Abstract
We evaluated the effectiveness of three different treatment groups at managing apple shoot blight, and the resulting canker incidence and canker length on wood caused by Erwinia amylovora. Preventative foliar sprays or trunk injections of giant knotweed extract (Regalia), oxytetracycline (Arbor-OTC or FireLine + Regulaid), or streptomycin (Agri-mycin/FireWall + Regulaid) were applied to mature ‘Fuji’ trees. Regalia and oxytetracycline were ineffective at reducing shoot blight severity, showing poor disease reductions of 18.2% and 24.3% compared to untreated controls across both years. Streptomycin was effective at controlling shoot blight severity when applied as a spray application, reducing necrosis by up to 93.9% across both years. Canker incidence was also poorly reduced by Regalia and oxytetracycline with an average decrease of 33.3% and 52.4%, respectively. Again, spray applications of streptomycin were most effective at reducing canker incidence (95.2%). When present, canker length was best controlled by spray applications of streptomycin, showing an average reduction of 95.7%. The effectiveness of Regalia and oxytetracycline was poor, reducing canker length by only 30.4% and 43.5%, respectively. Trunk injections of Regalia were consistently less effective than spray applications. Compared to their spray application counterpart, Regalia injections were, on average, 12.5%, 26.3%, and 25.1% less effective at reducing shoot blight severity, canker incidence, and canker length, respectively. Injected Arbor-OTC was more effective than spray applications of oxytetracycline. On average, Arbor-OTC injections were up to 28.3%, 40.1%, and 30% more effective at reducing shoot blight severity, canker incidence, and canker length compared to spray applications. Overall, Regalia and oxytetracycline were not as effective as streptomycin at controlling fire blight. The search for organic antibiotic alternatives for shoot blight and canker control continues, as cankers are increasing in economic importance by causing bearing wood and young tree death.
1. Introduction
Fire blight, caused by the bacterial pathogen Erwinia amylovora, is one of the most devastating diseases on pome fruit worldwide [1,2]. The bacteria are Gram-negative, facultative anaerobes that move via flagella [3]. They can grow between 4 °C and 37 °C and grow optimally at 28 °C but require at least 18 °C to cause blossom blight epidemics in field conditions [4]. Infection typically occurs on flowers and shoots, which manifests as blossom and shoot blight phases of the disease and can later progress into cankers on perennial wood. Fire blight cankers are necrotized patches of bark that develop from pathogen invasion of wood from an infection of flower or a shoot. Cankers harbor the bacteria as they overwinter and serve as main reservoirs of inoculum for infections next spring. Cells in cankers can transition into a viable, but non-culturable state, indicating interaction with a host immune system [5,6,7,8]. In the spring, orange ooze droplets can form on the canker, drawing insect vectors and becoming exposed to rain splash, that can spread the disease to flowers and shoots. The pathogen can also be spread by rain, wind, and insects to new blossoms and shoots, causing new infections [1,9,10].
Losses and management costs from fire blight can exceed $100 million dollars annually in the United States alone [11]. A 2000 epidemic of fire blight in Michigan resulted in a $42 million dollar removal of approximately 400,000 apple trees [12]. A further $68 million was lost in Washington and Oregon due to fire blight [13]. Beyond the United States, the destruction of 42 ha of trees occurred in Morocco in 2006 and was ultimately insufficient for the suppression of the disease in the area [14]. In Australia, during 1997, an estimated $13 million in revenue was lost during an epidemic at the Royal Botanic Gardens, 80% of which can be attributed to loss of trade [15]. Predictive models suggest that China, the world leader in apple and pear production, could be the next hotspot of a fire blight outbreak, with notable infections occurring only 200 km from the border [16].
Global climate change beckons in an age of warmer, wetter climate patterns. Since 1900, global surface temperatures have increased 1.1 °C, in part due to an increase in global greenhouse gas emissions. At just a 2 °C increase from 1900 conditions, the Intergovernmental Panel on Climate Change predicts at least a 30% increase in wettest day precipitation in key apple growing countries like China and upwards of 5 °C increases for the hottest days of the year [17]. We predict that global climate change will yield warmer and wetter springs and summers, which may lead to increasing outbreaks of fire blight and other plant pathogens [18,19,20,21]. We already face difficulties in the management of fire blight due to a lack of new, effective compounds on the market that are not antibiotics, coupled with few resistant cultivars that are time consuming and difficult to develop [22]. With warmer temperatures on the horizon and fire blight epidemics predicted to increase, the need for novel management strategies is now greater than ever.
A particularly alarming problem for apple and pear growers in the last 20 years is the abrupt occurrence of shoot blight during late spring or summer [23,24]. These shoot infections, originating from the last year’s cankers or current-year flower infections, lead to the death of young trees due to the development of girdling cankers on the wood of central leader and/or rootstock. In mature trees, fire blight cankers can girdle and kill bearing limbs or harbor the pathogen over the winter, allowing new infections in the following growing seasons. Shoot blight and canker phases of fire blight disease have increased in importance due to the introduction of high-density apple orchards with short fruit-bearing branches and small tree sizes [25]. These horticultural traits exacerbate the deadly impact of fire blight and require the development of new effective materials, spray programs, and strategies for preventive and post-infection management options in high-density orchards [26].
Historically, streptomycin and oxytetracycline have been used in the control of fire blight, but as resistant E. amylovora populations have emerged, new effective chemistries and biocontrol materials are in high demand [27]. Current management practices focus on preventative copper and antibiotic sprays, coupled with canopy management with prohexadione–calcium or pruning removal and other cultural practices that prevent the establishment of E. amylovora in orchards [11,23,26,28]. However, antibiotics and copper are often ineffective once the pathogen reaches the host xylem or cortical parenchyma [29]. In recent years, the development of materials that activate systemic resistance in plants has been of interest as an alternative to traditional pesticides. These materials, often referred to as systemic acquired resistance activators, or SARs, trigger a broad-spectrum immune response in their hosts [30]. Under normal conditions, a pathogen may elicit pattern-triggered or effector-triggered immune responses in plants during their infection period [31]. Successful infection requires the suppression of these host responses by the pathogen, but with the application of SAR activator materials, immune responses can be triggered before the pathogen can suppress the response. Trunk injections of SARs have been shown to be effective at controlling shoot and blossom blight in apple. Injections of potassium phosphites reduced shoot blight incidence by 70.8% and led to a significant reduction in blossom blight control of 25.1% on average and reduced incidence similarly to injected streptomycin [32]. However, injected SARs did not perform consistently in a year with a high infection pressure and have not reached the control levels of 92% to 99% expected with the spray application of antibiotics.
In the 1980s, a foliar spray developed from the extract of Reynotria sachalinensis (giant knotweed) became available under the name Milsana and was tested in the 1990s for efficacy against some fungal diseases such as Sphaerotheca fuliginea and Botrytis cinerea [33]. In 2009, the product was reformulated and marketed under the name Regalia and was labeled for foliar spray applications to control a wide range of fungal and bacterial diseases. Regalia relies on anthraquinones, a class of organic compounds based on the anthraquinone structure, which consists of three fused benzene rings forming a quinone derivative [34,35]. Emodin, an anthraquinone found in Regalia, has been suggested to play a role in the disruption of bacterial cell walls, and act as an inhibitor of the electron transport chain by disrupting interactions between ubiquinone and cytochrome b [36,37]. As a class, anthraquinones have been shown to be effective against a suite of Gram-negative bacteria, although their exact mode of action varies among derivatives, with some derivatives having multiple modes of action [38]. Regalia has also been suggested to trigger the SAR pathways to induce host resistance, but this view is contested by recent transcriptomic studies that show trehalose, an important signal molecule in plant microbe interactions, decreased in expression after the application of Regalia [39,40]. Despite the debate on Regalia’s mode of action, the compound shows promise in the control of fire blight on pome fruit [24].
