Effects of Microbial Biostimulants on Maize and Its Pest, the Western Corn Rootworm, Diabrotica virgifera virgifera
Abstract
1. Introduction
2. Materials and Methods
2.1. The Target Pest Diabrotica virgifera virgifera
2.2. Tested Microbial Biostimulants under Laboratory Conditions
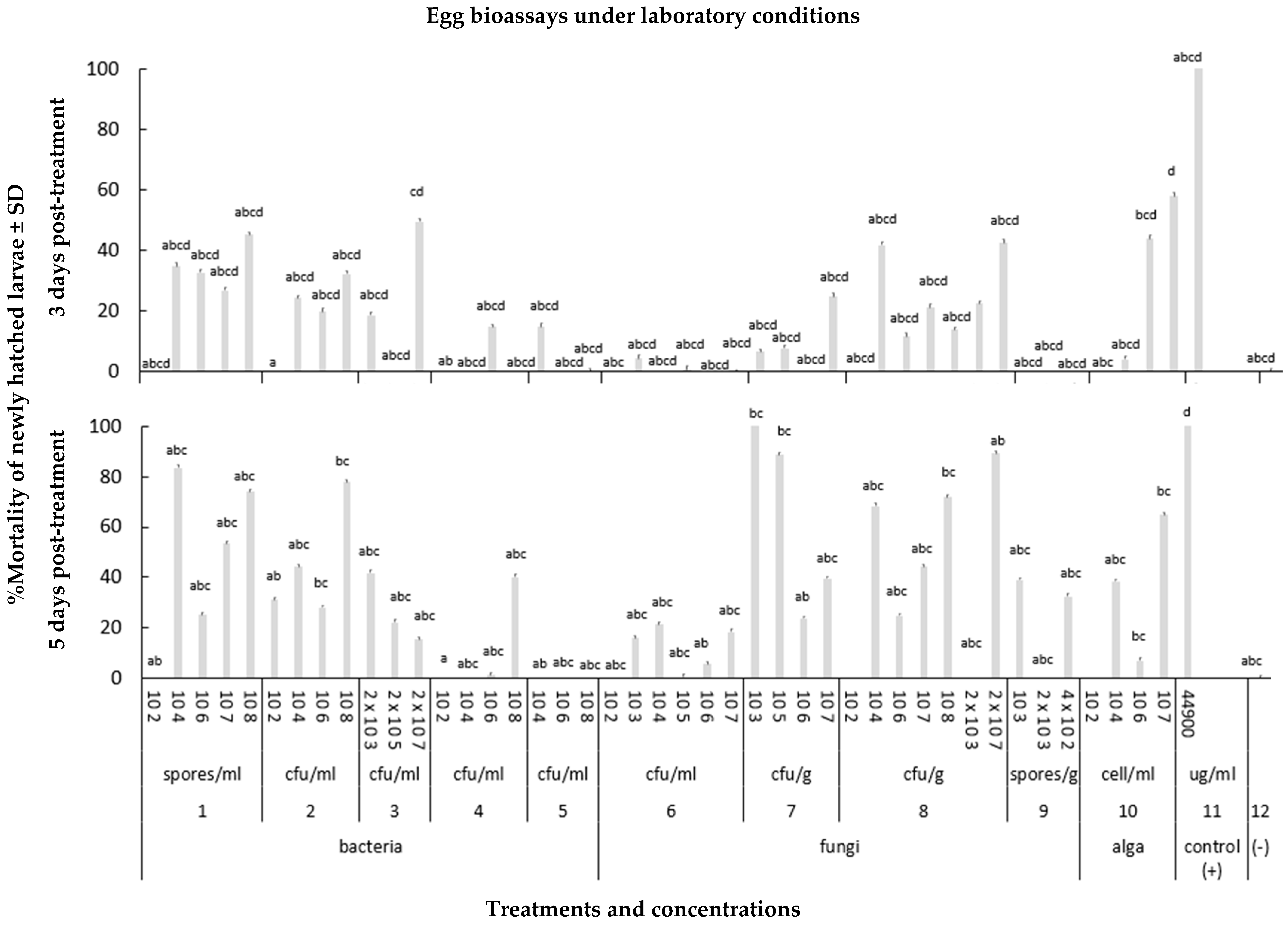
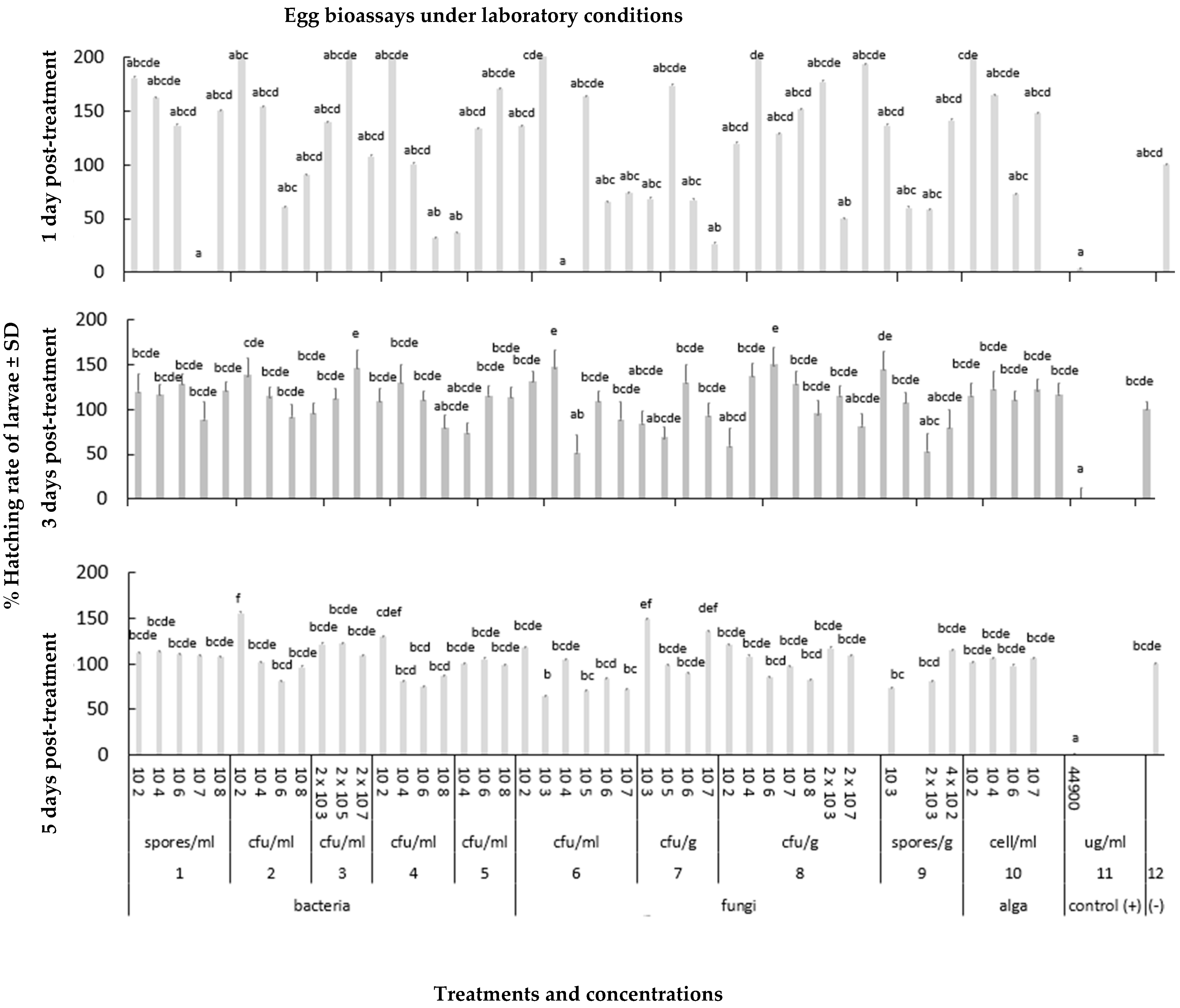
2.2.1. Egg Bioassays
2.2.2. Larvae Bioassays
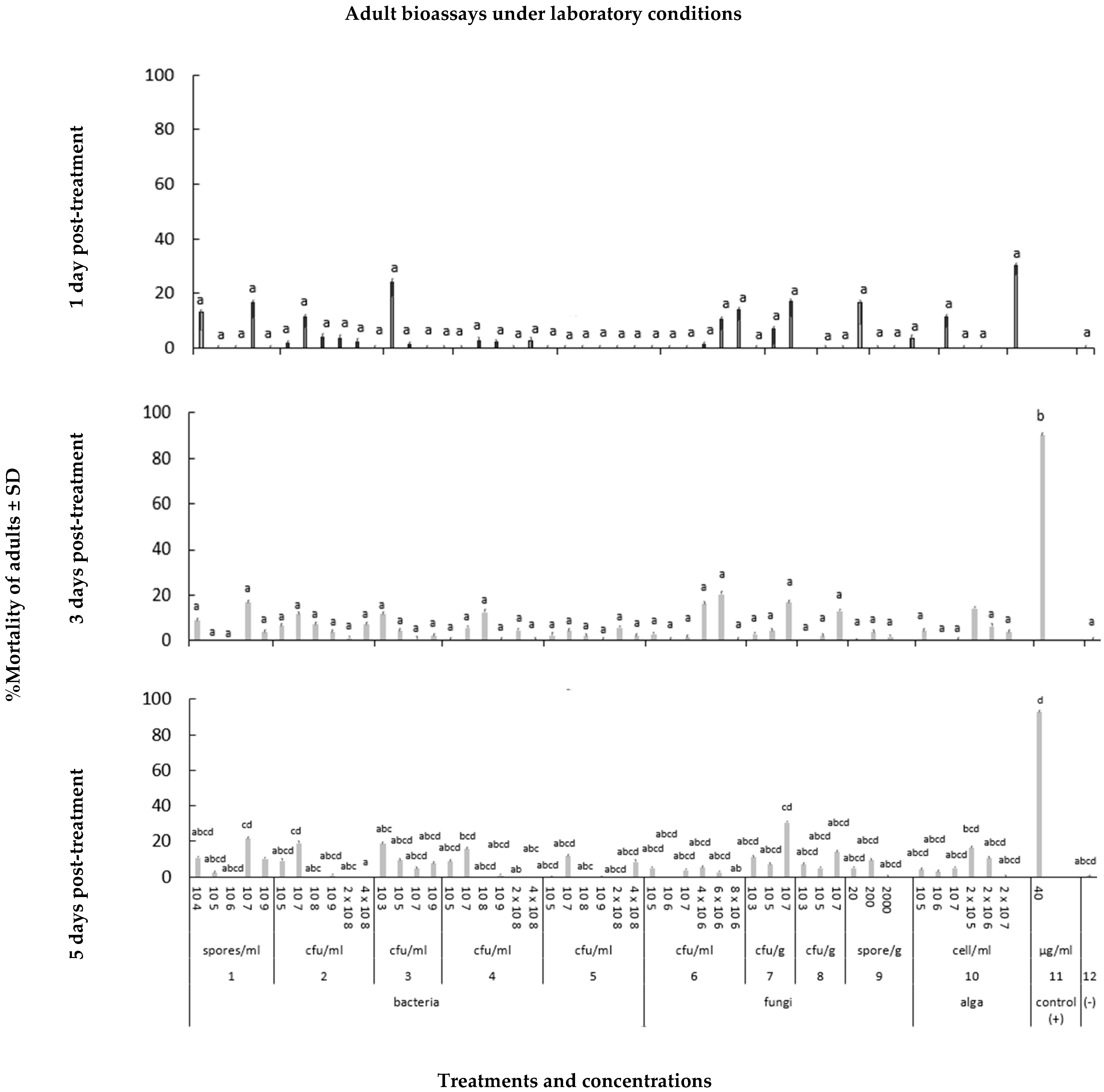
2.2.3. Adult Bioassays
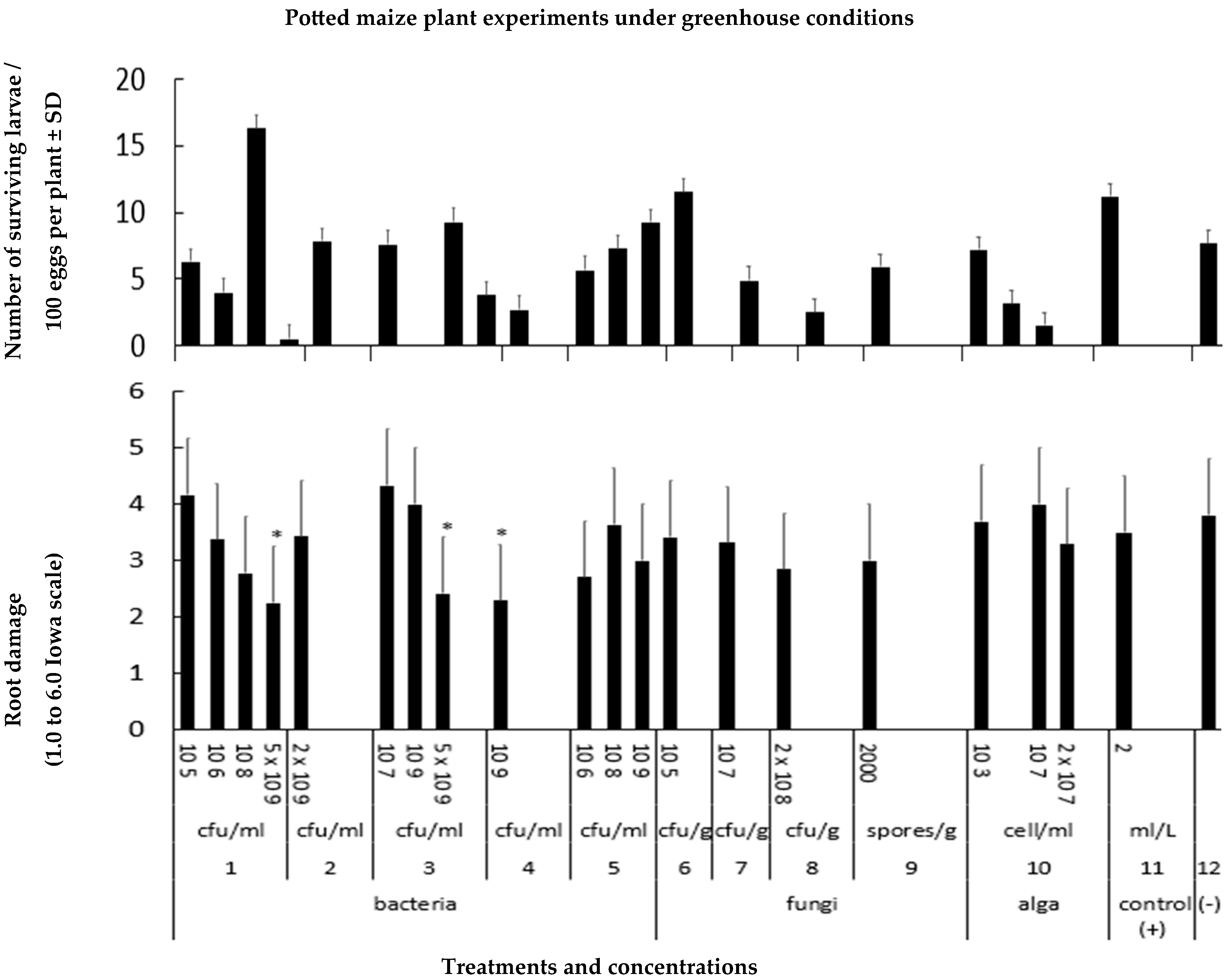
2.3. Testing Microbial Biostimulants under Greenhouse Conditions
Greenhouse Experiments
2.4. Data Analysis
3. Results
3.1. Effects of Microbial Biostimulants on Diabrotica virgifera virgifera Life Stages under Laboratory Conditions
3.1.1. Effects of Microbial Biostimulants on Diabrotica virgifera virgifera Eggs
3.1.2. Effects of Microbial Biostimulants on Diabrotica virgifera virgifera Larvae
3.1.3. Assessing the Effects of Microbial Biostimulants on Diabrotica virgifera virgifera Adults
3.2. Effects of Microbial Biostimulants on Maize and Diabrotica virgifera virgifera Larvae under Greenhouse Conditions
3.2.1. Effects of Microbial Biostimulants on Maize
3.2.2. Effects of Microbial Biostimulants on Diabrotica virgifera virgifera Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meinke, J.; Souza, D.; Siegfried, B.D. The use of insecticides to manage the western corn rootworm, Diabrotica virgifera virgifera, Le Conte: History, field-evolved resistance, and associated mechanisms. Insects 2021, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Bažok, R.; Lemić, D.; Chiarini, F.; Furlan, L. Western corn rootworm (Diabrotica virgifera virgifera LeConte) in Europe: Current status and sustainable pest management. Insects 2021, 12, 195. [Google Scholar] [CrossRef]
- Eben, A. Ecology and Evolutionary History of Diabrotica Beetles-Overview and Update. Insects 2022, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, S.; Toth, S. Entomopathogenic nematode application against root-damaging Diabrotica larvae in maize: What, when, and how. Microb. Nematode Control Invertebr. Pests IOBC-WPRS Bull. 2020, 150, 185–188. [Google Scholar]
