Optimizing Nitrogen Fertilizer Application for Synergistic Enhancement of Economic and Ecological Benefits in Rice–Crab Co-Culture Systems
Abstract
1. Introduction
2. Materials and Methods
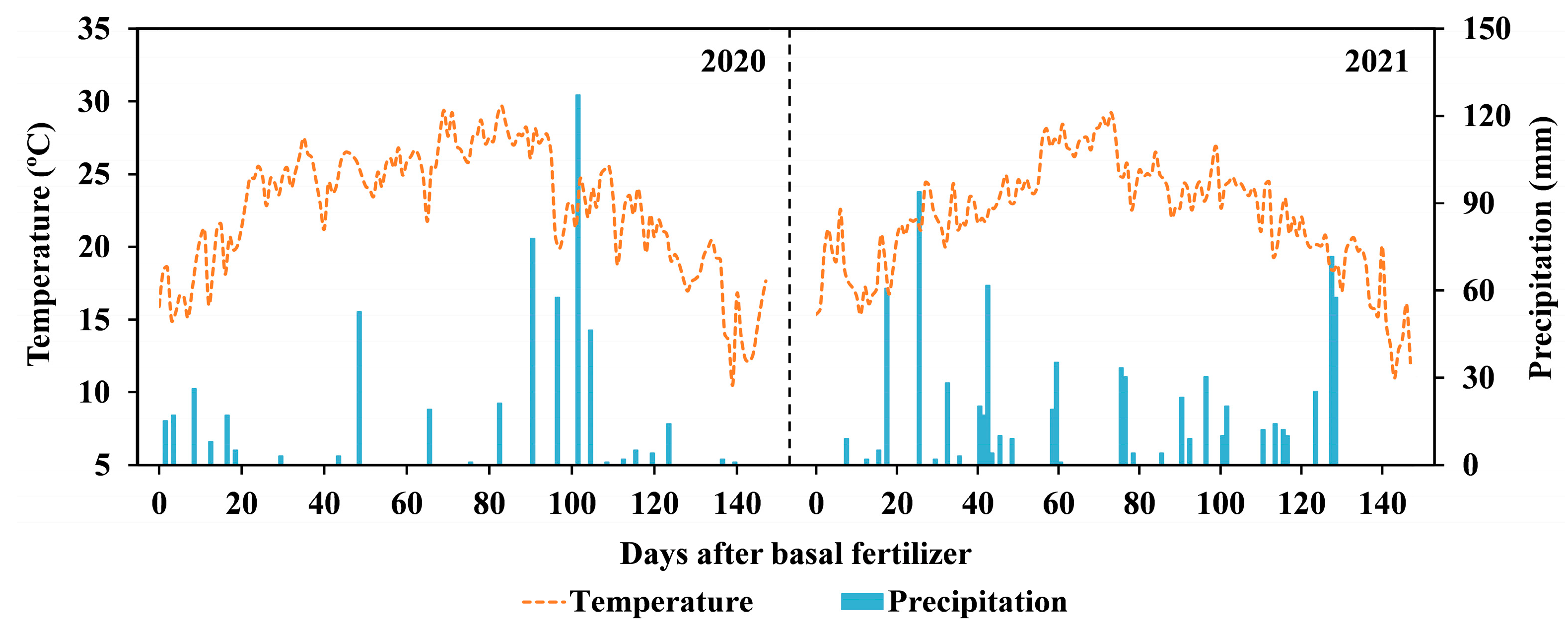
2.1. Study Site
2.2. Experimental Design
2.3. Sampling and Measurements
2.4. Calculation
2.5. Statistical Analysis
3. Results
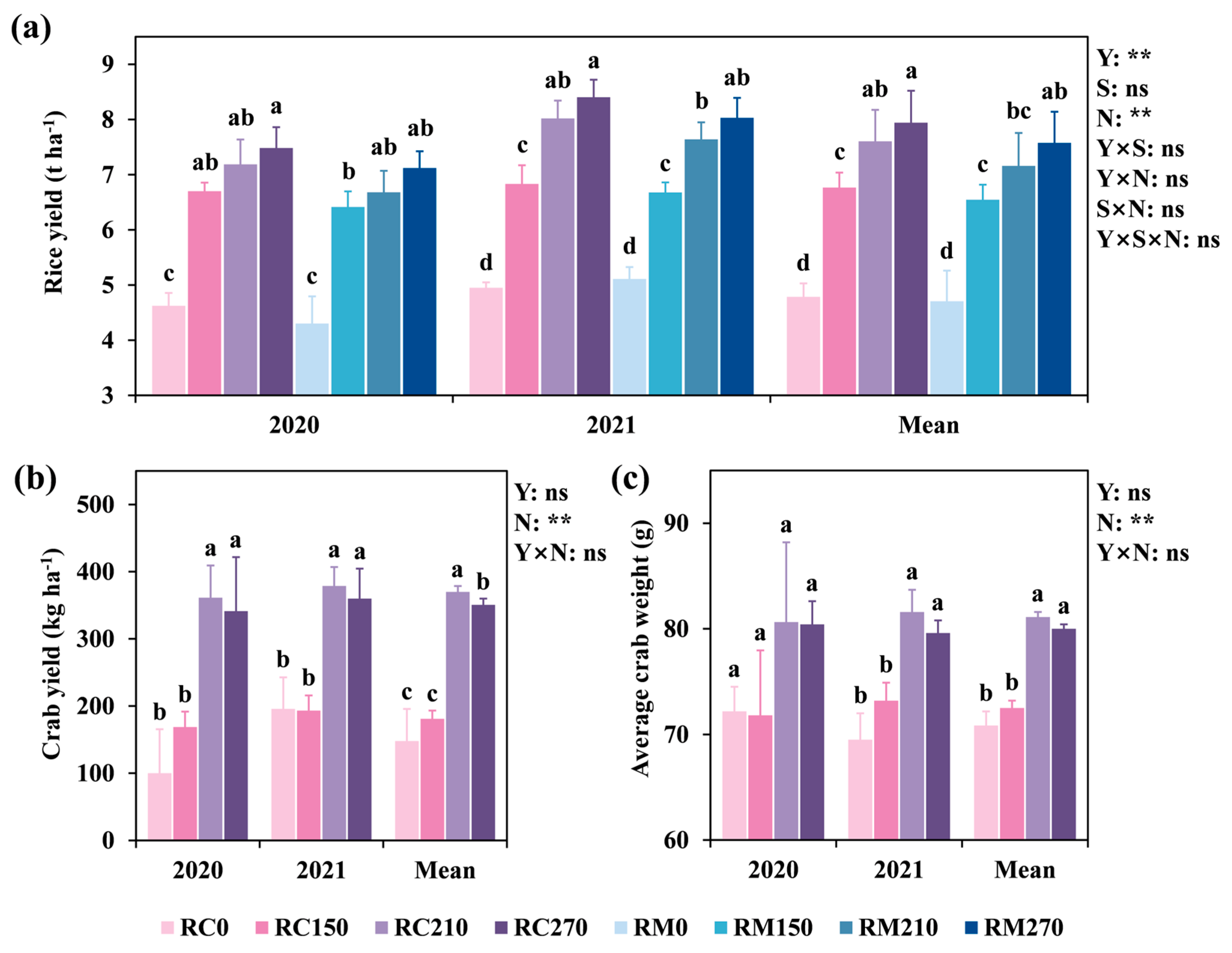
3.1. Rice and Crab Production
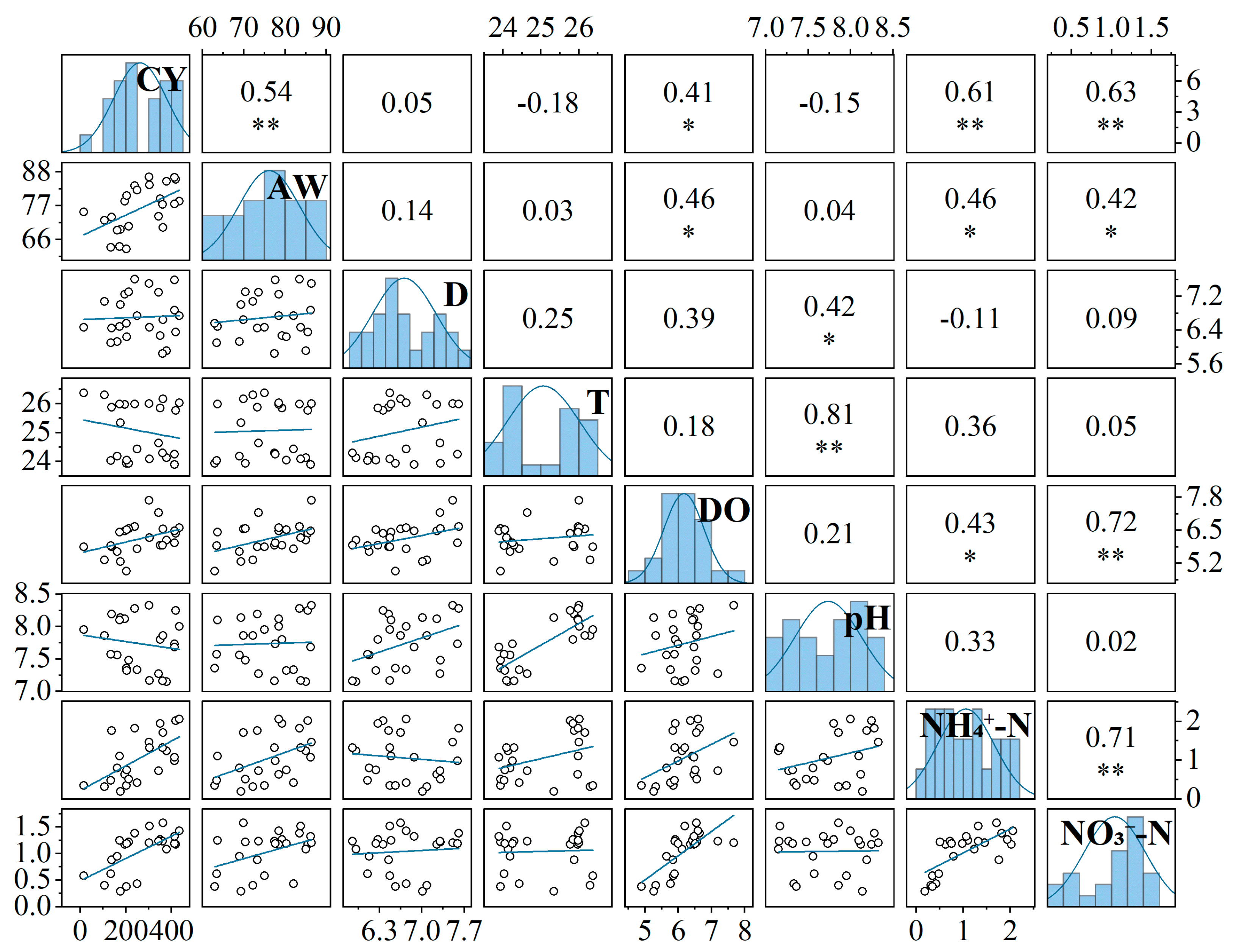
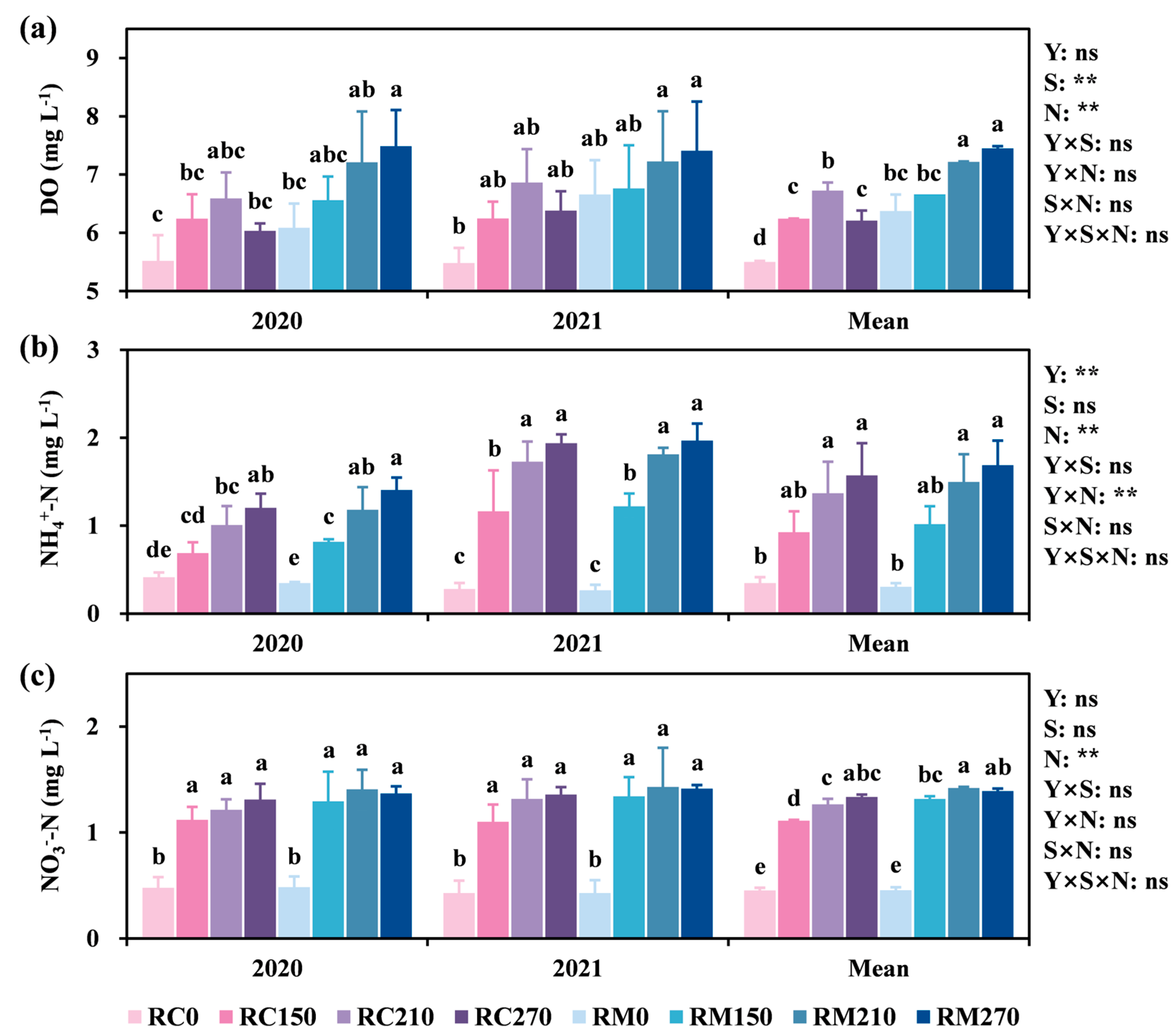
3.2. Effect of Surface Water Characteristics on Crab Production
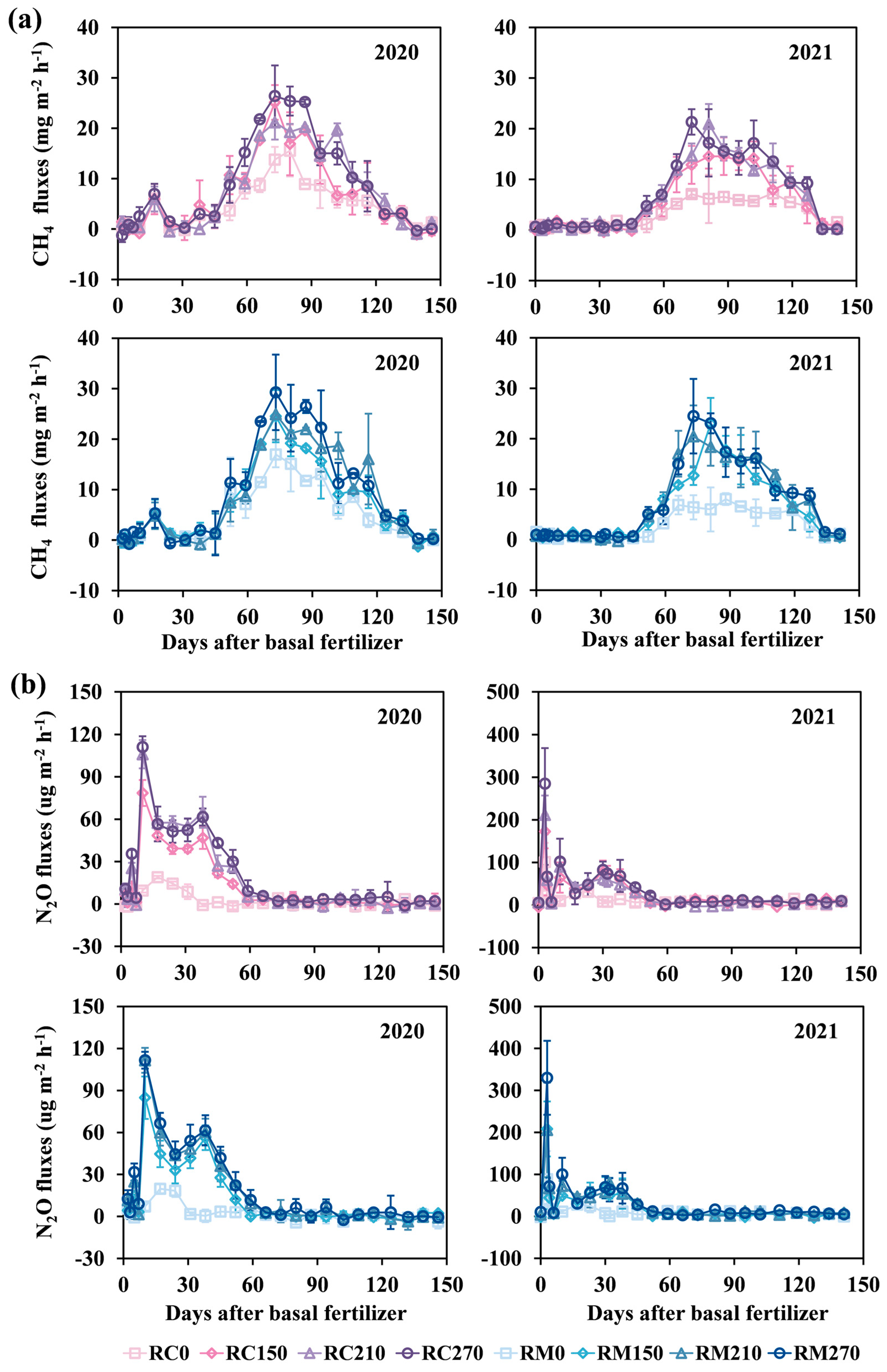
3.3. GHG Emissions, GWP, and GHGI
3.4. NEB and NEEB
4. Discussion
4.1. Optimizing N Application Potential for Improving Rice and Crab Production
4.2. Effects of the Optimizing N Application on GHG Emissions in RC System
4.3. Synergize Economic and Ecological Benefits with Optimizing N Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, X.; Batary, P.; Zou, Y.; Zhou, W.; Wang, G.; Liu, Z.; Bai, Y.; Gong, S.; Zhu, Z.; Settele, J.; et al. Agricultural diversification promotes sustainable and resilient global rice production. Nat. Food 2023, 4, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Elrys, A.S.; Zhao, C.; Cai, Z.; Zhang, J.; Mueller, C. Global patterns and controls of yield and nitrogen use efficiency in rice. Sci. Total Environ. 2023, 898, 165484. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Caro, D.; Qin, W.; Wang, C.; Yang, X.; Liu, R.; Zhang, L. Spatial heterogeneity of greenhouse gas emissions from cereal crop production in China. Environ. Chem. Lett. 2022, 20, 3371–3376. [Google Scholar] [CrossRef]
- Guo, C.; Liu, X.; He, X. A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ. 2022, 831, 154982. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Qin, Q.; Qian, X.; Wei, Y. Inequality across China’s Staple Crops in Energy Consumption and Related GHG Emissions. Ecol. Econ. 2018, 153, 17–30. [Google Scholar] [CrossRef]
- Hussain, S.; Peng, S.; Fahad, S.; Khaliq, A.; Huang, J.; Cui, K.; Nie, L. Rice management interventions to mitigate greenhouse gas emissions: A review. Environ. Sci. Pollut. R. 2015, 22, 3342–3360. [Google Scholar] [CrossRef]
- Linquist, B.; Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Global Change Biol. 2012, 18, 194–209. [Google Scholar] [CrossRef]
- Ge, L.; Sun, Y.; Li, Y.; Wang, L.; Guo, G.; Song, L.; Wang, C.; Wu, G.; Zang, X.; Cai, X.; et al. Ecosystem sustainability of rice and aquatic animal co-culture systems and a synthesis of its underlying mechanisms. Sci. Total Environ. 2023, 880, 163314. [Google Scholar] [CrossRef]
