havlík, P.; Schneider, U.A.; Böttcher, H.; Balkovič, J.; Obersteiner, M. The Dynamic Soil Organic Carbon Mitigation Potential of European Cropland. Glob. Environ. Chang. 2015, 35, 269–278. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S. IPCC 2022: Climate Change 2022: Impacts, Adaptation and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2022. [Google Scholar]
- Osička, J.; Černoch, F. European Energy Politics after Ukraine: The Road Ahead. Energy Res. Soc. Sci. 2022, 91, 102757. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global Status of Lignocellulosic Biorefinery: Challenges and Perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal Treatment of Biomass Feedstocks for Sustainable Production of Chemicals, Fuels, and Materials: Progress and Perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon Capture and Storage (CCS): The Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Isbell, F.; Reich, P.B.; Tilman, D.; Hobbie, S.E.; Polasky, S.; Binder, S. Nutrient Enrichment, Biodiversity Loss, and Consequent Declines in Ecosystem Productivity. Proc. Natl. Acad. Sci. USA 2013, 110, 11911–11916. [Google Scholar] [CrossRef]
- Bateman, I.; Balmford, A. Current Conservation Policies Risk Accelerating Biodiversity Loss. Nature 2023, 618, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A. The Ecological Role of Biodiversity in Agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Bridgewater, P.; Schmeller, D.S. The Ninth Plenary of the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES-9): Sustainable Use, Values, and Business (as Usual). Biodivers Conserv 2023, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- MIDAS, Marginal Lands and Industrial Crops for the European Bioeconomy, Horizon Europe Innovation Action. Available online: https://www.midas-bioeconomy.eu/ (accessed on 21 June 2023).
- Alexopoulou, E.; Elbersen, B.; Trindade, L.; Cosentino, S.L.; Monti, A.; Carmona, M.; Lewandowski, I.; Kyritsis, S.; Cocchi, M.; Papazoglou, E.G. The MIDAS Project: Utilization of Marginal Lands for Growing Sustainable Industrial Crops and Developing Innovative Bio-Based Products. In Proceedings of the European Biomass Conference and Exhibition, ETA-Florence Renewable Energies, Bologna, Italy, 5–9 June 2023; pp. 137–141. [Google Scholar]
- Anderson, E.; Arundale, R.; Maughan, M.; Oladeinde, A.; Wycislo, A.; Voigt, T. Growth and Agronomy of Miscanthus x Giganteus for Biomass Production. Biofuels 2011, 2, 71–87. [Google Scholar] [CrossRef]
- Ben Fradj, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European Bio-Economy: A Network Analysis. Ind. Crops Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- Winkler, B.; Mangold, A.; Von Cossel, M.; Clifton-Brown, J.; Pogrzeba, M.; Lewandowski, I.; Iqbal, Y.; Kiesel, A. Implementing Miscanthus into Farming Systems: A Review of Agronomic Practices, Capital and Labour Demand. Renew. Sustain. Energy Rev. 2020, 132, 110053. [Google Scholar] [CrossRef]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowski, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agronomy 2020, 10, 928. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical Characteristics, Crop Management and Potential of Silphium perfoliatum L. as a Renewable Resource for Biogas Production: A Review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- von Cossel, M.; Amarysti, C.; Wilhelm, H.; Priya, N.; Winkler, B.; Hoerner, L. The Replacement of Maize (Zea mays L.) by Cup Plant (Silphium perfoliatum L.) as Biogas Substrate and Its Implications for the Energy and Material Flows of a Large Biogas Plant. Biofuels Bioprod. Biorefining 2020, 14, 152–179. [Google Scholar] [CrossRef]
- Ende, L.M.; Laurer, M. Spontanvorkommen Der Silphie Im Bayreuther Raum: Birgt Diese Neue Bioenergiepflanze Ein Invasionspotenzial?—Spontaneous Occurences of the Cup Plant in the Bayreuth Region: Does This New Bioenergy Crop Have Invasive Potential? Nat. Und Landsch. 2020, 95, 310–315. [Google Scholar] [CrossRef]
- Grunwald, D.; Panten, K.; Schwarz, A.; Bischoff, W.-A.; Schittenhelm, S. Comparison of Maize, Permanent Cup Plant and a Perennial Grass Mixture with Regard to Soil and Water Protection. GCB Bioenergy 2020, 12, 694–705. [Google Scholar] [CrossRef]