Preliminary studies into the efficacy of Regalia on apple trees showed that at an 11.2 L/ha rate of application, shoot blight incidence could be reduced by 36% on average on mature ‘Honeycrisp’ cultivar [39]. A 35.7% control of shoot blight was also observed when Regalia was applied via trunk injection at a total volume of 76.8 fl oz/A [39]. In a recent study, five preventative spray applications of Regalia reduced canker incidence on pear by 100% compared to untreated controls. A fall trunk injection application of Regalia was not effective by allowing 38.5% canker incidence on wood but when injected in spring it achieved 89.8% control of canker incidence. This reduction was comparable to FireLine (oxytetracycline) and FireWall (streptomycin) spray applications, which provided 100% and 84.2% control of canker incidence, respectively [24]. Results from previous spray programs and trunk injection trials have guided us to develop an optimal application program for Regalia on apple trees as a method of controlling shoot blight and the resulting cankers.
We developed an application program of Regalia on 27-year-old (year 1) and 28-year-old Fuji apple trees (year 2) that decreases application rates but increases the number of applications. This concept is in line with a strategy that the frequent activation of immune responses is needed by plant extract or SAR materials to achieve the desired effect. Finally, our program focuses on spring applications of Regalia, which were shown by Borba et al. [24] to be more effective than fall applications on pear at reducing shoot blight and canker phases of fire blight. Our program’s efficacy was evaluated over two years (2022 and 2023) and compared to streptomycin and oxytetracycline antibiotics. We hypothesized that our previously designed spray application programs of Regalia on pear [24] would yield satisfactory control of shoot blight severity, canker incidence, and canker length on apple.
2. Materials and Methods
2.1. Bacterial Strain and Inoculum Prepartation
Inoculum was prepared using the E. amylovora strain Ea273, which was grown overnight in Luria–Bertani broth using a shaker incubator set at 28 °C [41]. Field inoculation solutions were prepared by diluting Ea273 liquid culture in water to a concentration of 2 × 108 CFU/mL using a micropipette. Concentrations were determined using a DEN-1 McFarland Densitometer (Grant Instruments, Shepreth, Royston UK) and McFarland Standard calibration set 0.5–0.4 (Pro-Lab Diagnostics, Georgetown, TX, USA). Same-day colony plate counts were used to confirm the concentrations of the field inoculation solutions. In 2022, colony plate counts confirmed that trees were inoculated with 6.5 × 108 CFU/mL and in 2023 plate counts confirmed that trees were inoculated with 1.46 × 109 CFU/mL. These concentrations are consistent with common ranges for the shoot blight severity and canker incidence control evaluations that we or others have used before [23,42].
2.2. Plant Material and Shoot Inoculations
Orchard experiments were performed at Virginia Tech’s Alson H. Smith Jr. Agricultural Research and Extension Center in Winchester VA, USA (N 39°6′40″, W 78°16′49″), in 2022 and 2023. In both years, inoculations were conducted on 27- and 28-year-old ‘Fuji BC-2’ M.9 trees (year 1 and year 2, respectively) planted 6.1 m between rows and 2 m between trees in a row. On 2 May 2022, and 25 April 2023, after applied treatments in Table 1, stems were inoculated with 40 µL of prepared Ea273 solution (2 × 108 CFU/mL in distilled water) into a sleeve cut made by a scalpel into the stem using a micropipette. A total of 10 shoots were inoculated per tree across 4 trees per treatment for a total of 40 shoots inoculated per treatment. Ea273 solution was kept on ice until inoculation. Tree location was randomly selected from a block of 64 trees. A buffer of one tree was present on each side of a tree in a row, but not across rows.

Table 1.
Preventive spray and trunk-injection treatments for shoot blight and canker management evaluated in 2022 and 2023. Treatments included 5% extract of giant knotweed R. sachalinensis (Regalia, EPA Reg. No. 84059-3) and two antibiotics, streptomycin (FireWall, EPA Reg. No. 80990-4) and oxytetracycline (Arbor-OTC, EPA Reg. No. 74578-7; FireLine, EPA Reg. No. 80990-6) to reduce shoot blight severity and the incidence of fire blight cankers on perennial wood of apple cultivar ‘Fuji’.
2.3. Experimental Design, Treatments, and Application Timing
Spray treatments (Table 1) were applied to the orchard dilute to drip (3741 L/ha) using a tractor-carried handgun sprayer (Pak-Blast 4 × 25-gal sprayer, 250 PSI, Rear’s Manufacturing, Coburg, OR, USA) to secure good coverage. Trunk injection treatments were conducted using a Quik-jet microinjection system (Arborjet Inc. Woburn, MA, USA) operating with hand-generated hydraulic pressure to deliver the low volumes of pesticide solution for injection, enabling faster application times. We drilled four cardinally oriented injection ports per tree (N, S, E, W), positioned at 10–15 cm above the ground line, 25.4 mm deep into the xylem tissue and 9.53 mm in diameter, with a cordless 1500 rpm drill (DeWalt Industrial Tool Co., Baltimore, MD, USA) [43]. Each port was sealed with an Arborplug® no. 4 (Arborjet Inc., Woburn, MA, USA), using a screwdriver-like plug set tool and a hammer, with the plug top surface located just below the bark level to allow port closure with cambium in the future. The tree trunk diameter-specific volumes of Arbor-OCT 10% solution in water (treatment #3, Table 1) were equally divided among four injection ports. In short, if the tree trunk diameter at 10–15 cm height from the ground line was 6.9 inches, the total injected volume per tree was 21.39 mL, with each of four injection ports receiving 5.3 mL of Arbor-OTC solution (3.1 mL of solution delivers 0.31 g for each 2.54 cm of trunk diameter).
A Regalia 50% solution volume in water of 4.84 mL per tree (treatment #1, Table 1) was equally divided into 1.21 mL and delivered into each of four injection ports per tree, delivering 2.42 mL of Regalia per tree or 0.605 mL of Regalia per injection port. This amount was injected twice on the same day, approximately 40 min apart, as per Table 1.
Maintenance sprays were conducted in the block prior to and after the inoculation in order to suppress the growth of unwanted pathogens and insects that may interfere with results. The maintenance sprays for both years are as follows.
For 2022: 4/6/2022 Inspire Super 0.88 L/ha + Manzate Pro-Stick 3.36 kg/ha + Assail 0.28 kg/ha; 4/21/2022 Inspire Super 0.88 L/ha + Manzate Pro-Stick 3.36 kg/ha + Sonoma 0.7 kg/ha; 5/4/2022 Inspire Super 0.88 L/ha + Manzate + Indar 0.56 kg/ha; 5/17/2022 Inspire Super 0.88 L/ha + Indar 0.56 kg/ha + Manzate 3.36 kg/ha + Diazinon 0.93 L/ha.
For 2023: 3/17/2023 Dormant cover spray with Biocover oil 56.1 L/ha; 3/30/2023 Avaunt 0.42 kg/ha + Vangaurd 0.35 kg/ha + Manzate Pro-Stick 3.36 kg/ha; 4/26/2023 Assail 0.56 kg/ha + Manzate Pro-Stick 3.36 kg/ha + Sonoma 20EW 0.28 kg/ha; 5/26/2023 Movento + Manzate Pro-Stick + Trionic 4SC + Aprovia + Altacor 0.31 kg/ha; 5/31/2023 Movento 0.66 L/ha + Altacor 0.28 kg/ha + Regulaid 0.125 mL/100 L; 6/13/2023 Captan 3.36 kg/ha + Topsin M 0.84 kg/ha + Aprovia 0.40 L/ha + Beseige 0.88 L/ha + Ethephon 1 pt/100; 6/29/2023 Captan 3.36 kg/ha + Omega 1.0 L/ha + Besiege 0.58 L/ha + Ethephon 1 pt/100; 7/11/2023 Diazinon 1.0 mL/100 L + Ziram 6.73 kg/ha.