- Chen, B.; Guo, L.; Tang, J.; Li, Y.; Li, C. Comprehensive impacts of different integrated rice-animal co-culture systems on rice yield, nitrogen fertilizer partial factor productivity and nitrogen losses: A global meta-analysis. Sci. Total Environ. 2024, 915, 169994. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Databases; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 1 May 2024).
- Qi, Z.; Liu, S.; Ning, B.; Wu, X. The history of rice-fish co-culture in China and its inspiration for the cooperation of the Lancang-Mekong countries. Aquac. Res. 2022, 53, 5761–5770. [Google Scholar] [CrossRef]
- Yu, X.; Hao, X.; Dang, Z.; Yang, L. China rice-fishery integrated farming industry development report. Chin. Fish. 2023, 8, 19–26. (In Chinese) [Google Scholar] [CrossRef]
- Ge, Y.; Shen, K. Current situation, problems and countermeasures of sustainable development for paddy field fishery in China. Guizhou Agr. Sci. 2021, 49, 78–84. (In Chinese) [Google Scholar] [CrossRef]
- Tang, J.; Li, W.; Lv, X.; Wang, Y.; Ding, X.; Tang, Y.; Li, J.; Zhang, J.; Du, J.; You, Y.; et al. Development status and rethinking of the integrated rice-fish system in China. China Rice 2020, 26, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Bao, J.; Jiang, H.; Li, X. Thirty years of rice-crab coculture in China—Research progress and prospects. Rev. Aquacult. 2022, 14, 1597–1612. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, Y.; Guo, J.; Dong, X.; An, D.; Shi, C.; Li, L.; Dong, Y.; Gao, Q. Co-culture of rice and aquatic animals mitigates greenhouse gas emissions from rice paddies. Aquacult. Int. 2024, 32, 1785–1799. [Google Scholar] [CrossRef]
- Zhang, Z.; Bu, D.; Qiang, S. A field investigation of long-term weed control via different types of rice-aquaculture integrated cultivations. J. Plant Prot. 2022, 49, 693–704. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Li, C. Study on the effect of rice-crab co-culture on the prevention and control of dominant weeds in Panjin rice area. North Rice 2019, 49, 24–26. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C.; Zhu, X.; Xu, Z.; Ren, D.; Meng, Y.; Liu, Y.; Tang, J. Dynamic non-target analysis and occurrence of herbicides residues in rice-crab co-culture systems in Jiangsu Province. J. Ecol. Rural Environ. 2022, 38, 933–943. (In Chinese) [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Hu, G.; Meng, S.; Fan, L.; Zheng, Y.; Chen, J.; Zhang, X. Risk assessment of chlorantraniliprole pesticide use in rice-crab coculture systems in the basin of the lower reaches of the Yangtze River in China. Chemosphere 2019, 230, 440–448. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Bashir, M.A.; Sun, Q.; Pan, J.; Wang, H.; Xu, Y.; Duan, N.; Liu, H. Optimal rice-crab co-culture system as a new paradigm to air-water-food nexus sustainability. J. Clean. Prod. 2021, 291, 125936. [Google Scholar] [CrossRef]