- Souza, D.; Peterson, J.A.; Wright, R.; Meinke, L.; Wright, R.J.; Meinke, L.J. Field efficacy of soil insecticides on pyrethroid-762 resistant western corn rootworms (Diabrotica virgifera virgifera LeConte). Pest Manag. Sci. 2020, 76, 827–833. [Google Scholar] [CrossRef]
- Souza, D.; Vieira, B.C.; Fritz, B.K.; Hoffmann, W.C.; Peterson, J.A.; Kruger, G.R.; Meinke, L.J. Western corn rootworm pyrethroid resistance confirmed by aerial application simulations of commercial insecticides. Sci. Rep. 2019, 9, 6713. [Google Scholar] [CrossRef] [PubMed]
- Furlan, L.; Kreutzweiser, D. Alternatives to neonicotinoid insecticides for pest control: Case studies in agriculture and forestry. Environ. Sci. Pollut. Res. 2015, 22, 135–147. [Google Scholar] [CrossRef]
- Ciosi, M.; Toepfer, S.; Li, H. European populations of Diabrotica virgifera virgifera are resistant to aldrin, but not to methyl-parathion. J. Appl. Entomol. 2009, 133, 307–314. [Google Scholar] [CrossRef]
- Parimi, S.; Meinke, L.J.; Nowatzki, T.M.; Chandler, L.D.; French, B.W.; Siegfried, B.D. Toxicity of insecticide-bait mixtures to insecticide resistant and susceptible western corn rootworms (Coleoptera: Chrysomelidae). Crop Prot. 2003, 22, 781–786. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Aleksandr, A.L.; Ildus, A.Y.; Patrick, B. Biostimulants in Plant Science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Tarigan, S.I.; Szabolcs, T.; Mark, S.; Jozsef, K.; Gyorgy, T.; Stefan, T. Biological control properties of microbial plant bio stimulants. A review. Biocontrol Sci. Technol. 2022, 32, 1351–1371. [Google Scholar] [CrossRef]
- Naseri, B.; Hamzavi, F. Effects of chemicaland bio-fertilizers on cowpea resistance to Cowpea Weevil, Callosobruchus maculatus (f.) (Coleoptera: Chrysomelidae). J. Stored Prod. Res. 2021, 92, 101785–101788. [Google Scholar] [CrossRef]
- Murray, C.; Markwick, N.; Kaji, R.; Poulton, J.; Martin, H.; Christeller, J. Expression of various biotin-binding proteins in transgenic Tobacco confers resistance to potato tuber moth, Phthorimaea operculella (Lepidopetera: Gelechiidae). Transgenic Res. 2010, 19, 1041–1051. [Google Scholar] [CrossRef]
