Strategic Light Use Efficiency Optimization of Hydroponic Lettuce Exposed to Different Photosynthetic Photon Flux Densities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Configuration and Environmental Conditions
2.2. Seedling Production in a Walk-In Vertical Farm
2.3. Transplant to Growth Chambers
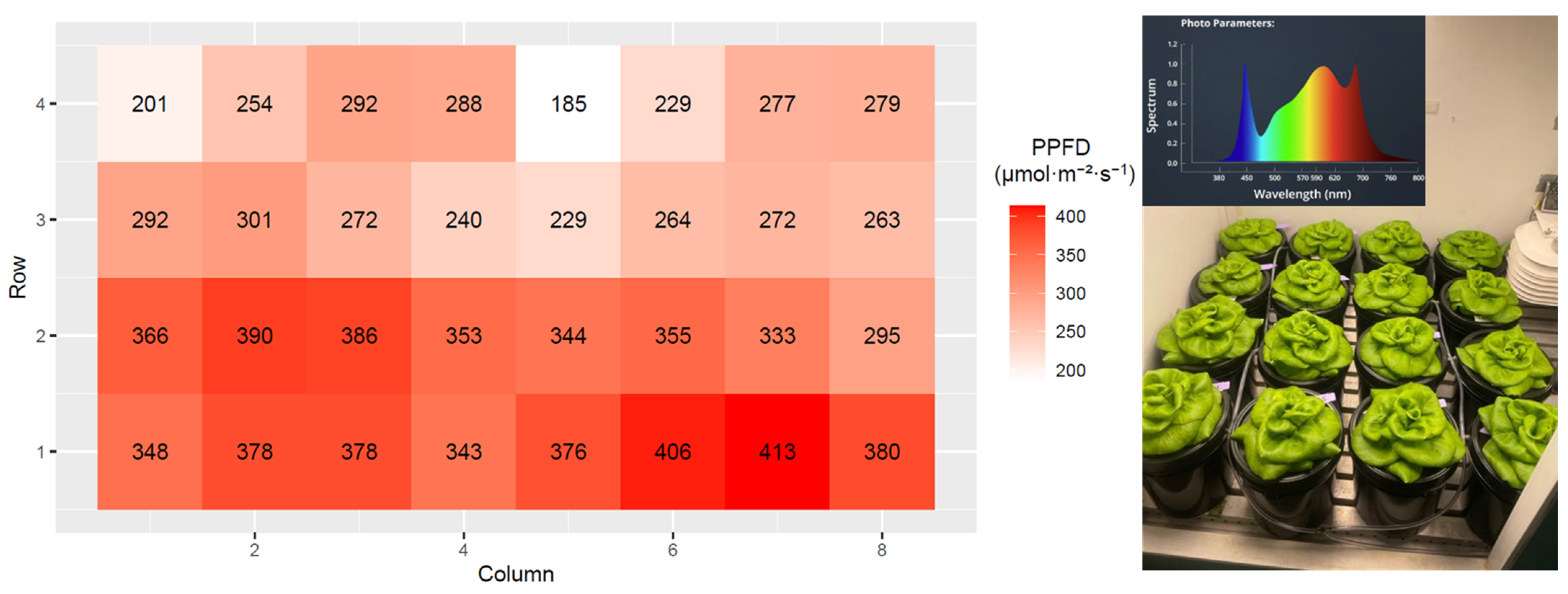