2.4. Measurement of Shoot Blight Severity, Canker Incidence, and Canker Severity
For each inoculated shoot, shoot blight severity percentage was calculated by multiplying the ratio of the necrotic shoot length (cm) to the total shoot length (cm) by 100. Canker incidence was a result of the development of E. amylovora infections on perennial wood after the pathogen successfully traveled down the inoculated shoot and reached the wood. Incidence percentage was calculated by dividing the number of cankers developed on wood by the total number of shoots inoculated on a per tree basis. This number was multiplied by 100 to obtain a canker incidence rating. Canker length was measured on all cankers that were observed on perennial wood that originated from inoculated shoots. Shoot blight severity, canker incidence, and canker length were observed on 5/31/22, 7/2/22, and 8/2/22 in 2022. In 2023, measurements were taken on 5/24/23, 6/24/23, and 7/24/23.
Mean shoot blight severity percentage, mean percentage of canker incidence, and mean canker length were calculated from 10 replicates and expressed for each replicate tree. The average shoot blight severity percentage, percentage of canker incidence, and canker length were calculated by averaging the four tree replicates per treatment. In total, each value per treatment represents data from 40 inoculated shoots that were measured.
2.5. Statistical Analysis
Treatment, time, and the interaction of treatment and time were considered fixed factors. The response variables analyzed by repeated measurements on the tree, as the experimental unit, were shoot blight severity (percentage), fire blight canker incidence (percentage), and canker length (centimeters). Random variables were the error associated with the tree, as an experimental unit, and the error associated with the time measurements taken on each tree. Data on shoot blight severity, canker incidence, and length were analyzed using the MIXED procedure in SAS Studio on Demand for Academics (SAS Institute, Cary, NC, USA).
2.5.1. Data Transformation
Shoot blight severity data from 2022 were square root-transformed to normalize the distribution of residuals and the main effects of treatment and time were analyzed using repeated measures best adjusted to a heterogeneous autoregressive covariance structure of the first order (p < 0.05). The shoot blight severity data from 2023 were arcsine-transformed to normalize the distribution of residuals and equalize their variances and the main effects of treatment and time were analyzed using repeated measures best adjusted to an unstructured covariance structure (p < 0.05). Treatment and time effects on shoot blight severity did not interact in both 2022 and 2023.
Data on the percentage of canker incidence in 2022 were square root-transformed to normalize the distribution of residuals, and the main effects of treatment and time effects were analyzed using repeated measures best adjusted to a heterogeneous autoregressive covariance structure of the first order (p < 0.05). Data on the percentage of canker incidence in 2023 were arcsine-transformed to normalize the distribution of residuals and equalize their variances and main effects were analyzed using repeated measures best adjusted to an unstructured covariance structure (p < 0.05). Data on canker length from 2022 were used untransformed and main effects were analyzed using repeated measures best adjusted to an unstructured covariance structure (p < 0.05). Data on canker length from 2023 were square root-transformed to normalize the distribution of residuals and the main effects of treatment and time were analyzed using repeated measures best adjusted to an unstructured covariance structure (p < 0.05).
2.5.2. Treatment Comparisons
All pairwise comparisons of spray programs were performed using the PDIFF option of LSMEANS, and means separation was completed by hand-drawing lines connecting the means of similar spray programs and assigning letters according to these lines. The separation of means was performed by hand-drawing lines connecting the means of similar spray programs and assigning letters according to these lines.
3. Results
3.1. Reduction in Shoot Blight Severity
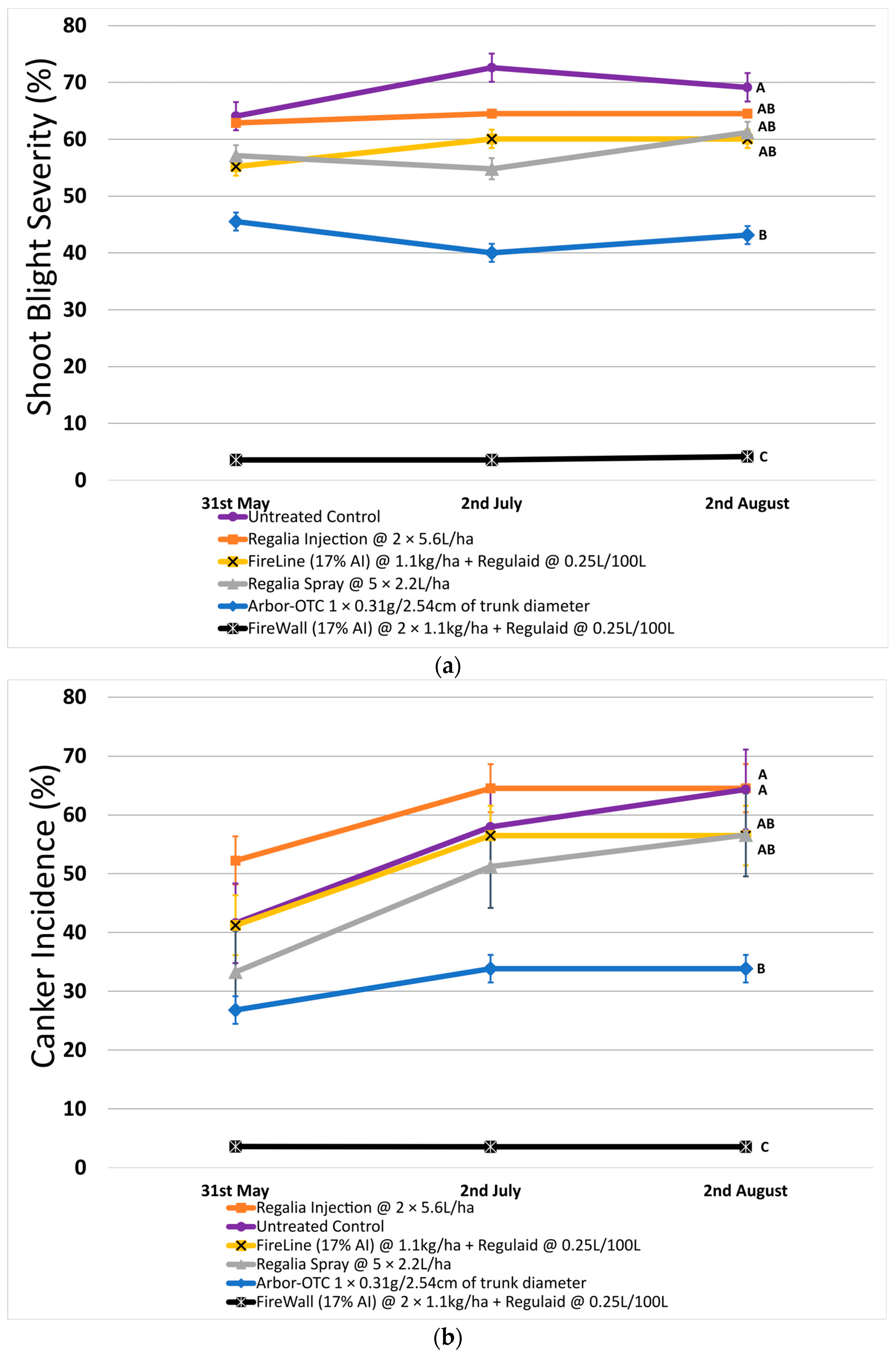
In 2022, shoot blight reached an average of 72.6% severity on untreated control. Shoot blight was the lowest on average after two preventative spray applications of FireWall + Regulaid, reaching only 4.2% severity (Figure 1a). Other control options were inconsistent (Arbor-OTC) or ineffective (Regalia) at successfully reducing shoot blight to an acceptable level.