- Lv, W.; Yuan, Q.; Lv, W.; Zhou, W. Effects of introducing eels on the yields and availability of fertilizer nitrogen in an integrated rice-crayfish system. Sci. Rep. 2020, 10, 14818. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Deng, Z.; Yin, C.; Xie, Y. Can integrated rice-duck farming reduce CH4 emissions? Environ. Sci. Pollut. R. 2020, 27, 1004–1008. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wang, H.; Sun, W.; Zhai, L.; Zhang, X.; Wang, N.; Rehim, A.; Raza, Q.U.A.; Liu, H. The implementation of rice-crab co-culture system to ensure cleaner rice and farm production. J. Clean. Prod. 2021, 316, 128284. [Google Scholar] [CrossRef]
- Das, P.; Jena, J.; Mishra, B.; Sarangi, N. Impact of varied frequency of nitrogen fertilization on the grow-out performance of Labeo rohita (Hamilton). Aquac. Res. 2011, 42, 1859–1868. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, W.; Li, W.; Zhao, Y.; Zhang, P.; Zhang, C. Food web structure and trophic levels in polyculture rice-crab fields. Chin. J. Oceanol. Limn. 2015, 33, 735–740. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nagelkerke, L.A.J.; Verdegem, M.C.J.; Wahab, M.A.; Verreth, J.A.J. Relationships among water quality, food resources, fish diet and fish growth in polyculture ponds: A multivariate approach. Aquaculture 2008, 275, 108–115. [Google Scholar] [CrossRef]
- Wang, A. Migration and Transformation of Nitrogen in Rice-Crab Culture Systems. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2018. [Google Scholar]
- Wang, A.; Ma, X.; Xu, J.; Lu, W. Methane and nitrous oxide emissions in rice-crab culture systems of northeast China. Aquac. Fish. 2019, 4, 134–141. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.; Zhou, Y.; Dou, Z.; Gao, W.; Yuan, X.; Gao, H.; Zhang, H. Effect of nitrogen application on greenhouse gas emissions and nitrogen uptake by plants in integrated rice-crayfish farming. Sci. Total Environ. 2023, 905, 167629. [Google Scholar] [CrossRef]
- Park, J.R.; Jang, Y.H.; Kim, E.G.; Lee, G.S.; Kim, K.M. Nitrogen Fertilization Causes Changes in Agricultural Characteristics and Gas Emissions in Rice Field. Sustainability 2023, 15, 3336. [Google Scholar] [CrossRef]
- Ren, H.; FU, L.; Wang, Y.; LV, X.; Li, X.; Sui, X. Effect of different nitrogen fertilizer application rates on the yield of super rice “Yanfeng 47”. Mod. Agric. Sci. Technol. 2016, 3, 30–31. (In Chinese) [Google Scholar] [CrossRef]
- Ma, P.; Lan, Y.; Lyu, T.; Li, F.; Yang, Z.; Sun, Y.; Ma, J. Nitrogen Fate and Efficiency of Fertilizer Application under a Rapeseed-Wheat-Rice Rotation System in Southwest China. Agronomy 2021, 11, 258. [Google Scholar] [CrossRef]
