Physiological and Transcriptomic Characterization of Rice Genotypes under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experiment Conditions
2.2. Sampling and Measurements
2.2.1. Yield Components
2.2.2. Chlorophyll Contents
2.2.3. Relative Water Contents
2.2.4. Gaseous Exchange
2.3. RNA-Seq Analysis
2.3.1. Extracting Total RNA and Constructing mRNA Libraries
2.3.2. Quality Control, Mapping of RNA Sequencing Reads, and Gene Annotations
2.4. Statistical Analysis
3. Results
3.1. Seed Yield, Yield Components, and Panicle Traits of a Single Plant
3.2. Leaf Chlorophyll Content and Relative Water Content (RWC) under Drought Stress and Recovery
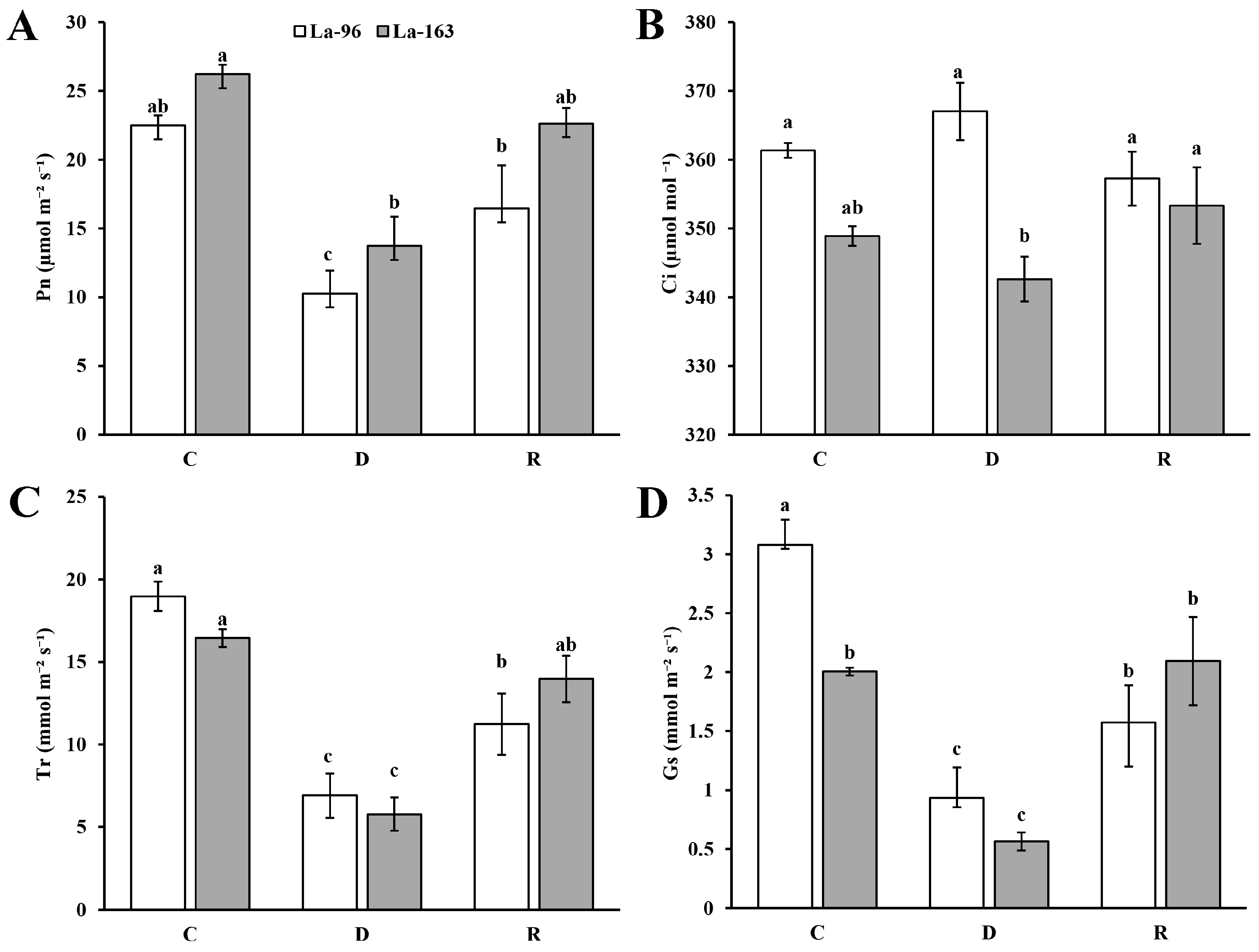
3.3. Photosynthetic Traits of Flag Leaves under Drought Stress and Recovery from Drought Treatment
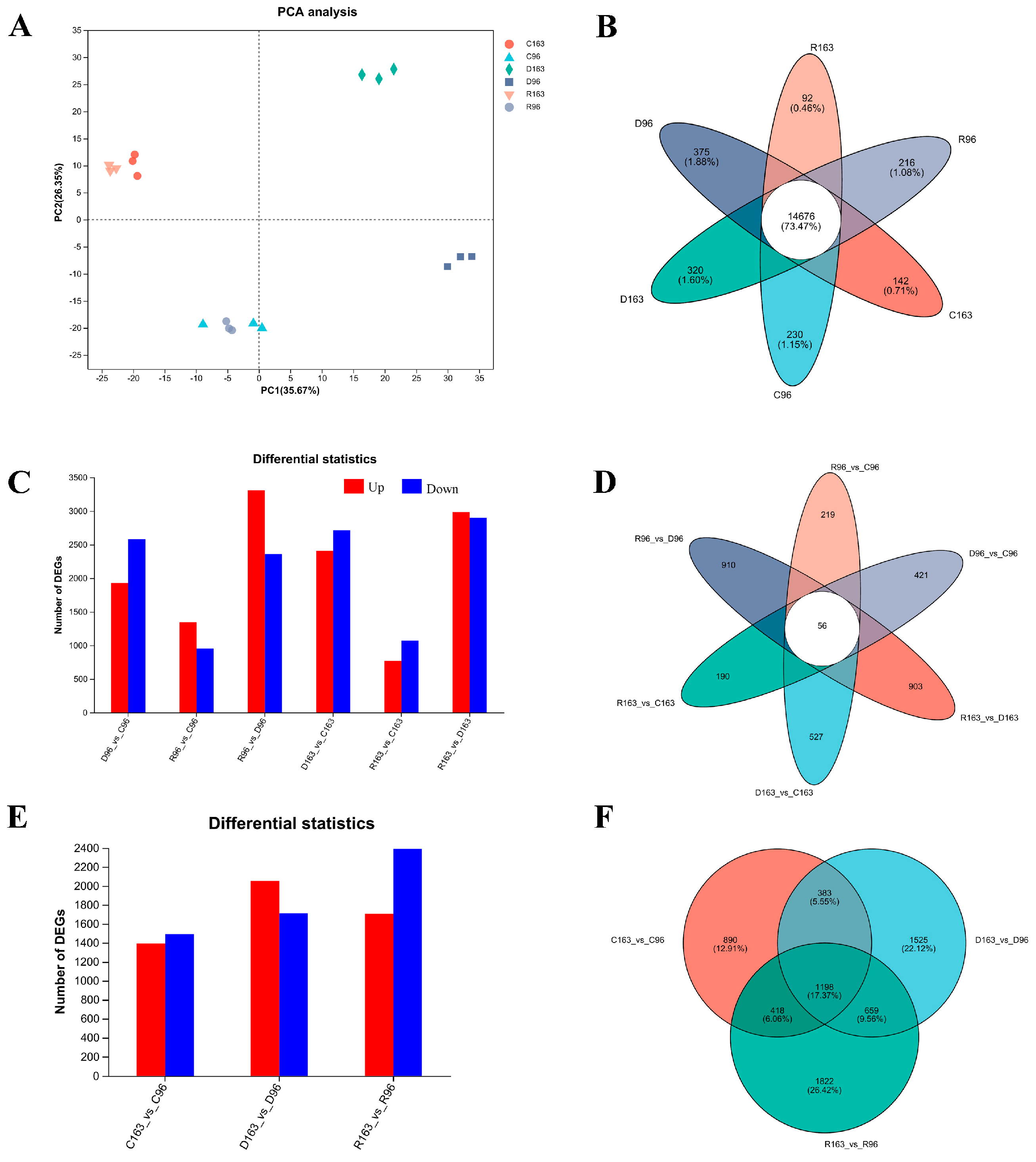
3.4. RNA Sequencing Analysis
3.4.1. Transcriptome Responses to Drought and Rewatering Recovery Response in the Leaves
3.4.2. GO Annotation under Drought Stress and Subsequent Recovery
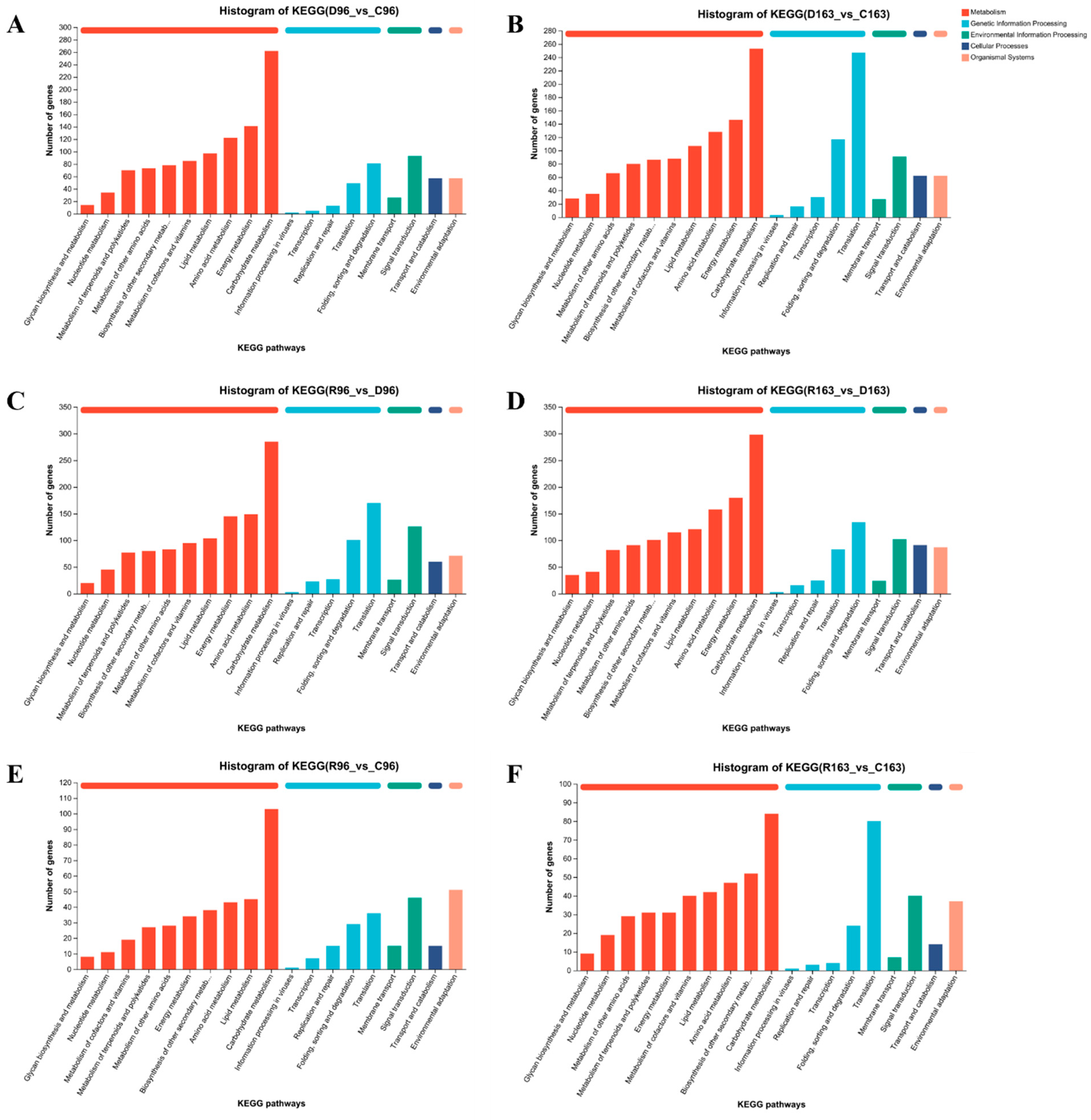
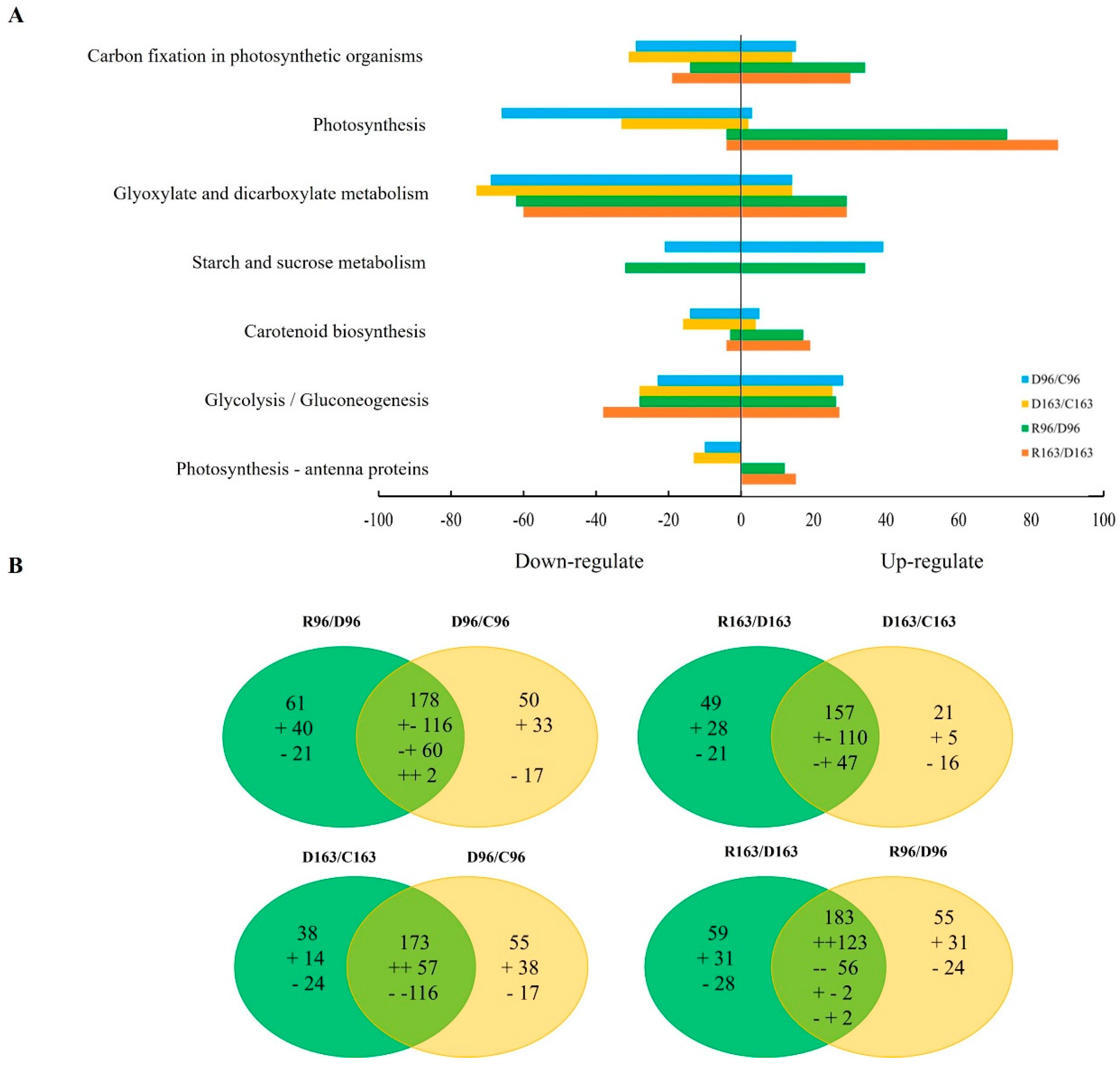
3.4.3. KEGG Pathway Annotation under Drought Stress and Subsequent Recovery
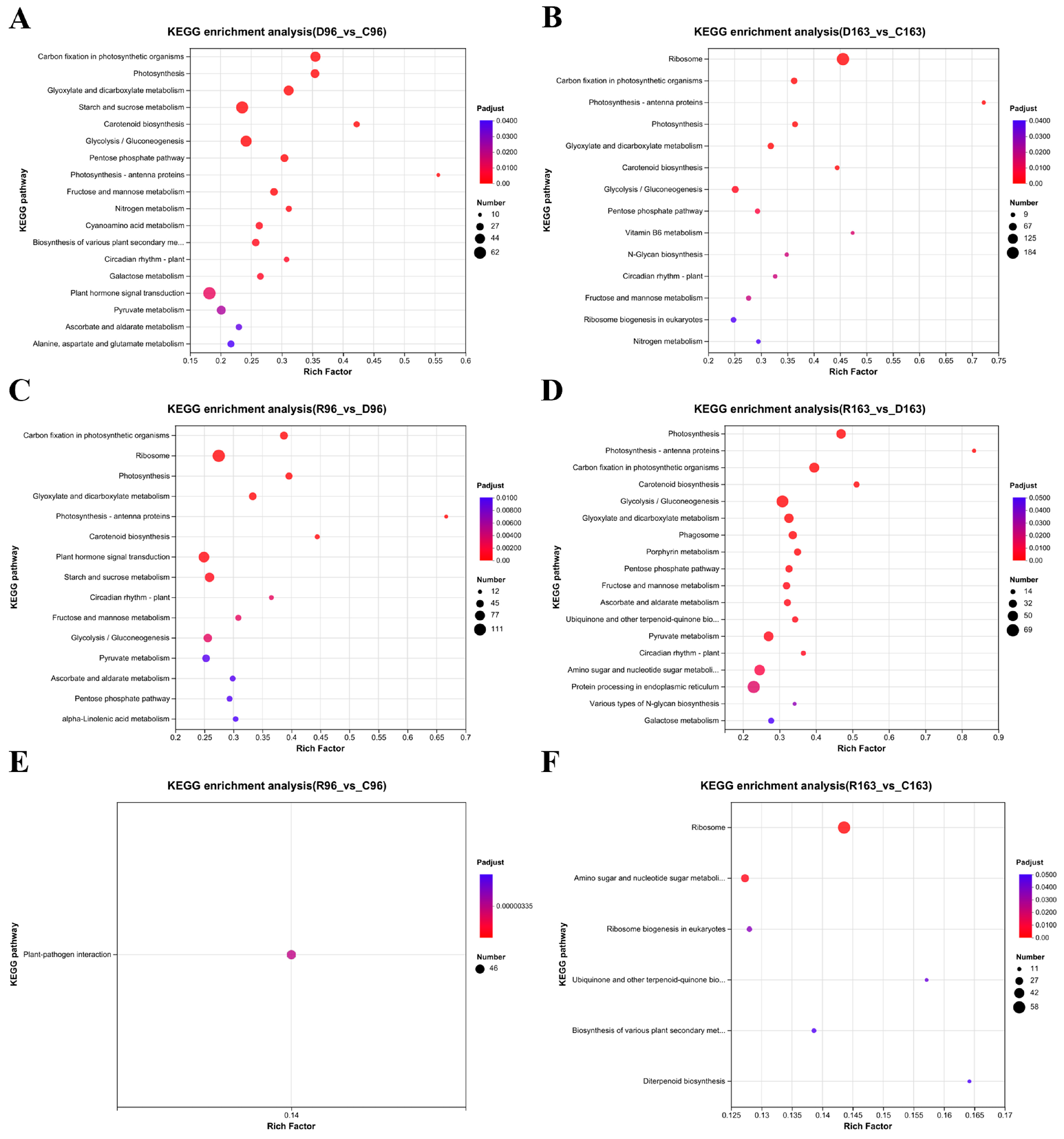
3.4.4. KEGG Pathway Enrichment under Drought Stress and Subsequent Recovery
3.4.5. Enriched DEGs for KEGG Pathway under Drought Stress and Successive Recovery
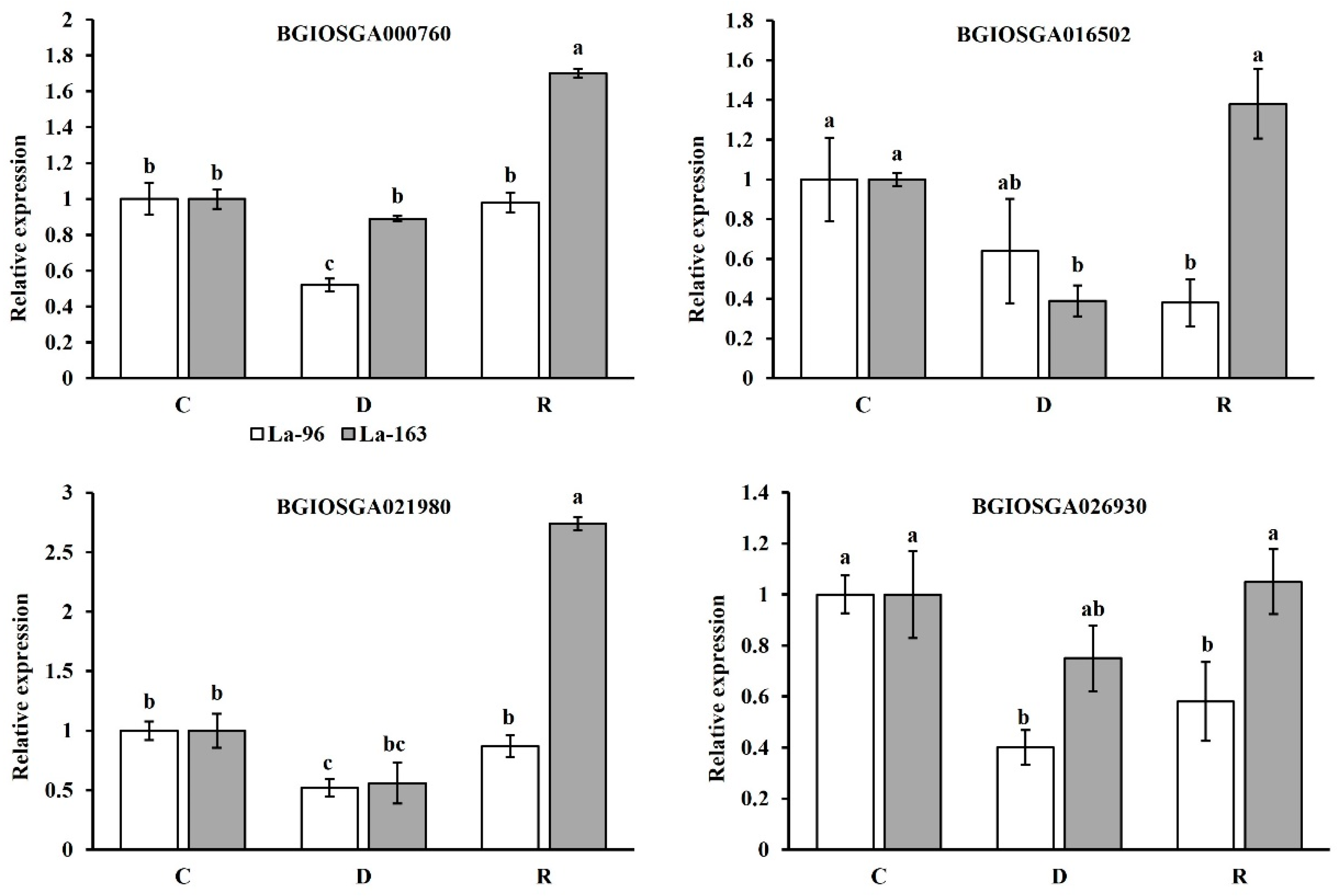