- Schoo, B.; Wittich, K.P.; Böttcher, U.; Kage, H.; Schittenhelm, S. Drought Tolerance and Water-Use Efficiency of Biogas Crops: A Comparison of Cup Plant, Maize and Lucerne-Grass. J. Agron. Crop Sci. 2017, 203, 117–130. [Google Scholar] [CrossRef]
- Ustak, S.; Munoz, J. Cup-Plant Potential for Biogas Production Compared to Reference Maize in Relation to the Balance Needs of Nutrients and Some Microelements for Their Cultivation. J. Environ. Manag. 2018, 228, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Krimmer, E.; Marzini, K.; Heidinger, I. Wild Plant Mixtures for Biogas: Promoting Biodiversity in a Production-Integrated Manner—Practical Trials for Ecological Enhancement of the Landscape. Naturschutz Landschaftsplanung 2021, 2. [Google Scholar] [CrossRef]
- Paltrinieri, S. Cultivated wilt plant mixtures—Description of their botanical appearance as a basis for the assessment of possible ecological potentials. J. Fur Kult. 2023, 75, 77–89. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Schmidt, J. Wild Plants Instead of Maize for Biogas—What Influences the Acceptance of This Biodiversity-Promoting Alternative Crop? Naturschutz Und Landschaftsplanung 2020, 52. [Google Scholar]
- Huth, E.; Paltrinieri, S.; Thiele, J. Bioenergy and Its Effects on Landscape Aesthetics–A Survey Contrasting Conventional and Wild Crop Biomass Production. Biomass Bioenergy 2019, 122, 313–321. [Google Scholar] [CrossRef]
- Mol, F.; Tamms, L.; Gerowitt, B. Biodiversität Einer Mehrjährigen Wildpflanzenmischung Für Die Biogasproduktion. Jul.-Kühn-Arch. 2018, 458, 238. [Google Scholar] [CrossRef]
- Kuhn, W.; Zeller, J.; Bretschneider-Herrmann, N.; Drenckhahn, K. Energy from Wild Plants—Practical Tips for the Cultivation of Wild Plants to Create Biomass for Biogas Generation Plants; Netzwerk Lebensraum Feldflur: Berlin, Germany, 2014; Volume 1, ISBN 978-3-936802-16-0. [Google Scholar]
- Becker, D.; Ilic, A.-M.; Reichardt, F.J.; Hartung, J.; Beck, J.; Jablonowski, N.D.; Lewin, E.; Von Cossel, M. Grower Perspectives on Perennial Wild Plant Mixtures for Biogas Production in Germany. Ind. Crops Prod. 2024, 220, 119126. [Google Scholar] [CrossRef]
- Fürst-Preiß, C.; Von Cossel, M. Biodiversity-Friendly Bioenergy—A Closer Look on Farmer’s Experiences with Perennial Wild Plant Mixture Cultivation for Biogas Production. In Biodiversity and Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 383–408. ISBN 978-0-323-95482-2. [Google Scholar]
- von Cossel, M. How to Reintroduce Arable Crops after Growing Perennial Wild Plant Species Such as Common Tansy (Tanacetum vulgare L.) for Biogas Production. Energies 2022, 15, 4380. [Google Scholar] [CrossRef]
- Kuhn, W. Expert Interview about the Cultivation of WPM and Potential Shift to Late Harvest Regime. 2022. [Google Scholar]
- Croghan, L.; Smith, A.; Tancos, M.; Anderson, N.; Becker, R. Benefits and Risks of Gene Drives for Invasive Plant Management—The Case for Common Tansy. Front. Agron. 2023, 5, 1290781. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.; Zengin, G.; Zheleva, D.; Mahomoodally, M.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.; et al. Tanacetum vulgare L. (Tansy) as an Effective Bioresource with Promising Pharmacological Effects from Natural Arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef] [PubMed]
- Rebele, F. Competition and Coexistence of Rhizomatous Perennial Plants along a Nutrient Gradient. Plant Ecol. 2000, 147, 77–94. [Google Scholar] [CrossRef]
- Kurhanova, I. Lice Infestation and Lice Control Remedies in the Ukraine. Ann. N. Y. Acad. Sci. 2006, 1078, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Dragland, S.; Rohloff, J.; Mordal, R.; Iversen, T.-H. Harvest Regimen Optimization and Essential Oil Production in Five Tansy (Tanacetum vulgare L.) Genotypes under a Northern Climate. J. Agric. Food Chem. 2005, 53, 4946–4953. [Google Scholar] [CrossRef]
- Pszczolkowski, M.A. Prospects of Codling Moth Management on Apples with Botanical Antifeedants and Repellents. Agriculture 2023, 13, 311. [Google Scholar] [CrossRef]
- Rausch, R. Arten-Portraits von Pflanzen oder Flechten (Translation: Species Portraits of Plants or Lichens)—Tanacetum vulgare. Available online: https://www.oekologie-seite.de/index.php?id=24&pid=2468 (accessed on 18 July 2024).