- Allan, R.P.; Arias, P.A.; Berger, S.; Canadell, J.G.; Cassou, C.; Chen, D.; Cherchi, A.; Connors, S.L.; Coppola, E.; Abigail Cruz, F.; et al. IPCC 2021: Summary for policymakers in Climate Change 2021. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Song, K.; Zhang, G.; Yu, H.; Huang, Q.; Zhu, X.; Wang, T.; Xu, H.; Lv, S.; Ma, J. Evaluation of methane and nitrous oxide emissions in a three-year case study on single rice and ratoon rice paddy fields. J. Clean. Prod. 2021, 297, 126650. [Google Scholar] [CrossRef]
- He, Z.; Hu, R.; Tang, S.; Wu, X.; Zhang, Y.; Xu, M.; Zhang, W.; Wu, L. New vegetable field converted from rice paddy increases net economic benefits at the expense of enhanced carbon and nitrogen footprints. Sci. Total Environ. 2024, 916, 170265. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hu, L.; Tang, J.; Wu, X.; Li, N.; Yuan, Y.; Yang, H.; Zhang, J.; Luo, S.; Chen, X. Ecological mechanisms underlying the sustainability of the agricultural heritage rice-fish coculture system. Proc. Natl. Acad. Sci. USA 2011, 108, E1381–E1387. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Guo, L.; Zhao, L.; Shi, X.; Ren, W.; Zhang, J.; Tang, J.; Chen, X. Productivity and the complementary use of nitrogen in the coupled rice-crab system. Agr. Syst. 2020, 178, 102742. [Google Scholar] [CrossRef]
- Anwar, K.; Joshi, R.; Morales, A.; Das, G.; Yin, X.; Anten, N.P.R.; Raghuvanshi, S.; Bahuguna, R.N.; Singh, M.P.; Singh, R.K.; et al. Genetic diversity reveals synergistic interaction between yield components could improve the sink size and yield in rice. Food Energy Secur. 2022, 11, e334. [Google Scholar] [CrossRef]
- Zhou, Q.; Yuan, R.; Zhang, W.; Gu, J.; Liu, L.; Zhang, H.; Wang, Z.; Yang, J. Grain yield, nitrogen use efficiency and physiological performance of indica/japonica hybrid rice in response to various nitrogen rates. J. Integr. Agr. 2023, 22, 63–79. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Qi, B.; Yu, S.; Yao, Z.; Zhu, G.; Fu, Q.; Li, C. Inhibiting release of phenanthrene from rice-crab coculture sediments to overlying water with rice stalk biochar: Performance and mechanisms. Sci. Total Environ. 2024, 908, 168385. [Google Scholar] [CrossRef]
- Leigh, C.; Hiep, L.H.; Stewart-Koster, B.; Vien, D.M.; Condon, J.; Sang, N.V.; Sammut, J.; Burford, M.A. Concurrent rice-shrimp-crab farming systems in the Mekong Delta: Are conditions (sub)optimal for crop production and survival? Aquac. Res. 2017, 48, 5251–5262. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Das, S.K.; Das, M.K.; Bag, M.P. Assessment of vermicompost as direct application manure in fish farming ponds. Turk. J. Fish. Aquat. Sci. 2010, 10, 47–52. [Google Scholar] [CrossRef]
- Terziyski, D.; Grozev, G.; Kalchev, R.; Stoeva, A. Effect of organic fertilizer on plankton primary productivity in fish ponds. Aquacult. Int. 2007, 15, 181–190. [Google Scholar] [CrossRef]