3.4.6. DEGs Induced by Drought Stress and Subsequent Recovery in La-96
3.4.7. DEGs Induced by Drought Stress and Subsequent Recovery in La-163
3.4.8. Common DEGs in Two Rice Lines under Drought and Recovery
3.4.9. Specific DEGs in Two Rice Lines under Drought and Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, M.A.; Dahu, N.; Hongning, T.; Qian, Z.; Yueming, Y.; Yiru, L.; Shimei, W. Drought Stress in Rice: Morpho-Physiological and Molecular Responses and Marker-Assisted Breeding. Front. Plant Sci. 2023, 14, 1215371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, Y.; Hassan, M.A.; Fang, W.; Wang, S. Comparative Physiological and Transcriptomic Characterization of Rice (Oryza Sativa L.) Root Seedlings under Phosphorus Deficiency. Plant Stress. 2024, 12, 100508. [Google Scholar] [CrossRef]
- At, L.; Core, T.H.E.; Disruption, O.F.; Action, P. Responding to the Impact of the COVID-19 Outbreak on Food Value Chains through Efficient Logistics; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Donde, R.; Kumar, J.; Gouda, G.; Gupta, M.K.; Mukherjee, M.; Baksh, S.Y.; Mahadani, P.; Sahoo, K.K.; Behera, L.; Dash, S.K. Assessment of Genetic Diversity of Drought Tolerant and Susceptible Rice Genotypes Using Microsatellite Markers. Rice Sci. 2019, 26, 239–247. [Google Scholar] [CrossRef]