2.4. Treatments
2.5. Projected Canopy Size Imaging
2.6. Data Collection
2.7. Experimental Design and Statistical Analysis
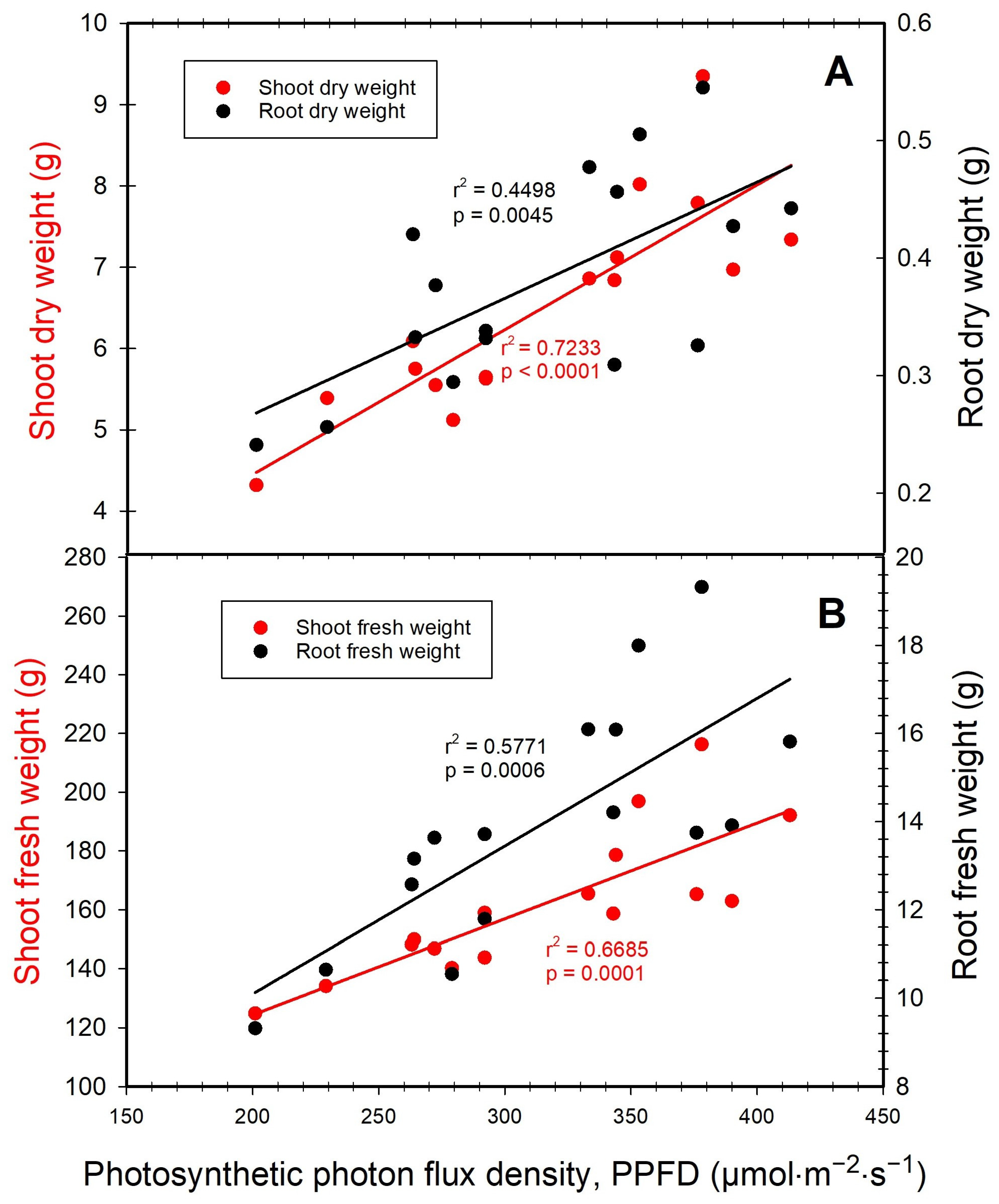
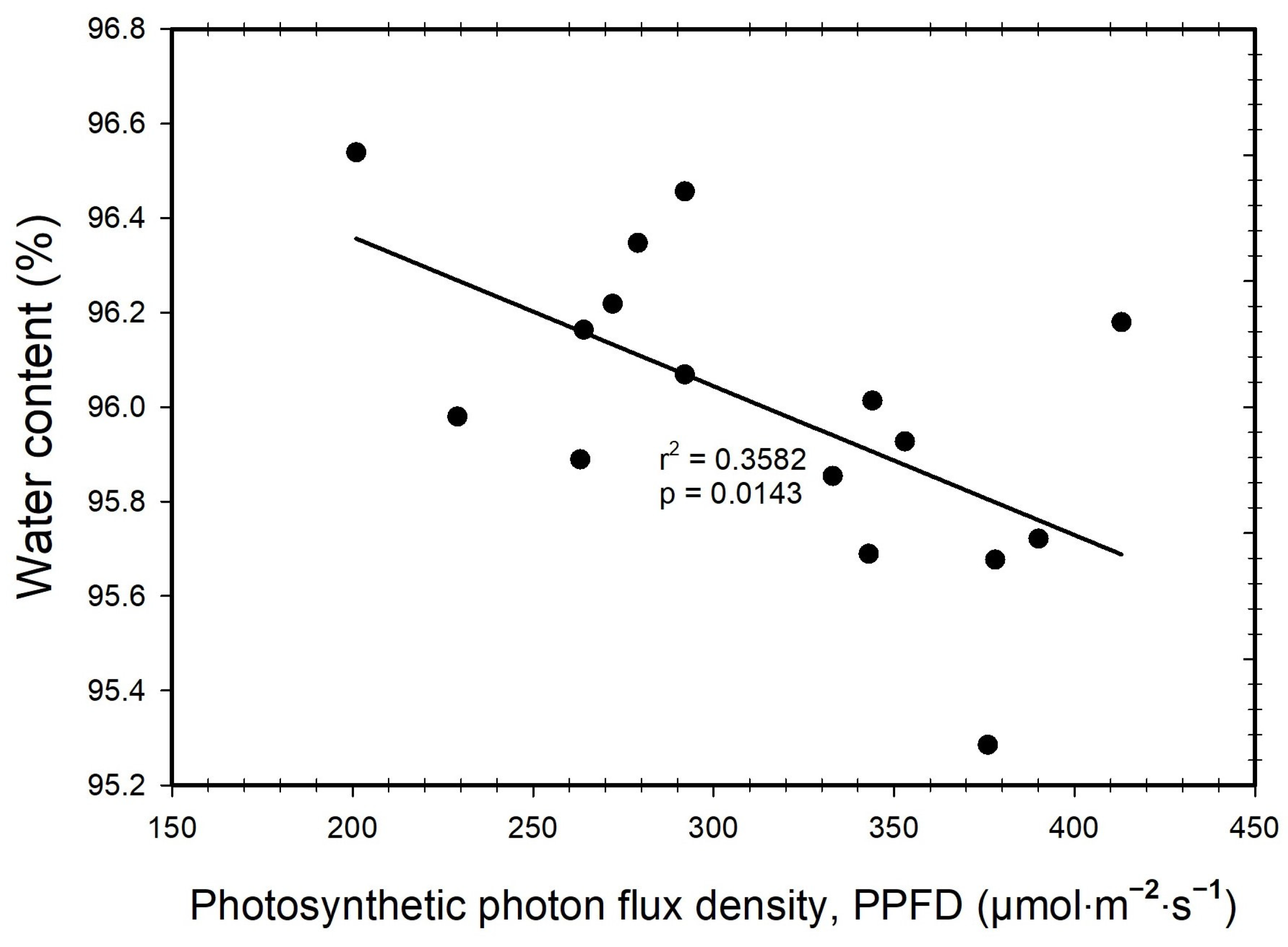
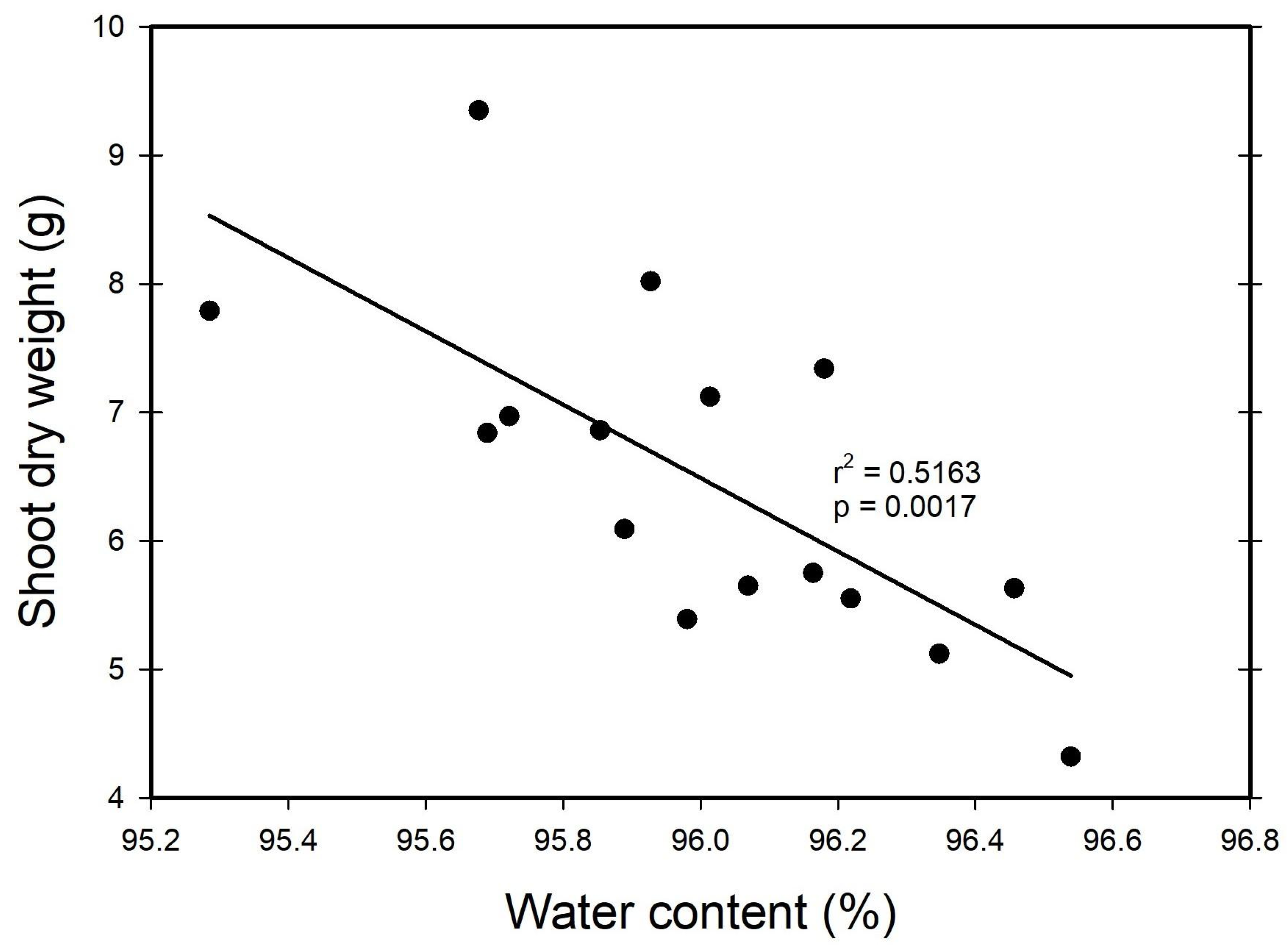
3. Results
3.1. Dry and Fresh Weights
3.2. Water Content