- Yan, W.; Lin, X.; Yao, Q.; Zhao, C. Arbuscular mycorrhizal fungi improve uptake and control efficacy of carbosulfan on Spodoptera frugiperda in maize plants. Pest Manag. Sci. 2021, 77, 2812–2819. [Google Scholar] [CrossRef]
- Gad, H.A.; Al-Anany, M.S.; Sameer, W.M.; Al-Anany, F.S. Control of Acanthoscelides obtectus with Trichoderma harzianum applied alone or in combination with diatomaceous earth on a stored common bean. Plant Prot. Sci. 2020, 56, 107–115. [Google Scholar] [CrossRef]
- Alınç, T.; Cusumano, A.; Peri, E.; Torta, L.; Colazza, S. Trichoderma harzianum Strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J. Chem. Ecol. 2021, 47, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Veena-Bhamrah, H.S. Studies on pathogenicity and management of mole cricket, Gryllotalpa gryllotalpa. Ann. Plant Prot. Sci. 2007, 15, 381–383. [Google Scholar]
- Purushothaman, T.; Mol, I. Micro-algae as bio-pesticides for the development of sustainable agriculture. Wide Spectr. 2020, 8, 35–48. [Google Scholar]
- Fei, X.; Zhang, Y.; Ding, L.; Xiao, S.; Xie, X.; Li, Y.; Deng, X. Development of an RNAi-based microalgal larvicide for the control of Aedes aegypti. Parasites Vectors 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Georce, B.W.; Ortman, E.E. Rearing the western corn rootworm in the laboratory. J. Econ. Entomol. 1965, 58, 375–377. [Google Scholar] [CrossRef]
- Branson, T.F.; Guss, P.L.; Krysan, J.L.; Sutter, G.R. Corn Rootworms: Laboratory Rearing and Manipulation; USDA ARS-NC: Peoria, IL, USA, 1975; Volume 28, p. 18. [Google Scholar]
- Singh, P.; Moore, R.F. Hancellook of Insect Rearing; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Toepfer, S.; Toth, S.; Szalai, M. Can the botanical azadirachtin replace phased-out soil insecticides in suppressing the soil insect pest Diabrotica virgifera virgifera. CABI Agric. Biosci. 2021, 2, 28. [Google Scholar] [CrossRef]
- Geisert, R.W.; Huynh, M.P.; Pereira, A.E.; Shapiro Ilan, D.I.; Hibbard, B.E. An improved bioassay for the testing of entomopathogenic nematode virulence to the Western corn rootworm (Diabrotica virgifera virgifera) (Coleoptera: Chrysomelidae): With focus on first instar larvae insect assessments. J. Econ. Entomol. 2023, 116, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Bernklau, E.J.; Bjostad, L.B.; Hibbard, B.E. Synthetic feeding stimulants enhance insecticide activity against western corn rootworm larvae, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J. Appl. Entomol. 2011, 135, 47–54. [Google Scholar] [CrossRef]