- Yuan, Q.; Lu, W.; Huang, W.; Sun, X.; Lu, W.; Zhou, W. Effects of different fertilization rates on the growth of loach and rice yield in rice-loach culture system. Acta Agric. 2020, 36, 17–22. (In Chinese) [Google Scholar] [CrossRef]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Malek, S.; Ahmad, S.M.S.; Singh, S.K.K.; Milow, P.; Salleh, A. Assessment of predictive models for chlorophyll-a concentration of a tropical lake. BMC Bioinform. 2011, 12, S12. [Google Scholar] [CrossRef]
- Wamboldt, J.J.; Wanamaker, A.D.; Morris, J.E. Commercial Diets in Phase I Palmetto Bass, Morone saxatilisx Morone chrysops, Production in Plastic-lined Ponds: Fertilizer or Feed? J. World Aquacult. Soc. 2015, 46, 490–504. [Google Scholar] [CrossRef]
- Song, H.; Zhu, Q.; Blanchet, J.P.; Chen, Z.; Zhang, K.; Li, T.; Zhou, F.; Peng, C. Central role of nitrogen fertilizer relative to water management in determining direct nitrous oxide emissions from global rice-based ecosystems. Global Biogeochem. Cycles 2023, 37, e2023GB007744. [Google Scholar] [CrossRef]
- Xing, G.; Shi, S.; Shen, G.; Du, L.; Xiong, Z. Nitrous oxide emissions from paddy soil in three rice-based cropping systems in China. Nutr. Cycl. Agroecosys. 2002, 64, 135–143. [Google Scholar] [CrossRef]
- Li, X.; Yuan, W.; Xu, H.; Cai, Z.; Yagi, K. Effect of timing and duration of midseason aeration on CH4 and N2O emissions from irrigated lowland rice paddies in China. Nutr. Cycl. Agroecosyst. 2021, 91, 293–305. [Google Scholar] [CrossRef]
- Hou, H.; Yang, S.; Wang, F.; Li, D.; Xu, J. Controlled irrigation mitigates the annual integrative global warming potential of methane and nitrous oxide from the rice-winter wheat rotation systems in Southeast China. Ecol. Eng. 2016, 86, 239–246. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Jiang, J.; Chen, N.; Yang, X.; Xiong, Z.; Zou, J. Methane and nitrous oxide emissions from rice seedling nurseries under flooding and moist irrigation regimes in Southeast China. Sci. Total Environ. 2012, 426, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Pump, J.; Conrad, R. Partitioning of CH4 and CO2 production originating from rice straw, soil and root organic carbon in rice microcosms. PLoS ONE 2012, 7, e49073. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Liu, S.; Zhu, J.; Zhao, L.; Qi, T.; Liang, J.; Luo, J.; Xiao, X.; Fan, X. Limited aerenchyma reduces oxygen diffusion and methane emission in paddy. J. Environ. Manag. 2021, 279, 111583. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Dash, P.K.; Swain, C.K.; Padhy, S.R.; Roy, K.S.; Neogi, S.; Berliner, J.; Adak, T.; Pokhare, S.S.; Baig, M.J.; et al. Mechanism of plant mediated methane emission in tropical lowland rice. Sci. Total Environ. 2019, 651, 84–92. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, L.; Deng, J.; Sha, Z.; Chu, C.; Zhou, D.; Wu, S.; Lv, W. Modeling CH4 and N2O emission patterns and mitigation potential from paddy fields in Shanghai, China with the DNDC model. Agr. Syst. 2020, 178, 102743. [Google Scholar] [CrossRef]