- Simkhada, K.; Thapa, R. Rice Blast, A Major Threat to the Rice Production and Its Various Management Techniques. Turk. J. Agric.-Food Sci. Technol. 2022, 10, 147–157. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought Resistance in Rice from Conventional to Molecular Breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Hassan, M.A.; Cheng, X.; Li, Y.; Liu, H.; Fang, W.; Zhu, Q.; Wang, S. QTL Mapping and Analysis for Drought Tolerance in Rice by Genome-Wide Association Study. Front. Plant Sci. 2023, 14, 1223782. [Google Scholar] [CrossRef]
- Sandhu, N.; Kumar, A. Bridging the Rice Yield Gaps under Drought: QTLs, Genes, and Their Use in Breeding Programs. Agronomy 2017, 7, 27. [Google Scholar] [CrossRef]
- Bhandari, U.; Gajurel, A.; Khadka, B.; Thapa, I.; Chand, I.; Bhatta, D.; Poudel, A.; Pandey, M.; Shrestha, S.; Shrestha, J. Morpho-Physiological and Biochemical Response of Rice (Oryza Sativa L.) to Drought Stress: A Review. Heliyon 2023, 9, e13744. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The Different Influences of Drought Stress at the Flowering Stage on Rice Physiological Traits, Grain Yield, and Quality. Sci. Rep. 2019, 9, 3742. [Google Scholar] [CrossRef]
- Lum, M.S.; Hanafi, M.; Rafii, Y.M.; Akmar, A. Effect of Drought Stress on Growth, Proline and Antioxidant Enzyme Activities of Upland Rice. J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Sabin, K.; Bijay, S.; Amrit, B.; Raman, G.D.; Bhuwan, S.; Priyanka, N.; Man, S.S.; Prasad, G. Screening of Different Rice Genotypes against (Pyricularia Grisea) Sacc. InNatural Epidemic Condition at Seedling Stage in Chitwan, Nepal. Adv. Crop Sci. Technol. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Rahman, M.T.; Islam, M.T.; Islam, M.O. Effect of Water Stress at Different Growth Stages on Yield and Yield Contributing Characters of Transplanted Aman Rice. Pak. J. Biol. Sci. 2002, 5, 169–172. [Google Scholar] [CrossRef]
- Boonjung, H.; Fukai, S. Effects of Soil Water Deficit at Different Growth Stages on Rice Growth and Yield under Upland Conditions. 2. Phenology, Biomass Production and Yield. Field Crops Res. 1996, 48, 47–55. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Li, H.B.; Li, G.Y.; Jin, Q.Y.; Tao, L.X.; Fu, G.F. Heat Stress-Reduced Kernel Weight in Rice at Anthesis Is Associated with Impaired Source-Sink Relationship and Sugars Allocation. Environ. Exp. Bot. 2018, 155, 718–733. [Google Scholar] [CrossRef]
- Xi, M.; Xu, Y.; Li, Z.; Hu, R.; Cheng, T.; Zhou, Y.; Tu, D.; Ji, Y.; Xu, X.; Sun, X.; et al. Progress and Challenges of Rice Ratooning Technology in Anhui Province, China. Crop Environ. 2023, 2, 81–86. [Google Scholar] [CrossRef]
- Xing, L.; Zhao, R.; Sun, H.; Li, M.; Tan, Z. Analysis of Drought Characteristics and Comparison of Historical Typical Years with 2022 Drought in the Yangtze River Basin. Nat. Hazards 2024, 120, 3699–3718. [Google Scholar] [CrossRef]