- Gemrich, E.G.; Dennis, M. Goldsberry. Laboratory bioassay to assess toxicity of insecticides to Diabrotica larvae. J. Econ. Entomol. 1982, 75, 220–222. [Google Scholar] [CrossRef]
- Sutter, G.R.; Branson, T.F.; Fisher, J.R.; Elliott, N.C. Effect of insecticides on survival, development, fecundity, and sex ratio in controlled infestations of western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1991, 84, 1905–1912. [Google Scholar] [CrossRef]
- Pleau, M.J.; Huesing, J.E.; Head, G.P.; Feir, D.J. Development of an artificial diet for the western corn rootworm. Entomol. Exp. Appl. 2002, 105, 1–11. [Google Scholar] [CrossRef]
- Moar, W.; Khajuria, C.; Pleau, M.; Ilagan, O.; Chen, M.; Jiang, C.; Price, P.; McNulty, B.; Clark, T.; Head, G. Cry3bb1-resistant western corn rootworm, Diabrotica virgifera virgifera (LeConte) does not exhibit cross-resistance to dvSnf7 dsRNA. PLoS ONE 2017, 12, e0169175. [Google Scholar] [CrossRef] [PubMed]
- Dulmage, H.T.; Yousten, A.A.; Singer, S.; Lacey, L.A. Guidelines for the production of Bacillus thuringiensis H-14 and Bacillus sphaericus. In UNDP/WHO/WHO Special Programme for Research and Training in Tropical Diseases; UNDP/WHO/WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Huynh, M.P.; Bernklau, E.J.; Coudron, T.A.; Shelby, K.S.; Bjostad, L.B.; Hibbard, B.E. Characterization of corn root factors to improve artificial diet for western corn rootworm (Coleoptera: Chrysomelidae) larvae. J. Insect Sci. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, S.; Gueldenzoph, C.; Ehlers, R.; Kuhlmann, U. Screening of entomopathogenic nematodes for virulence against the invasive western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) in Europe. Bull. Entomol. Res. 2005, 95, 473–482. [Google Scholar] [CrossRef]
- Brandson, T. Resistance in the grass tribe maydeae to larvae of the western corn rootworm. Ann. Entomol. Soc. Am. 1971, 64, 861–863. [Google Scholar] [CrossRef]