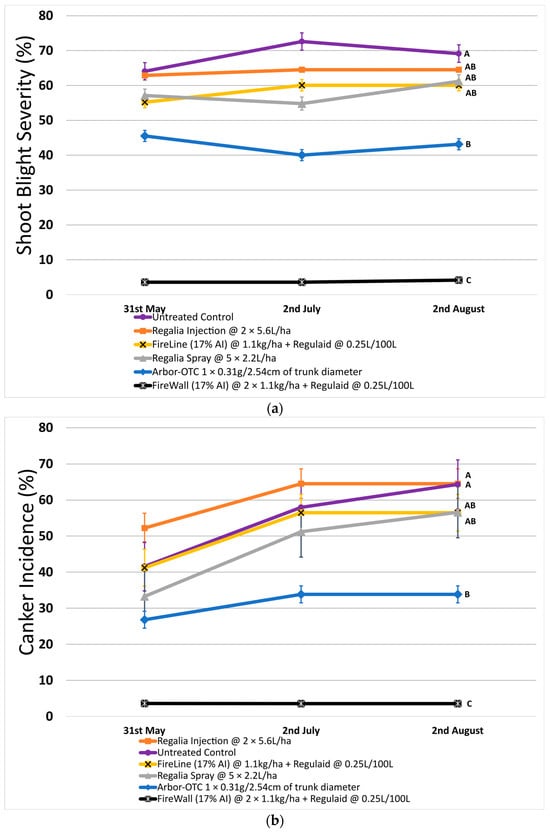
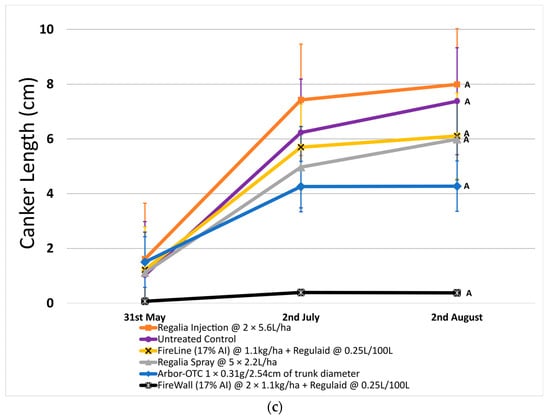
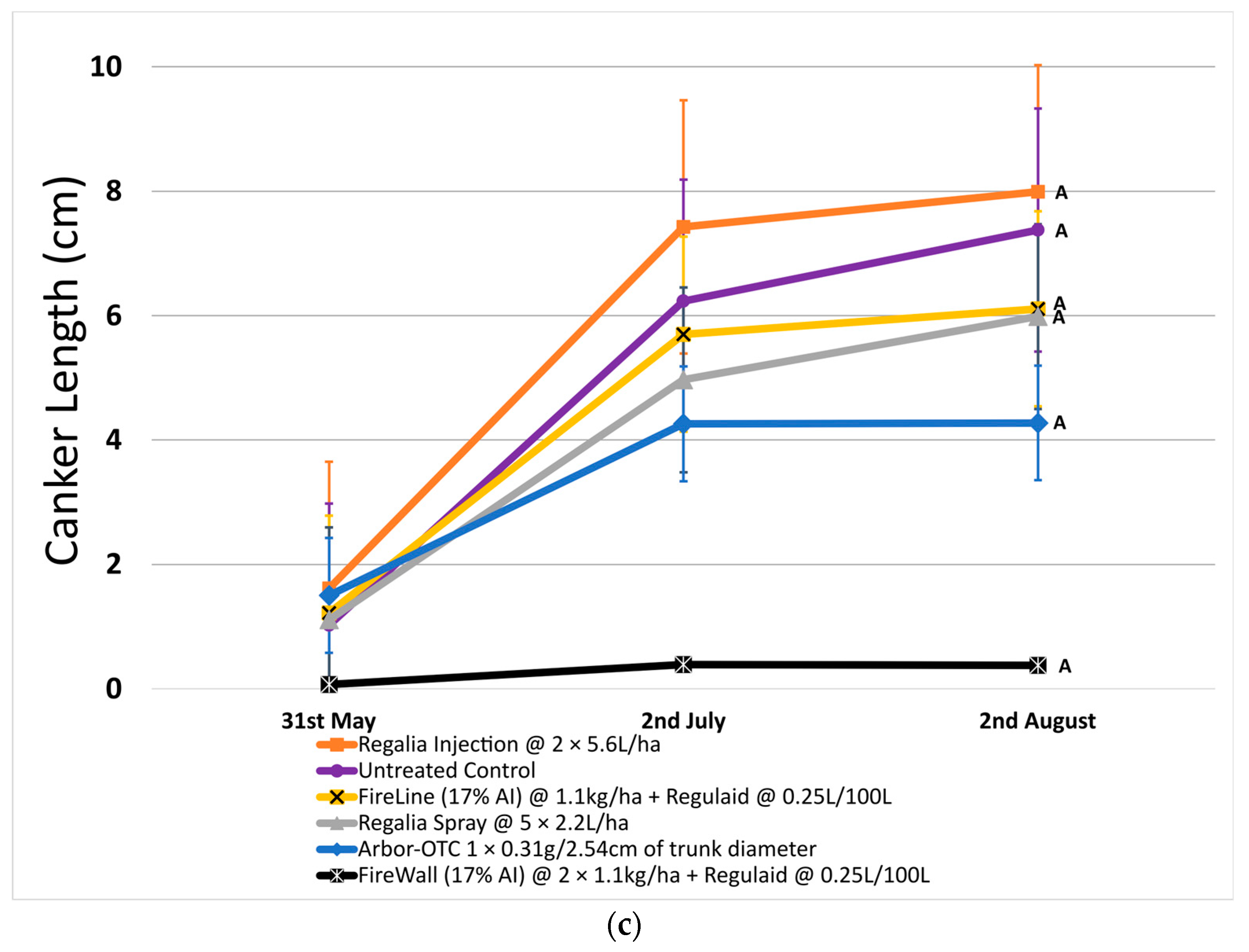
Figure 1.
Shoot blight severity, canker incidence, and canker length in 2022. Error bars represent the standard error of the mean. (a) Shoot blight severity results show the average ratio of necrosis to shoot length measured at three time points spaced one month apart across 4 replicates. (b) Percentage of canker incidence results show the average occurrence of cankers measured at three time points spaced one month apart across 4 replicates. (c) Canker length results show the average length of cankers measured at three time points spaced one month apart across 4 replicates. Treatment lines followed by different letters within each graph are significantly different (t-tests, p < 0.05).
Across both years, only FireWall + Regulaid consistently controlled shoot blight at a high level, 93.9% in 2022 and 81.4% in 2023. Regalia trunk injection and spray applications were not effective at reducing shoot blight when compared to untreated control. FireLine + Regulaid also showed no significant reduction in shoot blight severity when compared to the untreated control. In 2022, Arbor-OTC did show a significant reduction of 37.6% in shoot blight compared to untreated control, but shoot blight severity still reached 43.1% on average in August (Figure 1a).