- Rajendran, S.; Park, H.; Kim, J.; Park, S.; Shin, D.; Lee, J.H.; Song, Y.H.; Paek, N.C.; Kim, C.M. Methane emission from rice fields: Necessity for molecular approach for mitigation. Rice Sci. 2024, 31, 159–178. [Google Scholar] [CrossRef]
- Arth, I.; Frenzel, P.; Conrad, R. Denitrification coupled to nitrification in the rhizosphere of rice. Soil Boil. Biochem. 1998, 30, 509–515. [Google Scholar] [CrossRef]
- Ding, W.; He, P.; Zhang, J.; Liu, Y.; Xu, X.; Ullah, S.; Cui, Z.; Zhou, W. Optimizing rates and sources of nutrient input to mitigate nitrogen, phosphorus, and carbon losses from rice paddies. J. Clean. Prod. 2020, 256, 120603. [Google Scholar] [CrossRef]
- Dong, L.; Yang, T.; Ma, L.; Sun, F.; Yu, F.; Li, Z. Evaluation and benefit analysis of rice-crab cultivation modes in coastal rice areas of Liaoning. China Rice 2023, 29, 100–104. (In Chinese) [Google Scholar] [CrossRef]
- Wu, B.; Huang, L.; Wu, C.; Chen, J.; Chen, X.; He, J. Comparative Analysis of the Growth, Physiological Responses, and Gene Expression of Chinese Soft-Shelled Turtles Cultured in Different Modes. Animals 2024, 14, 962. [Google Scholar] [CrossRef]
- Shi, S.; Ma, Y.; Zhao, D.; Li, L.; Cao, C.; Jiang, Y. The differences in metabolites, starch structure, and physicochemical properties of rice were related to the decrease in taste quality under high nitrogen fertilizer application. Int. J. Biol. Macromol. 2023, 253, 126546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ying, C. Effect of the rice-azolla imbircata cropping on phosphorus absorption and phosphorus fertilizer use efficiency in Rice. Fujian Agr. Sci. Technol. 2024, 55, 55–59. (In Chinese) [Google Scholar]
- Zhang, Y.; Chen, L.; Wang, M.; Lu, J.; Zhang, H.; Heroux, P.; Wang, G.; Tang, L.; Liu, Y. Evaluating micro-nano bubbles coupled with rice-crayfish co-culture systems: A field study promoting sustainable rice production intensification. Sci. Total Environ. 2024, 933, 173162. [Google Scholar] [CrossRef] [PubMed]


| Y | S | N (kg ha−1) | Number of Productive Tillers (plant−1) | Filled Grains (spike−1) | Seed Setting (%) | 1000-Grain Weight (g) | Harvest Index (%) |
|---|---|---|---|---|---|---|---|
| 2020 | RC | 0 | 9.3 ± 0.3 e | 93.3 ± 2.9 ab | 93.3 ± 0.7 a | 28.0 ± 0.2 a | 45.9 ± 1.1 a |
| 150 | 12.9 ± 0.3 bc | 97.0 ± 6.5 ab | 93.3 ± 1.5 a | 28.4 ± 0.3 a | 43.9 ± 0.4 ab | ||
| 210 | 14.2 ± 0.2 ab | 91.0 ± 1.3 ab | 93.0 ± 1.0 a | 28.4 ± 0.2 a | 44.3 ± 2.7 ab | ||
| 270 | 14.5 ± 0.4 a | 87.8 ± 5.3 b | 94.3 ± 0.9 a | 28.4 ± 0.2 a | 41.5 ± 1.1 bc | ||