- Hills, T.M.; Peters, D.C. A method of evaluating post planting insecticide treatments for control of western corn rootworrn larvae. J. Ecoll. Entomol. 1971, 64, 764–765. [Google Scholar] [CrossRef]
- Dent, D.R.; Walton, M.P. Methods in Ecological and Agricultural Entomology; CAB International: Wallingford, UK, 1998. [Google Scholar]
- Fischhoff, I.R.; Keesing, F.; Ostfeld, R.S. The tick biocontrol agent Metarhizium brunneum (= M. anisopliae) (strain F52) does not reduce non-target arthropods. PLoS ONE 2017, 12, e0187675. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, P.R.; Gray, C.D. SPSS for Windows Made Simple; Psychology Press Ltd.: East Sussex, UK, 2000. [Google Scholar]
- Wang, Y.; Liu, H.; Shen, Z.; Miao, Y.; Wang, J.; Jiang, X.; Qirong, S.; Rong, L. Richness and antagonistic effects co-affect plant growth promotion by synthetic microbial consortia. Appl. Soil Ecol. 2022, 170, 104300. [Google Scholar] [CrossRef]
- Mulock, B.S.; Chandler, L.D. Effect of Beauveria bassiana on the fecundity of western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Biol. Control 2001, 22, 16–21. [Google Scholar] [CrossRef][Green Version]
- Tawidian, P.; Kang, Q.; Michel, K. The potential of a new Beauveria bassiana isolate for mosquito larval control. J. Med. Entomol. 2023, 60, 131–147. [Google Scholar] [CrossRef]
- Clark, T.B.; Kellen, W.R.; Fukuda, T.; Lindegren, J.E. Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquitoes. J. Invertebr. Pathol. 1968, 11, 1–7. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Khachatourians, G.G. Infection sites of the entomopathogenic fungus Beauveria bassiana in the larvae of the mosquito Aedes aegypti. Entomol. Exp. Appl. 1991, 59, 19–27. [Google Scholar] [CrossRef]
- Bitencourt, R.D.O.B.; dos Santos Mallet, J.R.; Mesquita, E.; Gôlo, P.S.; Fiorotti, J.; Bittencourt, V.R.E.P.; da Costa Angelo, I. Larvicidal activity, route of interaction and ultrastructural changes in Aedes aegypti exposed to entomopathogenic fungi. Acta Trop. 2021, 213, 105732. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Khachatourians, G.G. Larvicidal activity of blastospores and conidiospores of Beauveria bassiana (strain GK 2016) against age groups of Aedes aegypti. Vet. Parasitol. 1990, 37, 155–162. [Google Scholar] [CrossRef]