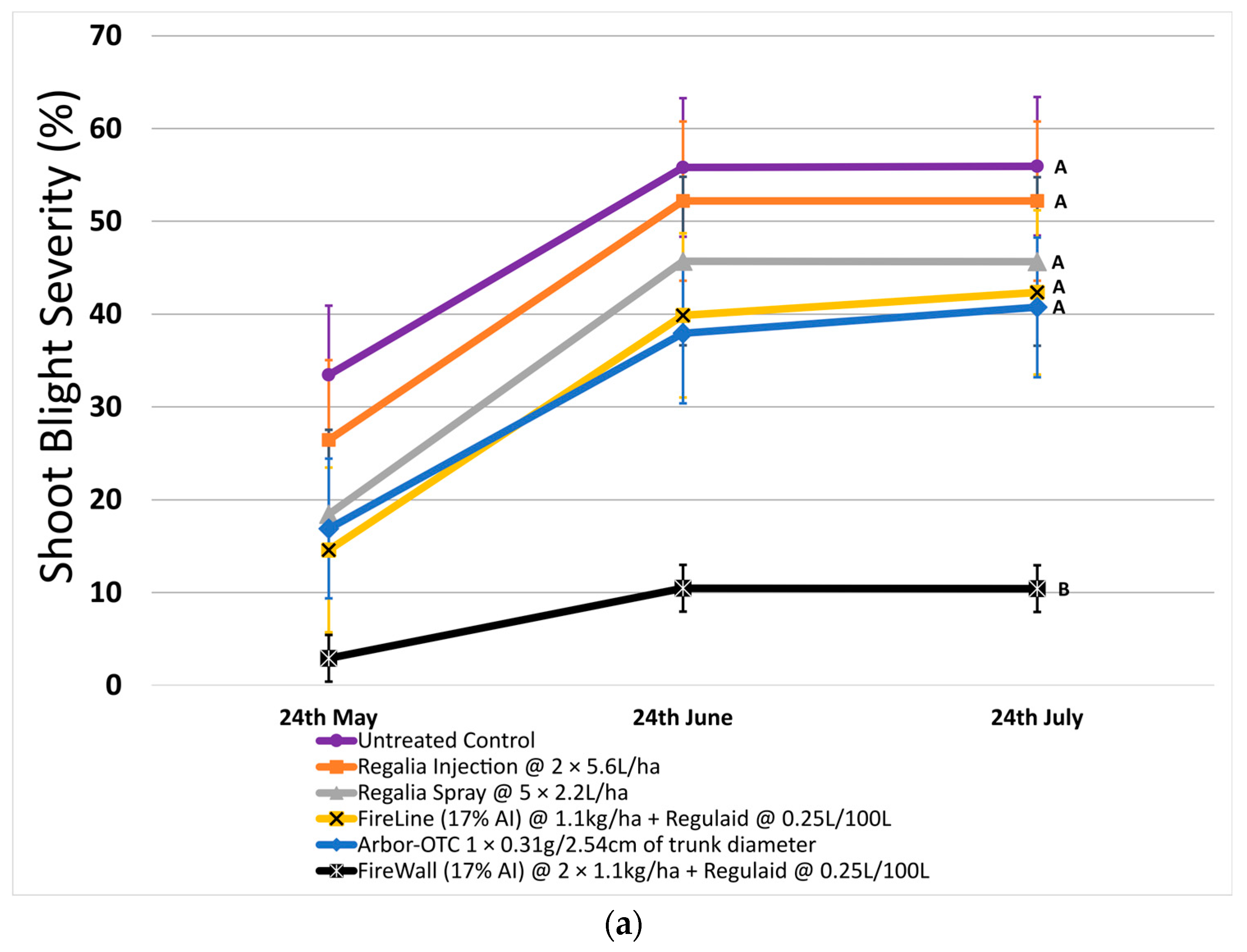
In 2023, shoot blight was less severe on untreated control trees (55.9% on average) (Figure 2a). FireWall + Regulaid was the most effective treatment at reducing shoot blight, allowing an average of only 10.4% shoot blight in August (Figure 2a). Once again, Regalia sprays and trunk injections were not significantly different from untreated control at reducing shoot blight severity. This time, Arbor-OTC was not effective (severity of 40.7%) when compared to untreated control at reducing shoot blight severity.
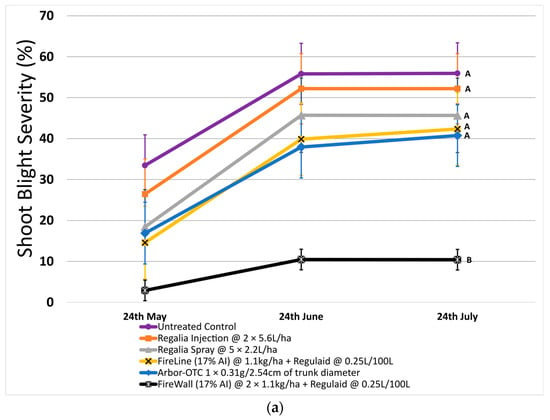
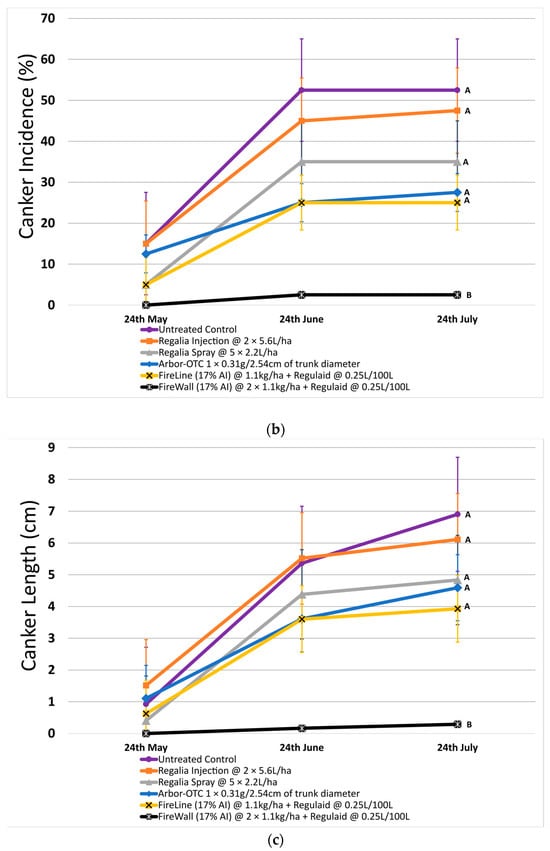
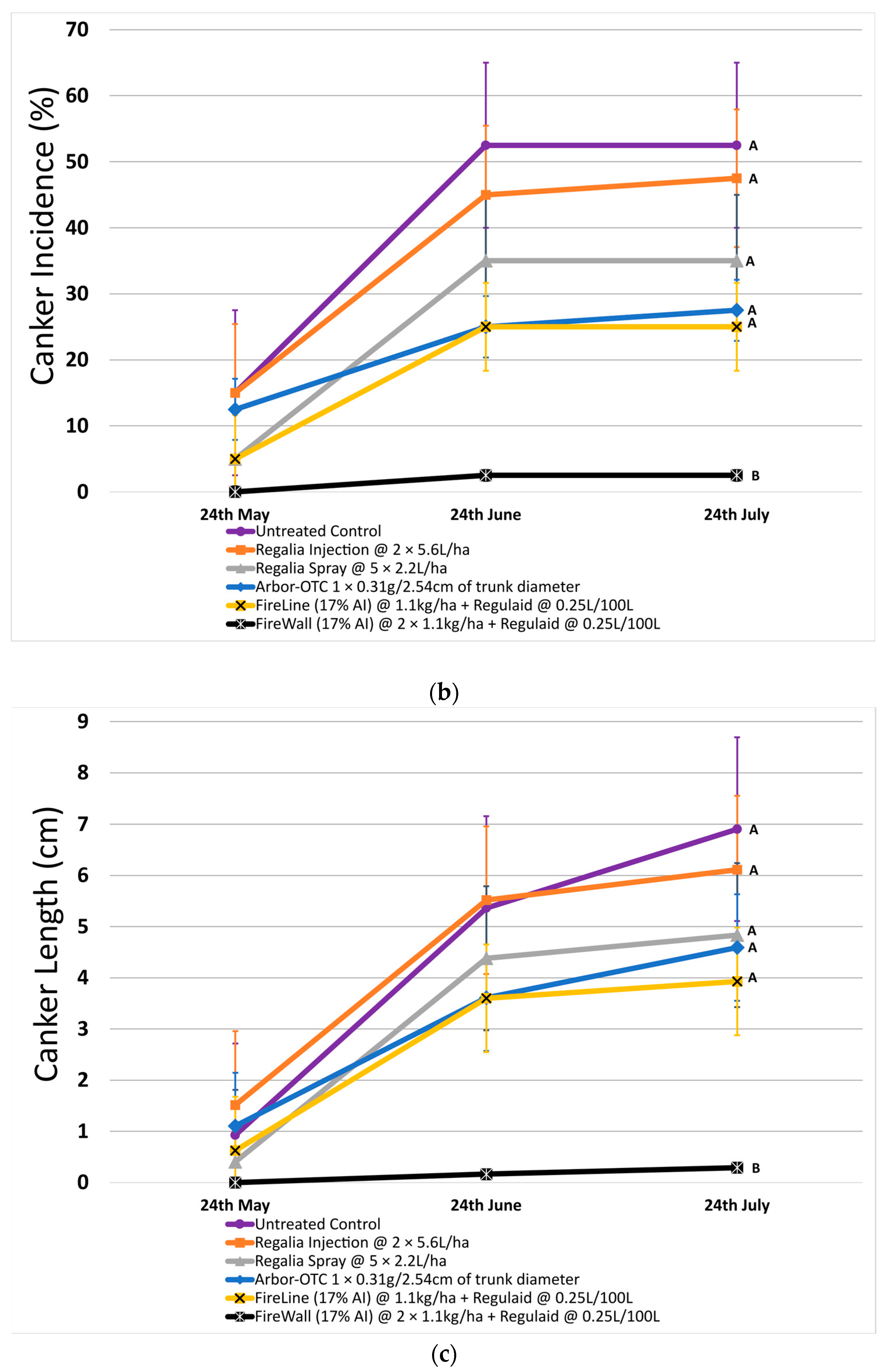
Figure 2.