- Pantuwan, G.; Fukai, S.; Cooper, M.; Rajatasereekul, S.; O’Toole, J.C. Yield Response of Rice (Oryza Sativa L.) Genotypes to Drought under Rainfed Lowland: 3. Plant Factors Contributing to Drought Resistance. Field Crops Res. 2002, 73, 181–200. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of Various Drought Stresses and Subsequent Recovery on Proline, Total Soluble Sugar and Starch Metabolisms in Rice (Oryza Sativa L.) Varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Ravikumar, G.; Manimaran, P.; Voleti, S.R.; Subrahmanyam, D.; Sundaram, R.M.; Bansal, K.C.; Viraktamath, B.C.; Balachandran, S.M. Stress-Inducible Expression of AtDREB1A Transcription Factor Greatly Improves Drought Stress Tolerance in Transgenic Indica Rice. Transgenic Res. 2014, 23, 421–439. [Google Scholar] [CrossRef]
- Jin, J.; Duan, J.; Shan, C.; Mei, Z.; Chen, H.; Feng, H.; Zhu, J.; Cai, W. Ethylene Insensitive3-Like2 (OsEIL2) Confers Stress Sensitivity by Regulating OsBURP16, the β Subunit of Polygalacturonase (PG1β-like) Subfamily Gene in Rice. Plant Sci. 2020, 292, 110353. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh-Moridani, M.; Esfahani, M.; Aalami, A.; Moumeni, A.; Khaledian, M. Profiling the Physiological Response of Upland and Lowland Rice (Oryza Sativa L.) Genotypes to Water Deficit. J. Crop Sci. Biotechnol. 2022, 25, 289–300. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.; Chen, X.; Li, M.; Wang, J.; Xu, Z.; Feng, Z.; Chen, Z.; Zuo, S. Analysis of Blast Resistance Genes and Neck Blast Resistance of Japonica Rice Varieties/Lines Recently Developed in Jiangsu Province. Chin. J. Rice Sci. 2020, 34, 413–424. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Moonmoon, S.; Islam, M. Effect of Drought Stress at Different Growth Stages on Yield and Yield Components of Six Rice (Oryza Sativa L.) Genotypes. Fundam. Appl. Agric. 2017, 2, 285–289. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain-Filling Problem in ‘Super’ Rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef]
- Poethig, R.S. Vegetative Phase Change and Shoot Maturation in Plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar] [CrossRef]
- Mirshad, P.P.; Puthur, J.T. Drought Tolerance of Bioenergy Grass Saccharum Spontaneum L. Enhanced by Arbuscular Mycorrhizae. Rhizosphere 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.; Niu, X.; Wang, L.; Li, L.; Yuan, Q.; Pei, X. Research on LncRNA Related to Drought Resistance of Shanlan Upland Rice. BMC Genom. 2022, 23, 336. [Google Scholar] [CrossRef]
- Moin, M.; Bakshi, A.; Madhav, M.S.; Kirti, P.B. Expression Profiling of Ribosomal Protein Gene Family in Dehydration Stress Responses and Characterization of Transgenic Rice Plants Overexpressing RPL23A for Water-Use Efficiency and Tolerance to Drought and Salt Stresses. Front. Chem. 2017, 5, 97. [Google Scholar] [CrossRef]
- Ban, N.; Nissen, P.; Hansen, J.; Moore, P.B.; Steitz, T.A. The Complete Atomic Structure of the Large Ribosomal Subunit at 2.4 A Resolution. Science 2000, 289, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.L.; Videler, H.; Santos, C.; Ballesta, J.P.G.; Robinson, C.V. Mass Spectrometry of Ribosomes from Saccharomyces Cerevisiae: Implications for Assembly of the Stalk Complex. J. Biol. Chem. 2004, 279, 42750–42757. [Google Scholar] [CrossRef] [PubMed]
- Fromont-Racine, M.; Senger, B.; Saveanu, C.; Fasiolo, F. Ribosome Assembly in Eukaryotes. Gene 2003, 313, 17–42. [Google Scholar] [CrossRef]
- Barakat, A.; Szick-Miranda, K.; Chang, I.F.; Guyot, R.; Blanc, G.; Cooke, R.; Delseny, M.; Bailey-Serres, J. The Organization of Cytoplasmic Ribosomal Protein Genes in the Arabidopsis Genome. Plant Physiol. 2001, 127, 398–415. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription Factors and Regulation of Photosynthetic and Related Metabolism under Environmental Stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, D.; Guo, J.; Wu, H.; Jin, M.; Lu, Q.; Lu, C.; Zhang, L. A Psb27 Homologue in Arabidopsis Thaliana Is Required for Efficient Repair of Photodamaged Photosystem II. Plant Mol. Biol. 2006, 61, 567–575. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Wu, J.; Mowers, R.; Zhou, H.P.; Meghji, M.; Primavesi, L.F.; Paul, M.J.; Chen, X.; Gao, Y.; Haque, E.; et al. Expression of Trehalose-6-Phosphate Phosphatase in Maize Ears Improves Yield in Well-Watered and Drought Conditions. Nat. Biotechnol. 2015, 33, 862–869. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Do Choi, Y.; Kochian, L.V.; Wu, R.J. Trehalose Accumulation in Rice Plants Confers High Tolerance Levels to Different Abiotic Stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef]