- Farida, B.; Sonia, H.; Hakima, M.K.; Fatma, B.; Fatma, H. Histological changes in the larvae of the domestic mosquito Culex pipiens treated with the entomopathogenic fungus Beauveria bassiana. Sci. Res. Essays 2018, 13, 1–10. [Google Scholar]
- Dematheis, F.; Kurtz, B.; Vidal, S.; Smalla, K. Multitrophic interactions among Western Corn Rootworm, Glomus intraradices and microbial communities in the rhizosphere and endorhiza of maize. Front. Microbiol. 2013, 4, 357. [Google Scholar] [CrossRef] [PubMed]
- Podder, D.; Ghosh, S.K. A new application of Trichoderma asperellum as an anopheline larvicide for ecofriendly management in medical science. Sci. Rep. 2019, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Tan, M.; Wu, S.; Zheng, L.; Wang, Q.; Wang, G.; Yan, S. Defense responses of arbuscular mycorrhizal fungus-colonized poplar seedlings against gypsy moth larvae: A multiomics study. Hortic. Res. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Sharma, P.R.; Prasad, B.N. Effect of Bradyrhizobium japonicum on Chlorophyll Content, Nodulation, and Plant Growth in Soybean. Korean J. Crop Sci. 2005, 50, 265–267. [Google Scholar]
- Armijo, G.; Kraiser, T.; Medina, M.P.; Gras, D.E.; Zúñiga, A.; González, B.; Gutiérrez, R.A. Arabidopsis thaliana interaction with Ensifer meliloti can support plant growth under N-deficiency. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sorokan, A.; Svetlana, V.; Galina, B.; Igor, M. Endophytic strain Bacillus subtilis 26 D increase levels of phytohormones and repairs growth of potato plants after colorado potato beetle damage. Plants 2021, 20, 923. [Google Scholar] [CrossRef]
- Abdel-Salam, M.S.; Ameen, H.H.; Soliman, G.M.; Elkelany, U.S.; Asar, A.M. Improving the nematicidal potential of Bacillus amyloliquefaciens and Lysinibacillus sphaericus against the root-knot nematode Meloidogyne incognita using protoplast fusion technique. Egypt. J. Biol. Pest Control 2018, 28, 1–6. [Google Scholar] [CrossRef]
- Levine, E.; Oloumi-Sadeghi, H. Management of diabroticite rootworms in corn. Annu. Rev. Entomol. 1991, 36, 229–255. [Google Scholar] [CrossRef]