Shoot blight severity, canker incidence, and canker length in 2023 after preventive spray and trunk injection treatments. Error bars represent the standard error of the mean. (a) Shoot blight severity results show the average ratio of necrosis to shoot length measured at three time points spaced one month apart across 4 replicates. (b) The percentage of canker incidence results show the average occurrence of cankers measured at three time points spaced one month apart across 4 replicates. (c) Canker length results show the average length of cankers measured at three time points spaced one month apart across 4 replicates. Treatment lines followed by different letters within each graph are significantly different (t tests, p < 0.05).
3.2. Reduction in Canker Incidence on Perennial Wood
In 2022, canker incidence in untreated control trees was 64.3% on average. Regalia showed no significant reduction in canker incidence when applied as a spray application (incidence of 56.6%) or through trunk injection (incidence of 64.5%) when compared to untreated control. Canker incidence was significantly reduced by 47.4% with Arbor-OTC and by 94.4% with FireWall + Regulaid (incidence of 3.6%) (Figure 1b). FireLine + Regulaid was also ineffective at reducing canker incidence compared to untreated control (incidence of 56.5%).
In 2023, canker incidence in untreated control at 52.5% was just 18.4% lower compared to 2022. Only FireWall + Regulaid was successful at reducing canker incidence by 95.2% compared to untreated control, allowing only 2.5% canker incidence. Regalia spray applications, Regalia trunk injection, Arbor-OTC, and FireLine + Regulaid were all ineffective at reducing canker incidence when compared to untreated control. They allowed 35%, 47.5%, 27.5%, and 25% canker incidence, respectively (Figure 2b).
Across both years, Regalia was ineffective at reducing canker incidence. Arbor-OTC showed inconsistent results, with mild control in 2022 and no control in 2023. FireWall + Regulaid showed good control in both years. FireLine + Regulaid failed to control canker incidence across both years.
3.3. Reduction in Canker Length
In 2022, untreated control reached a canker length of 7.4 cm on average. FireWall + Regulaid showed the best control of canker length, reaching only 0.4 cm in length on average. Regalia trunk injections showed no canker length control, reaching cankers 8.0 cm in size. Regalia spray treatments, FireLine + Regulaid, and Arbor-OTC also showed poor control of canker length, with average growth reaching 6.0 cm, 6.1 cm, and 4.3 cm, respectively. Results in 2023 showed similar trends, with untreated control reaching an average length of 6.9 cm. Again, FireWall + Regulaid showed the best control allowing only 0.3 cm of canker size development on average. Regalia spray treatments, trunk injections, FireLine + Regulaid, and Arbor-OTC showed no control of canker length, allowing growths of 4.8 cm, 6.1 cm, 3.9 cm, and 4.6 cm, respectively, which were not significantly different from untreated control.
3.4. Comparison of Trunk Injection and Spray Applications
Trunk injections overall were not seen to be statistically any more or less effective than spray applications. Numerical differences do suggest that trunk injections of Regalia were less effective at reducing the severity of fire blight infections. Shoot blight severity was up to 12.7% more severe with trunk injections of Regalia than with the spray applications. Similarly, canker incidence and canker length increased by up to 26.3% and 25.1%, respectively. Arbor-OTC trunk injections were numerically more effective, but not significantly different from spray applications of oxytetracycline (FireLine). Spray applications of this antibiotic had higher shoot blight severity by up to 39.4%, canker incidence by up to 67.2% and canker length by up to 42.9% when compared to trunk injection applications of oxytetracycline (Arbor-OTC).
4. Discussion
Streptomycin in a mixture with a penetrating surfactant was highly effective at controlling shoot blight severity and cankers in both years. Streptomycin has been used widely by growers to control blossom blight, but its high efficacy in the prevention of canker development from the infected shoots has not been shown before. Due to streptomycin-resistant strains of E. amylovora which have emerged in several states of the USA, this antibiotic is not recommended for shoot blight control [44]. Recent studies, however, highlight that streptomycin may induce viable but nonculturable cells (VBNC) in laboratory-grown strains of E. amylovora, allowing them to persist after initial applications. Subsequent treatments of streptomycin or oxytetracycline did not reduce the counts of VBNC in solution, but copper was able to kill these cells when applied 3 days after initial streptomycin treatments [45]. Though these findings have not been applied to a field study, they suggest that streptomycin alone should not be used to control endemic populations of E. amylovora, such as those present in cankers.
Our results highlighted that Regalia sprays applied at high rates or applied as a trunk injection were not as effective as antibiotics such as streptomycin. In both years and at each time point, there was no significant reduction in shoot blight severity, canker incidence, or canker length when trees were treated with Regalia. Our results on Regalia are consistent with those found in Aćimović and Meredith [39] in showing that Regalia showed little to no control of shoot blight incidence when applied as a spray application. More frequent applications at a lower rate, delivering a cumulative rate of 11.2 L/ha, were used in the current study compared to the 2017 study (3 × 4.68 L/ha and 2 × 4.68 L/ha, respectively) [39]. Yuan et al. [40] highlight the inability of Regalia to trigger an SAR in apple through RNA sequencing of apple leaves. Compared to prohexadione–calcium (Apogee, Kudos), Regalia fails to provide the upregulation of defense-related genes, which suggests that its antimicrobial activity on apple is likely limited only to the anthraquinones present in the mixture [38]. Therefore, differences between this study and the 2017 study are likely due to differences in environmental conditions, rather than cultivar response or application rates. Based on several years of data, we can conclude that Regalia alone is not an effective method for controlling shoot blight and canker phases of fire blight disease under high infection pressure conditions. Despite this, there may be a role for Regalia to serve as a tank mixture for other anti-bacterials that may jointly be effective at controlling fire blight.
Our study highlighted that Arbor-OTC had inconsistent results and showed some reduction in shoot blight severity and canker incidence in 2022 but did not reduce canker length. In 2023, Arbor-OTC was ineffective at controlling shoot blight severity and cankers. In the past, trunk injections of oxytetracycline have been effective at controlling shoot blight severity, controlling it by 82% and blossom blight incidence by 60.7% [26,32]. It is likely that the past success of trunk-injected oxytetracycline is due to the age of the trees used in the study. In the current study, our ‘Fuji’ trees were older (27 and 28 years) than the 14-year-old ‘Gala’ and 11-year old ‘Jonathan’ trees used in a 2015 study [26]. The older trees may require more than four injection ports to adequately distribute oxytetracycline, which could have led to poor disease reduction. We believe the difference between 2022 and 2023 that we observed could be explained by the previously described ultraviolet degradation of oxytetracycline [46,47], rendering it less effective over time.
Finally, oxytetracycline applied as a foliar spray was not very effective at reducing fire blight. Since it is bacteriostatic, oxytetracycline slows the rate of bacterial growth rather than killing the pathogen outright, which allows it to prevent pathogen development if applied preventatively [13,48,49,50]. Further studies suggest that strains of E. amylovora (strains 33-1, 32-10) are developing dual resistance to both oxytetracycline and streptomycin, further stressing the need for the development of alternative control materials and practices [51]. In addition, oxytetracycline is a light sensitive material that had can degrade when exposed to sufficient sunlight, reducing its longevity on the host and changing the application times to optimal low-light conditions (early morning or evening) [46,47]. These factors make oxytetracycline a decreasingly viable option for controlling fire blight outbreaks.