- Koster, K.L.; Leopold, A.C. Sugars and Desiccation Tolerance in Seeds. Plant Physiol. 1988, 88, 829–832. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought Stress Condition Increases Root to Shoot Ratio via Alteration of Carbohydrate Partitioning and Enzymatic Activity in Rice Seedlings. Acta Physiol. Plant 2015, 37, 9. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Hassan, M.A.; Li, Y.; Fang, W.; Wu, J.; Wang, S. Physiological and Transcriptomic Characterization of Rice Genotypes under Drought Stress. Agronomy 2024, 14, 2247. https://doi.org/10.3390/agronomy14102247
Zhu Q, Hassan MA, Li Y, Fang W, Wu J, Wang S. Physiological and Transcriptomic Characterization of Rice Genotypes under Drought Stress. Agronomy. 2024; 14(10):2247. https://doi.org/10.3390/agronomy14102247
Chicago/Turabian StyleZhu, Qian, Muhammad Ahmad Hassan, Yiru Li, Wuyun Fang, Jingde Wu, and Shimei Wang. 2024. "Physiological and Transcriptomic Characterization of Rice Genotypes under Drought Stress" Agronomy 14, no. 10: 2247. https://doi.org/10.3390/agronomy14102247
APA StyleZhu, Q., Hassan, M. A., Li, Y., Fang, W., Wu, J., & Wang, S. (2024). Physiological and Transcriptomic Characterization of Rice Genotypes under Drought Stress. Agronomy, 14(10), 2247. https://doi.org/10.3390/agronomy14102247