| RM | 0 | 7.7 ± 0.3 f | 106.0 ± 9.5 a | 92.7 ± 2.3 a | 27.8 ± 0.6 a | 39.8 ± 2.4 c | |
| 150 | 10.9 ± 0.2 d | 100.0 ± 5.3 ab | 94.7 ± 0.9 a | 28.3 ± 0.1 a | 44.2 ± 0.9 ab | ||
| 210 | 12.1 ± 1.5 cd | 94.8 ± 3.6 ab | 93.0 ± 1.2 a | 28.3 ± 0.4 a | 41.4 ± 1.3 bc | ||
| 270 | 12.9 ± 0.4 bc | 88.1 ± 5.3 b | 94.7 ± 0.9 a | 27.7 ± 0.5 a | 39.1 ± 0.9 c | ||
| 2021 | RC | 0 | 8.8 ± 0.2 c | 113.1 ± 1.7 a | 91.8 ± 0.1 a | 26.6 ± 0.6 a | 47.9 ± 0.8 a |
| 150 | 12.7 ± 0.2 b | 109.5 ± 9.0 a | 88.6 ± 0.8 b | 26.4 ± 0.2 a | 45.9 ± 0.9 b | ||
| 210 | 14.6 ± 0.4 a | 110.9 ± 7.3 a | 88.7 ± 0.9 b | 26.5 ± 0.7 a | 49.4 ± 0.6 a | ||
| 270 | 15.6 ± 0.8 a | 110.7 ± 4.3 a | 88.7 ± 0.6 b | 26.2 ± 1.0 a | 44.8 ± 0.8 bc | ||
| RM | 0 | 9.4 ± 0.3 c | 109.5 ± 6.2 a | 87.2 ± 0.4 c | 26.6 ± 0.6 a | 44.8 ± 0.9 bc | |
| 150 | 12.8 ± 0.4 b | 108.5 ± 0.1 a | 85.8 ± 0.2 d | 26.4 ± 0.7 a | 43.3 ± 0.6 cd | ||
| 210 | 14.5 ± 0.6 a | 109.3 ± 1.6 a | 85.8 ± 0.3 d | 26.3 ± 0.9 a | 45.0 ± 0.7 bc | ||
| 270 | 15.3 ± 0.4 a | 107.6 ± 6.2 a | 85.0 ± 0.6 d | 26.2 ± 0.1 a | 42.5 ± 0.6 d | ||
| F value | Y | 23.2 ** | 31.0 ** | 222.7 ** | 24.3 ** | 75.4 ** | |
| S | 12.1 ** | 0.2 | 17.6 ** | 0.1 | 70.6 ** | ||
| N | 106.9 ** | 1.1 | 1.3 | 0.1 | 14.6 ** | ||
| Y × S | 14.9 ** | 1.7 | 24.6 ** | 0.1 | 0.3 | ||
| Y × N | 1.1 | 0.8 | 5.8 ** | 0.1 | 5.7 ** | ||
| S × N | 0.2 | 0.2 | 0.9 | 0.0 | 4.4 * | ||
| Y × S × N | 0.1 | 0.3 | 0.1 | 0.0 | 3.4 * | ||
| Y | S | N (kg ha−1) | GNU (kg ha−1) | NRE (%) | NPFP (kg) |
|---|---|---|---|---|---|
| 2020 | RC | 0 | 44.7 ± 2.3 b | - | - |
| 150 | 62.7 ± 1.5 a | 25.8 ± 1.4 a | 44.7 ± 1.0 a | ||
| 210 | 65.4 ± 4.1 a | 23.3 ± 1.1 a | 34.2 ± 2.1 b | ||
| 270 | 67.2 ± 3.4 a | 22.6 ± 1.4 a | 27.7 ± 1.4 c | ||
| RM | 0 | 40.2 ± 4.6 b | - | - | |
| 150 | 60.0 ± 2.6 a | 24.0 ± 2.1 a | 42.8 ± 1.9 a | ||
| 210 | 63.1 ± 3.7 a | 22.2 ± 1.9 a | 31.8 ± 1.9 b | ||
| 270 | 66.6 ± 2.8 a | 22.2 ± 1.2 a | 26.4 ± 1.1 c | ||
| 2021 | RC | 0 | 42.6 ± 0.5 d | - | - |
| 150 | 65.0 ± 2.6 c | 31.0 ± 0.6 a | 45.5 ± 2.3 a | ||
| 210 | 79.2 ± 2.6 ab | 28.9 ± 1.0 b | 38.2 ± 1.5 b | ||
| 270 | 83.4 ± 3.8 a | 28.2 ± 1.1 b | 31.1 ± 1.2 c | ||
| RM | 0 | 43.7 ± 1.8 d | - | - | |
| 150 | 61.6 ± 3.5 c | 27.1 ± 1.1 b | 44.5 ± 1.2 a | ||
| 210 | 73.6 ± 3.0 b | 24.1 ± 0.6 c | 36.4 ± 1.5 b | ||
| 270 | 77.1 ± 3.4 b | 24.2 ± 0.5 c | 29.7 ± 1.3 c | ||
| F value | Y | 42.4 ** | 58.2 ** | 21.6 ** | |
| S | 8.0 ** | 27.1 ** | 6.5 * | ||
| N | 163.4 ** | 11.0 ** | 197.4 ** | ||
| Y × S | 0.2 | 9.6 ** | 0.1 | ||
| Y × N | 9.5 ** | 0.1 | 1.8 | ||
| S × N | 0.2 | 0.2 | 0.1 | ||
| Y × S × N | 1.2 | 0.3 | 0.0 | ||
| Y | S | N (kg ha−1) | CH4 Emissions (kg ha−1) | N2O Emissions (kg ha−1) | GWP (kg CO2-eq ha−1) | GHGI (kg CO2-eq kg−1) |
|---|---|---|---|---|---|---|