- Kiss, J.; Edwards, C.R.; Berger, H.K.; Cate, P.; Cean, M.; Cheek, S.; Vahala, O. Monitoring of western corn rootworm (Diabrotica virgifera virgifera LeConte) in Europe 1992–2003. In Western Corn Rootworm: Ecology and Management; CAB International: Wallingford, UK, 2005; pp. 29–39. [Google Scholar]
- Meinke, L.J.; Sappington, T.W.; Onstad, D.W.; Guillemaud, T.; Miller, N.J.; Komáromi, J.; Toth, F. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric. For. Entomol. 2009, 11, 29–46. [Google Scholar] [CrossRef]




| Number | Active Ingredients | Trade Name | Active Ingredient in Product | Formulation | Tested Dosage Range | |||
|---|---|---|---|---|---|---|---|---|
| Eggs | Larvae | Adults | Unit | |||||
| Bacteria | ||||||||
| 1 | Bacillus amyloliquefaciens | CAPITO BIO® | 5 × 109 spore/mL | liquid | 102–108 | 101–108 | 104–109 | spore/mL |
| 2 | Bradyrhizobium japonicum | Phylazonit® NG | 2 × 109 cfu/mL | liquid | 102–108 | 103–108 | 105–109 | cfu/mL |
| 3 | Bacillus subtilis | AmazoN® | 5 × 109 cfu/g | granule | 2 × 103–2 × 107 | 101–108 | 103–109 | cfu/mL |
| 4 | Ensifer meliloti | RhizoFix® RF-50 | 1 × 109 cfu/mL | liquid | 102–108 | 104–108 | 105–109 | cfu/mL |
| 5 | Rhizobium leguminosarum | RhizoFix® RF-40 | 1 × 109 cfu/mL | liquid | 2 × 102–2 × 106 | 105–109 | 105–2 × 108 | cfu/mL |
| Fungi | ||||||||
| 6 | Trichoderma asperellum | Hi-spore® | 3.5 × 107 cfu/g | liquid | 102–107 | 1.103–2 × 107 | 105–107 | cfu/mL |
| 7 | Beauveria bassiana | Bora R® | 5 m/m % | powder | 103–107 | 103–106 | 103–107 | cfu/g |
| 8 | Trichoderma harzianum | Tricho Immun® | 2 × 108 cfu/g | powder | 102–108 | 103–107 | 103–107 | cfu/g |
| 9 | Rhizophagus irregularis | LALRISE® MAX WP | 2000 spore/g | powder | 4 × 102–2 × 103 | 2.101–2.107 | 2 × 101–2 × 103 | spore/g |
| Algae | ||||||||
| 10 | Chlorella vulgaris | Bioplasma Algatrágya® | 2 × 107 cell/mL | liquid | 102–107 | 103–107 | 105–2 × 107 | cell/mL |
| Positive and negative controls | ||||||||
| 11 | Imidacloprid | Confidor ® 200SL | 200 mg/mL | liquid | 44,900 | 1 | 40 | µg/mL |
| 12 | Untreated control (sterilized tap water) | |||||||
| Number | Active Ingredients | Trade Name | Active Ingredient Concentration in Product | Formulation | Dose Tested |
|---|---|---|---|---|---|