Managing the development of fire blight cankers is a critical step to prevent widespread and ongoing losses in pome fruit orchards. E. amylovora populations in cankers can reach up to 107 cells/g in warmer months but fall in population by 1–2 orders of magnitude during colder months [7]. Larger and more frequent cankers throughout the orchard provide a greater magnitude of inoculum for subsequent infection and give the pathogen a higher chance of survival [7]. When cankers reach a sufficient size, they may girdle the stem, or the rootstock of a tree, which can kill the branch of an older tree, or totally destroy a young (1–9 years old) tree [52]. Effective pruning is a good way to keep fire blight cankers in check, but the process is labor intensive [28]. Biorational materials that can decrease cankers per acre are key to developing a sustainable program for future fire blight management strategies and reducing dependency on antibiotics and pruning labor costs.
Antibiotic resistance and the diminishing number of effective materials on the market are a looming issue for traditional means of fire blight control. Through the investigation of alternative management strategies, we can begin to develop a more comprehensive and dynamic spray program for growers to manage resistance and extend the market longevity of prominent antibiotics. We recognize that current management strategies rely heavily on these antibiotics and there are few alternative measures to manage fire blight to the same degree. In the future, we suspect these programs will continue to incorporate antibiotics, but will hopefully be supplemented by SARs, enzymes, and other biological and biorational control measures.
Author Contributions
Conceptualization, S.G.A.; methodology, S.G.A.; formal analysis, S.G.A. and M.C.B.; investigation, N.B., M.C.B. and S.G.A.; resources, S.G.A.; data curation M.C.B., S.G.A. and N.B., writing—original draft preparation, N.B. and S.G.A.; writing—review and editing, S.G.A., M.C.B. and N.B.; visualization, N.B. and S.G.A.; supervision, S.G.A.; project administration, S.G.A.; funding acquisition, S.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Crop Protection and Pest Management Program, project accession number 1028841 (to S.G.A.), by the USDA National Institute of Food and Agriculture, Specialty Crop Research Initiative project Accession No. 1031507, Grant No. 2023-51181-41319/2023-05675 (to S.G.A.), by the Bowman Trust startup fund 444746 and the Virginia Tech faculty startup fund 440905, in 2021–2022 (to S.G.A.), by the cost-share College of Agriculture and Life Sciences Equipment Trust Fund Phase 35 at Virginia Tech in 2022 to S.G.A., and by S.G.A’s unrestricted field trial funds.
Data Availability Statement
Data are contained within the article. The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank Marrone Bio Innovations Inc. for the donation of Regalia material for this project and Vivien Wong, Julie Wong, and Fernanda Ferreira for assisting in collecting the data.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Van Der Zwet, T.; Keil, H.L.; Keil, H.L. Fire Blight: A Bacterial Disease of Rosaceous Plants; US Department of Agriculture, Beltsville Agricultural Research Center: Beltsville, MD, USA, 1979.
- Winslow, C.-E.; Broadhurst, J.; Buchanan, R.; Krumwiede, C., Jr.; Rogers, L.; Smith, G. The Families and Genera of the Bacteria Final Report of the Committee of the Society of American Bacteriologists on Characterization and Classification of Bacterial Types. J. Bacteriol. 1920, 5, 191–229. [Google Scholar] [CrossRef] [PubMed]
- Billing, E.; Baker, L.A.E.; Crosse, J.E.; Garrett, C.M.E. Characeristics of English Isolates of Erwinia amylovora (Burrill) Winslow et al. J. Appl. Bacteriol. 1961, 24, 195–211. [Google Scholar] [CrossRef]
- Santander, R.D.; Biosca, E.G. Erwinia amylovora Psychrotrophic Adaptations: Evidence of Pathogenic Potential and Survival at Temperate and Low Environmental Temperatures. PeerJ 2017, 5, e3931. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, S.; Balaž, J.; Aćimović, D.; Reeb, P. High Magnitude of Fire Blight Symptom Development and Canker Formation from July Onwards on Two Apple Cultivars under Severe Natural Infections. J. Plant Pathol. 2014, 96, 159–168. [Google Scholar]
- Santander, R.D.; Català-Senent, J.F.; Marco-Noales, E.; Biosca, E.G. In Planta Recovery of Erwinia amylovora Viable but Nonculturable Cells. Trees 2012, 26, 75–82. [Google Scholar] [CrossRef]
- Santander, R.D.; Khodadadi, F.; Meredith, C.L.; Rađenović, Ž.; Clements, J.; Aćimović, S.G. Fire Blight Resistance, Irrigation and Conducive Wet Weather Improve Erwinia amylovora Winter Survival in Cankers. Front. Microbiol. 2022, 13, 1009364. [Google Scholar] [CrossRef]
- Dhar, B.C.; Delgado Santander, R.; Aćimović, S.G. Improved Canker Processing and Viability Droplet Digital PCR Allow Detection of Erwinia Amylovora Viable Nonculturable Cells in Apple Bark. Microorganisms 2024, 12, 376. [Google Scholar] [CrossRef]
- Cellini, A.; Giacomuzzi, V.; Donati, I.; Farneti, B.; Rodriguez-Estrada, M.T.; Savioli, S.; Angeli, S.; Spinelli, F. Pathogen-Induced Changes in Floral Scent May Increase Honeybee-Mediated Dispersal of Erwinia amylovora. ISME J. 2019, 13, 847–859. [Google Scholar] [CrossRef]
- Slack, S.M.; Zeng, Q.; Outwater, C.A.; Sundin, G.W. Microbiological Examination of Erwinia amylovora Exopolysaccharide Ooze. Phytopathology 2017, 107, 403–411. [Google Scholar] [CrossRef]
- Norelli, J.L.; Jones, A.L.; Aldwinckle, H.S. Fire Blight Management in the Twenty-First Century: Using New Technologies That Enhance Host Resistance in Apple. Plant Dis. 2003, 87, 756–765. [Google Scholar] [CrossRef]
- Longstroth, M. The 2000 Fire Blight Epidemic in Southwest Michigan Apple Orchards. Compact Fruit Tree 2001, 34, 16–19. [Google Scholar]
- Stockwell, V.; Johnson, K.; Loper, J. Biological Control of Fire Blight: Understanding Interactions among Introduced and Indigenous Microbial Communities. In Phyllosphere Microbiol; American Phytopathological Society: St. Paul, MI, USA, 2002; pp. 225–239. [Google Scholar]
- Doukkali, L.; Radouane, N.; Ezrari, S.; Tahiri, A.; Tazi, B.; Guenoun, F.; Amiri, S.; Lahlali, R. Lessons Learnt from the Fire Blight Epidemics: A Mini Review. Indian Phytopathol. 2022, 75, 611–625. [Google Scholar] [CrossRef]
- Rodoni, B.; Merriman, P.; McKirdy, S.; Wittwer, G. Costs Associated with Fire Blight Incursion Management and Predicted Costs of Future Incursions. Acta Hortic. 2006, 704, 55. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Wang, L.; Geng, G.; Zhao, W.; Hu, B.; Zhao, Y. Fire Blight Disease, a Fast-Approaching Threat to Apple and Pear Production in China. J. Integr. Agric. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- IPCC. 2023: Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Ault, T.R.; Schwartz, M.D.; Zurita-Milla, R.; Weltzin, J.F.; Betancourt, J.L. Trends and Natural Variability of Spring Onset in the Coterminous United States as Evaluated by a New Gridded Dataset of Spring Indices. J. Clim. 2015, 28, 8363–8378. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S. Net Carbon Uptake Has Increased through Warming-Induced Changes in Temperate Forest Phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A. European Phenological Response to Climate Change Matches the Warming Pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of Climate Change on Plant Diseases—Opinions and Trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Gusberti, M.; Klemm, U.; Meier, M.S.; Maurhofer, M.; Hunger-Glaser, I. Fire Blight Control: The Struggle Goes On. A Comparison of Different Fire Blight Control Methods in Switzerland with Respect to Biosafety, Efficacy and Durability. Int. J. Environ. Res. Public Health 2015, 12, 11422–11447. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Meredith, C.L.; Santander, R.D.; Khodadadi, F. Proof of Concept for Shoot Blight and Fire Blight Canker Management with Postinfection Spray Applications of Prohexadione-Calcium and Acibenzolar-s-Methyl in Apple. Plant Dis. 2021, 105, 4095–4105. [Google Scholar] [CrossRef]