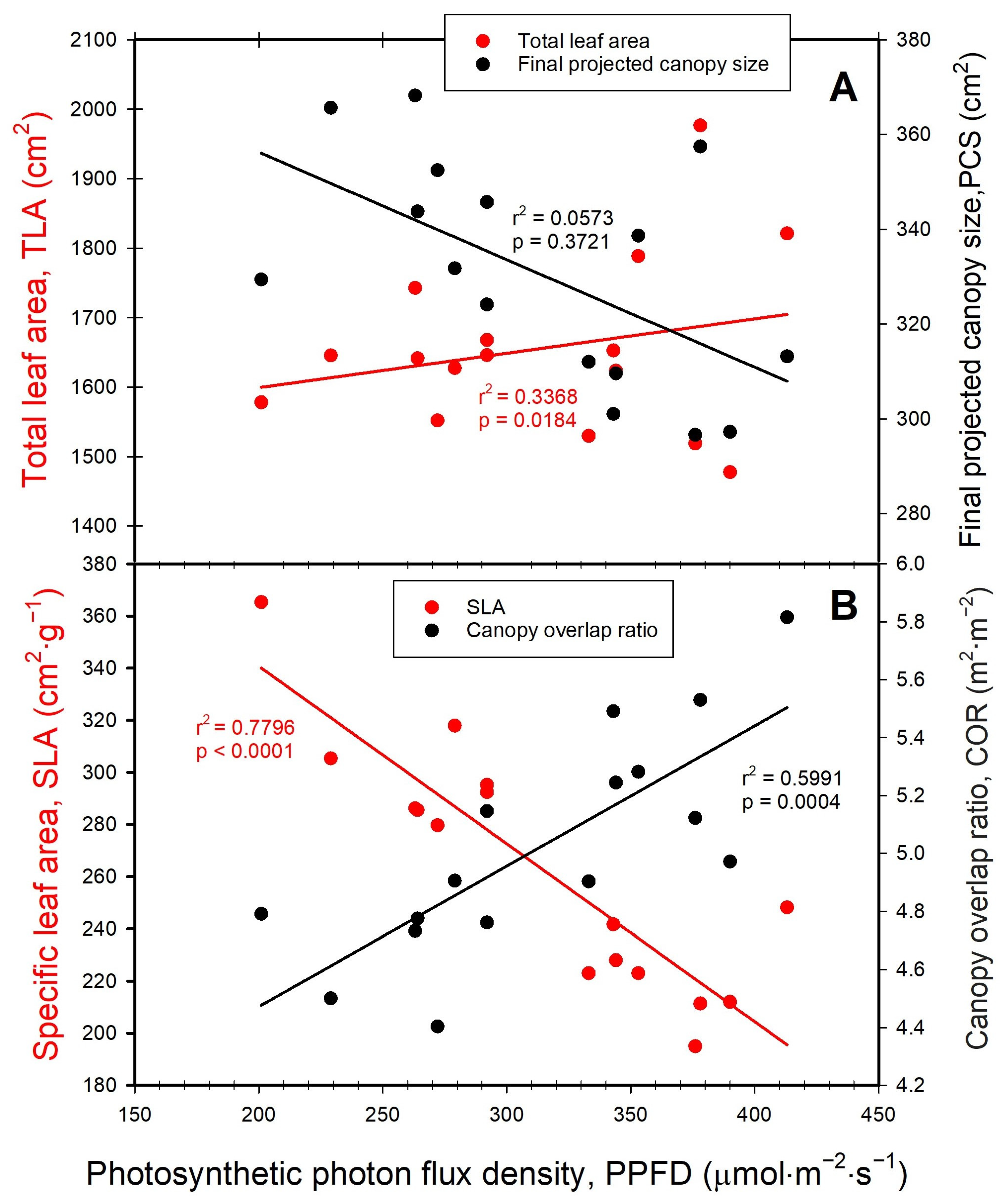
3.3. Leaf Area, Projected Canopy Size, Canopy Overlap Ratio
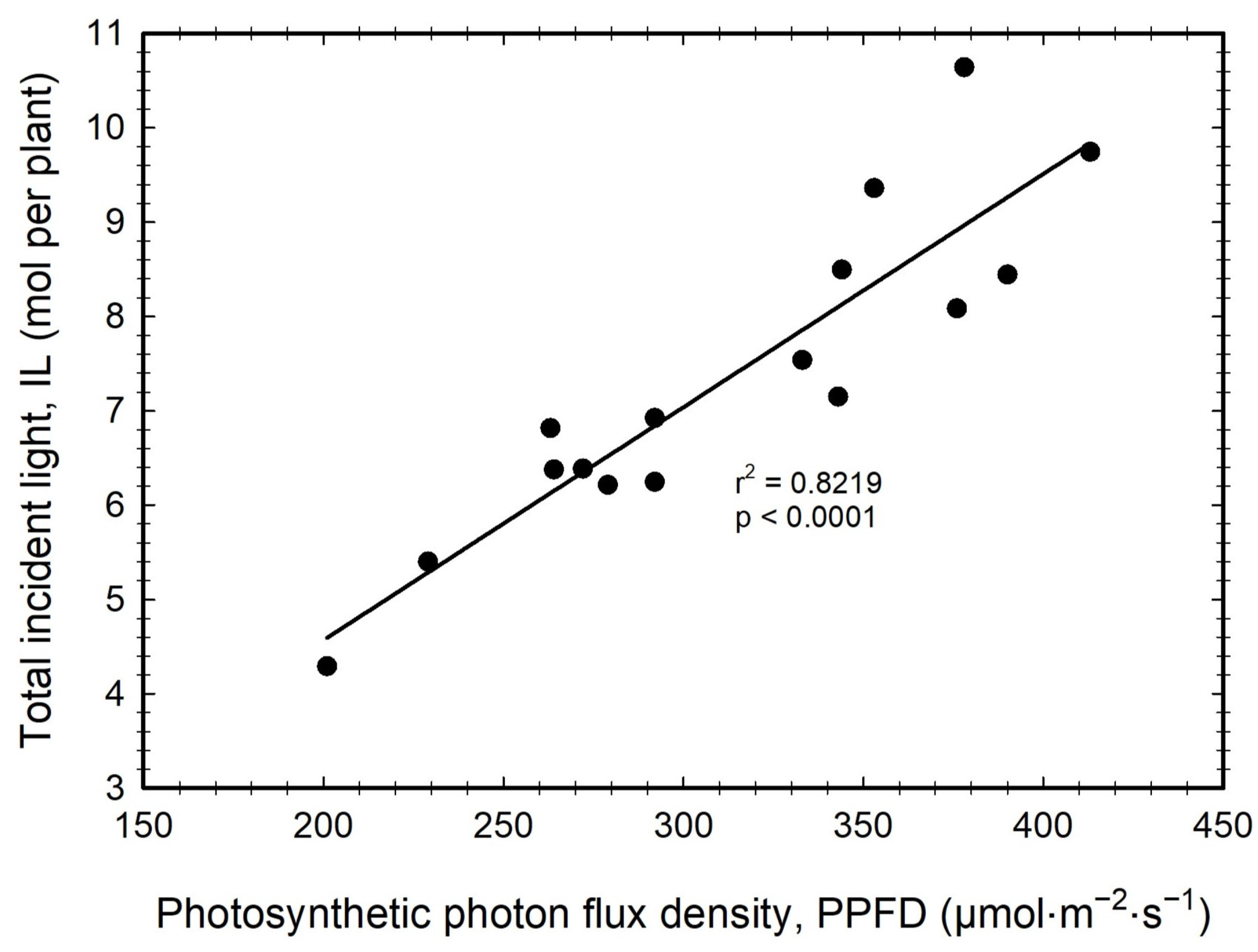
3.4. Total Incident Light
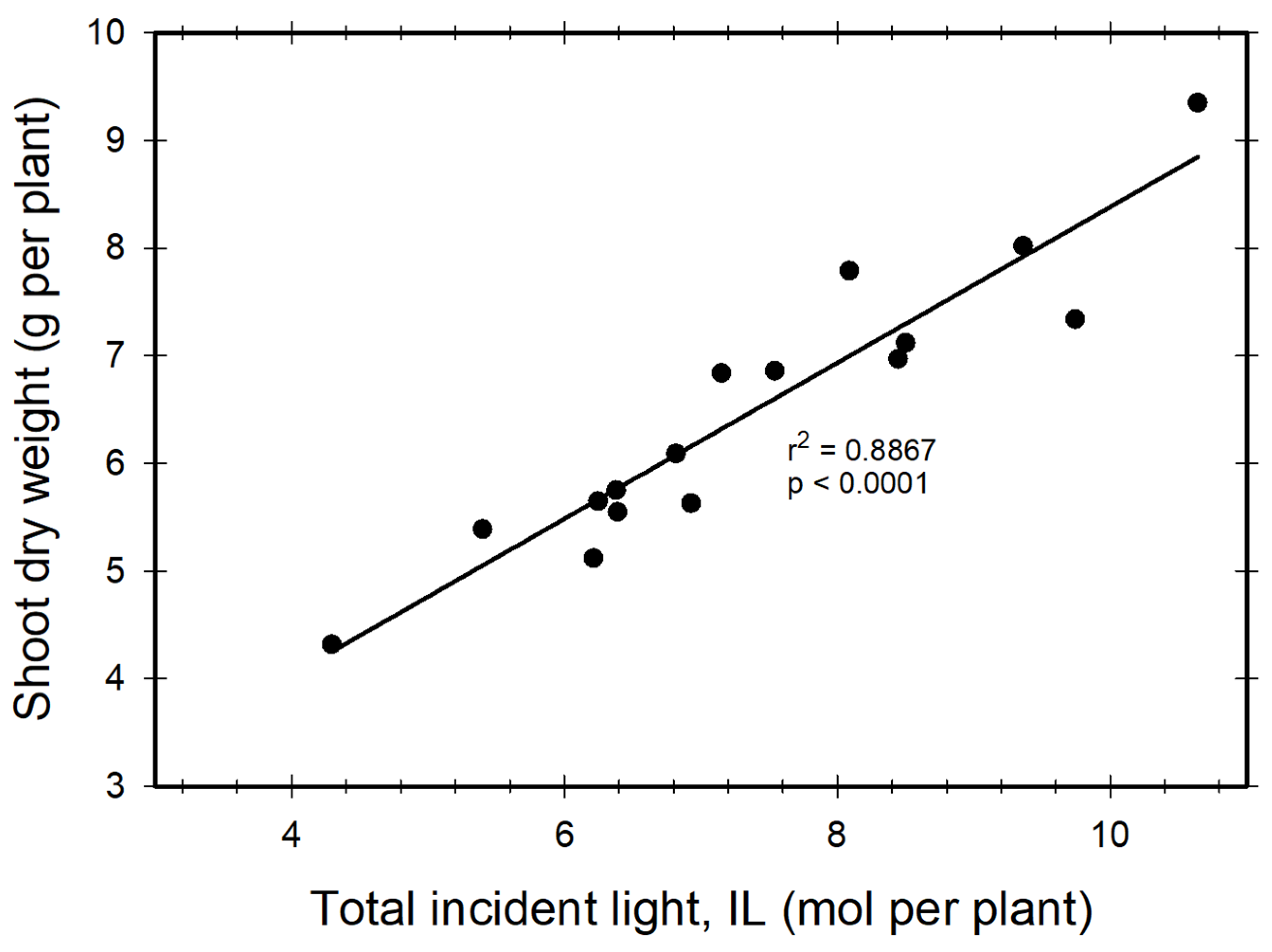
3.5. Light Use Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortSci. Horts 