| Bacteria | |||||
| 1 | Bacillus amyloliquefaciens | CAPITO BIO® | 5 × 109 spore/mL | liquid | 104; 106; 108 spore/mL |
| 2 | Bradyrhizobium japonicum | Phylazonit ® NG | 2 × 109 cfu/mL | liquid | 2 × 109 cfu/mL |
| 3 | Bacillus subtilis | AmazoN® | 5 × 109 cfu/g | granule | 5 × 109; 107; 109 cfu/g |
| 4 | Ensifer meliloti | RhizoFix® RF-50 | 1 × 109 cfu/mL | liquid | 109 cfu/mL |
| 5 | Rhizobium leguminosarum | RhizoFix® RF-40 | 1 × 109 cfu/mL | liquid | 104; 106; 108 cfu/mL |
| Fungi | |||||
| 6 | Trichoderma asperellum | Hi-spore® | 3.5 × 107 cfu/g | liquid | 105 cfu/g |
| 7 | Beauveria bassiana | Bora R® | 5 m/m % | powder | 107 cfu/g |
| 8 | Trichoderma harzianum | Tricho Immun® | 2 × 108 cfu/g | powder | 2 × 108 cfu/g |
| 9 | Rhizophagus irregularis | LALRISE® MAX WP | 2000 spore/g | powder | 2000 spores/g |
| Algae | |||||
| 10 | Chlorella vulgaris | Bioplasma Algatrágya® | 2 × 107 cell/mL | liquid | 103; 105; 107 cell/mL |
| Positive and negative controls | |||||
| 11 | NPK | Bionova® Soil Supermix | NPK 7-3-6; 2 mL/L | liquid | 2 mL/L |
| 12 | Untreated control (unsterilized tap water) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarigan, S.I.; Kiss, J.; György, T.; Doan, N.P.Y.; Toepfer, S. Effects of Microbial Biostimulants on Maize and Its Pest, the Western Corn Rootworm, Diabrotica virgifera virgifera. Agronomy 2024, 14, 2239. https://doi.org/10.3390/agronomy14102239
Tarigan SI, Kiss J, György T, Doan NPY, Toepfer S. Effects of Microbial Biostimulants on Maize and Its Pest, the Western Corn Rootworm, Diabrotica virgifera virgifera. Agronomy. 2024; 14(10):2239. https://doi.org/10.3390/agronomy14102239
Chicago/Turabian StyleTarigan, Sri Ita, Jozsef Kiss, Turóczi György, Nhu Phuong Y Doan, and Stefan Toepfer. 2024. "Effects of Microbial Biostimulants on Maize and Its Pest, the Western Corn Rootworm, Diabrotica virgifera virgifera" Agronomy 14, no. 10: 2239. https://doi.org/10.3390/agronomy14102239
APA StyleTarigan, S. I., Kiss, J., György, T., Doan, N. P. Y., & Toepfer, S. (2024). Effects of Microbial Biostimulants on Maize and Its Pest, the Western Corn Rootworm, Diabrotica virgifera virgifera. Agronomy, 14(10), 2239. https://doi.org/10.3390/agronomy14102239