- Borba, M.C.; Meredith, C.L.; Dhar, B.C.; Aćimović, S.G. Proof of Concept for Management of Shoot Blight and Fire Blight Cankers on Pear with Preventive Spray Applications of Giant Knotweed Extract. Front. Hortic. 2023, 1, 1082284. [Google Scholar] [CrossRef]
- Rezzonico, F.; Emeriewen, O.F.; Zeng, Q.; Peil, A.; Smits, T.H.M.; Sundin, G.W. Burning Questions for Fire Blight Research: I. Genomics and Evolution of Erwinia amylovora and Analyses of Host-Pathogen Interactions. J. Plant Pathol. 2024, 106, 797–810. [Google Scholar] [CrossRef]
- Aćimović, S.G.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Evaluation of Trunk-Injected Bactericides and Prohexadione-Calcium for Environmentally Friendly Control of Fire Blight (Erwinia Amylovora) in Apples; Plant Protection Society of Serbia (PPSS): Zlatibor, Serbia, 2015; ISBN 978-86-83017-27-0. [Google Scholar]
- McGhee, G.C.; Sundin, G.W. Evaluation of Kasugamycin for Fire Blight Management, Effect on Nontarget Bacteria, and Assessment of Kasugamycin Resistance Potential in Erwinia amylovora. Phytopathology 2011, 101, 192–204. [Google Scholar] [CrossRef]
- DuPont, S.T.; Munir, M.; Cox, K.; Johnson, K.; Peter, K.; Baro, A. Evaluation of Pruning Therapies in Apple Trees with Fire Blight. J. Plant Pathol. 2023, 105, 1695–1709. [Google Scholar] [CrossRef]
- Koczan, J.M.; McGrath, M.J.; Zhao, Y.; Sundin, G.W. Contribution of Erwinia amylovora Exopolysaccharides Amylovoran and Levan to Biofilm Formation: Implications in Pathogenicity. Phytopathology 2009, 99, 1237–1244. [Google Scholar] [CrossRef]
- Gao, H.; Guo, M.; Song, J.; Ma, Y.; Xu, Z. Signals in Systemic Acquired Resistance of Plants against Microbial Pathogens. Mol. Biol. Rep. 2021, 48, 3747–3759. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, S.G.; Zeng, Q.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Control of Fire Blight (Erwinia amylovora) on Apple Trees with Trunk-Injected Plant Resistance Inducers and Antibiotics and Assessment of Induction of Pathogenesis-Related Protein Genes. Front. Plant Sci. 2015, 6, 16. [Google Scholar] [CrossRef]
- Daayf, F.; Schmitt, A.; Bélanger, R. The Effects of Plant Extracts of Reynoutria sachalinensis on Powdery Mildew Development and Leaf Physiology of Long English Cucumber. Plant Dis. 1995, 79, 577–580. [Google Scholar] [CrossRef]
- Zhang, X.; Thuong, P.T.; Jin, W.; Su, N.D.; Sok, D.E.; Bae, K.; Kang, S.S. Antioxidant Activity of Anthraquinones and Flavonoids from Flower of Reynoutria sachalinensis. Arch. Pharmacal Res. 2005, 28, 22–27. [Google Scholar] [CrossRef]
- Su, H. Regalia® Bioprotectant in Plant Disease Management. Outlooks Pest Manag. 2012, 23, 30–34. [Google Scholar] [CrossRef]
- Liu, M.; Peng, W.; Qin, R.; Yan, Z.; Cen, Y.; Zheng, X.; Pan, X.; Jiang, W.; Li, B.; Li, X. The Direct Anti-MRSA Effect of Emodin via Damaging Cell Membrane. Appl. Microbiol. Biotechnol. 2015, 99, 7699–7709. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular Mechanism of Emodin Action: Transition from Laxative Ingredient to an Antitumor Agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef]
- Malmir, M.; Serrano, R.; Silva, O. Anthraquinones as Potential Antimicrobial Agents-A Review. In Antimicrobial Research: Novel Bioknowledge and Educational Programs; Mendez-Vilas, A., Ed.; Formatex Research Center S.L.: Badajoz, Spain, 2017; pp. 55–61. [Google Scholar]
- Aćimović, S.G.; Meredith, C.L. Evaluation of Newer Biologicals and the SAR-Activator Candidate Regalia in Fire Blight Control Applied by Spraying or Trunk Injection. Fruit Q. 2017, 25, 25–29. [Google Scholar]
- Yuan, X.; Gdanetz, K.; Outwater, C.A.; Slack, S.M.; Sundin, G.W. Evaluation of Plant Defense Inducers and Plant Growth Regulators for Fire Blight Management Using Transcriptome Studies and Field Assessments. Phytopathology 2023, 113, 2152–2164. [Google Scholar] [CrossRef]
- Bertani, G. Studies on Lysogenesis I. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Temple, T.N. Comparison of Methods of Acibenzolar-S-Methyl Application for Post-Infection Fire Blight Suppression in Pear and Apple. Plant Dis. 2016, 100, 1125–1131. [Google Scholar] [CrossRef]
- Aćimović, S.G.; VanWoerkom, A.H.; Reeb, P.D.; Vandervoort, C.; Garavaglia, T.; Cregg, B.M.; Wise, J.C. Spatial and Temporal Distribution of Trunk-Injected Imidacloprid in Apple Tree Canopies. Pest Manag. Sci. 2014, 70, 1751–1760. [Google Scholar] [CrossRef]
- de León Door, A.P.; Romo Chacón, A.; Acosta Muñiz, C. Detection of Streptomycin Resistance in Erwinia amylovora Strains Isolated from Apple Orchards in Chihuahua, Mexico. Eur. J. Plant Pathol. 2013, 137, 223–229. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, H.S.; Park, D.H. Persistence and Viable but Non-Culturable State Induced by Streptomycin in Erwinia amylovora. Front. Microbiol. 2024, 15, 1346300. [Google Scholar] [CrossRef]
- Slack, S.M.; Walters, K.J.; Outwater, C.A.; Sundin, G.W. Effect of Kasugamycin, Oxytetracycline, and Streptomycin on in-Orchard Population Dynamics of Erwinia amylovora on Apple Flower Stigmas. Plant Dis. 2021, 105, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Christiano, R.; Reilly, C.; Miller, W.; Scherm, H. Oxytetracycline Dynamics on Peach Leaves in Relation to Temperature, Sunlight, and Simulated Rain. Plant Dis. 2010, 94, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.; Jones, A. Epidemiology and Genetic Analysis of Streptomycin-Resistant Erwinia amylovora from Michigan and Evaluation of Oxytetracycline for Control. Phytopathology 1994, 84, 627–633. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic Use in Plant Agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, V.; Duffy, B. Use of Antibiotics in Plant Agriculture. Rev. Sci. Tech.-Off. Int. Epizoot. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Sundin, G.W.; Peng, J.; Brown, L.E.; Zeng, Q.; Förster, H.; Adaskaveg, J.E. A Novel IncX Plasmid Mediates High-Level Oxytetracycline and Streptomycin Resistance in Erwinia amylovora from Commercial Pear Orchards in California. Phytopathology 2023, 113, 2165–2173. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Santander, R.D.; Meredith, C.L.; Pavlović, Ž.M. Fire Blight Rootstock Infections Causing Apple Tree Death: A Case Study in High-Density Apple Orchards with Erwinia amylovora Strain Characterization. Front. Hortic. 2023, 2, 1082204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).