| 2020 | RC | 0 | 181.1 ± 16.0 c | 0.09 ± 0.02 c | 5098 ± 453 c | 1.10 ± 0.04 d |
| 150 | 263.0 ± 31.0 b | 0.48 ± 0.07 b | 7495 ± 854 b | 1.12 ± 0.10 cd | ||
| 210 | 292.0 ± 3.5 ab | 0.65 ± 0.06 a | 8353 ± 93 ab | 1.17 ± 0.08 bcd | ||
| 270 | 330.6 ± 31.1 a | 0.72 ± 0.02 a | 9449 ± 875 a | 1.26 ± 0.06 abcd | ||
| RM | 0 | 199.8 ± 30.4 c | 0.09 ± 0.04 c | 5621 ± 847 c | 1.31 ± 0.13 abc | |
| 150 | 274.3 ± 29.4 b | 0.49 ± 0.08 b | 7814 ± 823 b | 1.22 ± 0.08 abcd | ||
| 210 | 313.0 ± 15.4 ab | 0.64 ± 0.06 a | 8934 ± 438 ab | 1.34 ± 0.03 ab | ||
| 270 | 340.6 ± 23.0 a | 0.69 ± 0.10 a | 9724 ± 620 a | 1.37 ± 0.10 a | ||
| 2021 | RC | 0 | 117.8 ± 1.7 d | 0.36 ± 0.04 c | 3397 ± 60 d | 0.69 ± 0.02 b |
| 150 | 207.5 ± 21.1 c | 0.75 ± 0.07 b | 6011 ± 579 c | 0.89 ± 0.12 a | ||
| 210 | 248.1 ± 12.7 ab | 0.75 ± 0.06 b | 7148 ± 377 ab | 0.89 ± 0.04 a | ||
| 270 | 265.2 ± 7.5 a | 0.95 ± 0.08 a | 7678 ± 194 a | 0.91 ± 0.04 a | ||
| RM | 0 | 109.3 ± 21.4 d | 0.33 ± 0.05 c | 3152 ± 616 d | 0.62 ± 0.12 b | |
| 150 | 228.8 ± 14.2 bc | 0.69 ± 0.08 b | 6594 ± 420 bc | 0.99 ± 0.09 a | ||
| 210 | 267.5 ± 26.6 a | 0.82 ± 0.03 ab | 7711 ± 738 a | 1.01 ± 0.13 a | ||
| 270 | 282.5 ± 7.1 a | 0.93 ± 0.12 a | 8159 ± 230 a | 1.02 ± 0.06 a | ||
| F value | Y | 63.7 ** | 70.9 ** | 59.9 ** | 135.6 ** | |
| S | 3.5 | 0.1 | 3.5 | 11.9 ** | ||
| N | 83.6 ** | 106.6 ** | 90.4 ** | 9.1 ** | ||
| Y × S | 0.0 | 0.0 | 0.0 | 1.7 | ||
| Y × N | 0.9 | 0.9 | 0.9 | 5.1 ** | ||
| S × N | 0.1 | 0.1 | 0.2 | 0.3 | ||
| Y × S × N | 0.3 | 0.4 | 0.3 | 1.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, H.; Wang, H.; Du, X.; Bashir, M.A.; Zhang, X.; Sun, W.; An, M.; Liu, H. Optimizing Nitrogen Fertilizer Application for Synergistic Enhancement of Economic and Ecological Benefits in Rice–Crab Co-Culture Systems. Agronomy 2024, 14, 2219. https://doi.org/10.3390/agronomy14102219
Xu Y, Li H, Wang H, Du X, Bashir MA, Zhang X, Sun W, An M, Liu H. Optimizing Nitrogen Fertilizer Application for Synergistic Enhancement of Economic and Ecological Benefits in Rice–Crab Co-Culture Systems. Agronomy. 2024; 14(10):2219. https://doi.org/10.3390/agronomy14102219
Chicago/Turabian StyleXu, Yang, Hao Li, Hongyuan Wang, Xinzhong Du, Muhammad Amjad Bashir, Xiushuang Zhang, Wentao Sun, Miaoying An, and Hongbin Liu. 2024. "Optimizing Nitrogen Fertilizer Application for Synergistic Enhancement of Economic and Ecological Benefits in Rice–Crab Co-Culture Systems" Agronomy 14, no. 10: 2219. https://doi.org/10.3390/agronomy14102219
APA StyleXu, Y., Li, H., Wang, H., Du, X., Bashir, M. A., Zhang, X., Sun, W., An, M., & Liu, H. (2024). Optimizing Nitrogen Fertilizer Application for Synergistic Enhancement of Economic and Ecological Benefits in Rice–Crab Co-Culture Systems. Agronomy, 14(10), 2219. https://doi.org/10.3390/agronomy14102219