- Von Cossel, M.; Lewandowski, I. Perennial Wild Plant Mixtures for Biomass Production: Impact of Species Composition Dynamics on Yield Performance over a Five-Year Cultivation Period in Southwest Germany. Eur. J. Agron. 2016, 79, 74–89. [Google Scholar] [CrossRef]
- Elbersen, B.; Van Verzandvoort, M.; Boogaard, S.; Mucher, S.; Cicarelli, T.; Elbersen, W.; Mantel, S.; Bai, Z.; MCallum, I.; Iqbal, Y.; et al. Definition and Classification of Marginal Lands Suitable for Industrial Crops in Europe (EU Deliverable); Wageningen University and Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal Agricultural Land Low-Input Systems for Biomass Production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Confalonieri, R.; Jones, B.; Van Diepen, K.; Van Orshoven, J. Scientific Contribution on Combining Biophysical Criteria Underpinning the Delineation of Agricultural Areas Affected by Specific Constraints: Methodology and Factsheets for Plausible Criteria Combinations; Terres, J.-M., Hagyo, A., Wania, A., Eds.; Publications Office of the European Union: Luxembourg, 2014; ISBN 978-92-79-44340-4. [Google Scholar]
- VDLUFA. Methodenbuch Band III Futtermittel (Grundwerk 1976); VDLUFA—Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten e.V.: Speyer, Germany, 1976. [Google Scholar]
- Kenward, M.G.; Roger, J.H. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- Piepho, H.-P. An Algorithm for a Letter-Based Representation of All-Pairwise Comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ciesielczuk, T.; Poluszynska, J.; Rosik-Dulewska, C.; Sporek, M.; Lenkiewicz, M. Uses of Weeds as an Economical Alternative to Processed Wood Biomass and Fossil Fuels. Ecol. Eng. 2016, 95, 485–491. [Google Scholar] [CrossRef]
- Scordia, D.; Papazoglou, E.G.; Kotoula, D.; Sanz, M.; Ciria, C.S.; Pérez, J.; Maliarenko, O.; Prysiazhniuk, O.; von Cossel, M.; Greiner, B.E.; et al. Towards Identifying Industrial Crop Types and Associated Agronomies to Improve Biomass Production from Marginal Lands in Europe. GCB Bioenergy 2022, 14, 710–734. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lebendig, F.; Müller, M.; Hieber, C.; Iqbal, Y.; Cohnen, J.; Jablonowski, N. Improving Combustion Quality of Miscanthus by Adding Biomass from Perennial Flower-Rich Wild Plant Species. Renew. Sustain. Energy Rev. 2022, 168, 112814. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lebendig, F.; Müller, M.; Hieber, C.; Iqbal, Y.; Cohnen, J.; Jablonowski, N.D. Comparison of Thermochemical Conversion and Anaerobic Digestion of Perennial Flower-Rich Herbaceous Wild Plant Species for Bioenergy Production. Bioresour. Technol. 2021, 340, 125724. [Google Scholar] [CrossRef]
- ISO/DIS 17225-7:2020; DIN Solid Biofuels—Fuel Specifications and Classes—Part 7: Graded Non-Woody Briquettes. Deutsches Institut für Normung e.V.: Berlin, Germany, 2020.
- Piatkowska, E.; Biel, W.; Witkowicz, R.; Kepinska-Pacelik, J. Chemical Composition and Antioxidant Activity of Asteraceae Family Plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- von Cossel, M.; Heinzel, K.; Patiño Lordello, G.; Aron Winkler, A.; Lauria, M.V.; Gandamalla, G.; Jablonowski, N.D. Exploring the Potential of Perennial Nectar-Producing Wild Plants for Pellet Combustion. Adv. Sustain. Syst. 2024, 8, 2300599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).