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Rai, N.; Morales, L.O.; Aphalo, P.J. Perception of solar UV radiation by plants: Photoreceptors and mechanisms. Plant Physiol. 2021, 186, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M.; Meinen, E.; Nijs, E.M.F.M.; Raaphorst, M.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–104. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Cabrera-Bosquet, L.; Fournier, C.; Brichet, N.; Welcker, C.; Suard, B.; Tardieu, F. High-throughput estimation of incident light, light interception and radiation-use efficiency of thousands of plants in a phenotyping platform. New Phytol. 2016, 212, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Gavhane, K.P.; Hasan, M.; Singh, D.K.; Kumar, S.N.; Sahoo, R.N.; Alam, W. Determination of optimal daily light integral (DLI) for indoor cultivation of iceberg lettuce in an indigenous vertical hydroponic system. Sci. Rep. 2023, 13, 10923. [Google Scholar] [CrossRef]
- Jishi, T. LED Lighting Technique to Control Plant Growth and Morphology. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 211–222. [Google Scholar]
- Kim, C.; van Iersel, M.W. Morphological and Physiological Screening to Predict Lettuce Biomass Production in Controlled Environment Agriculture. Remote Sens. 2022, 14, 316. [Google Scholar] [CrossRef]
- Pei, Y.; Dong, J.; Zhang, Y.; Yuan, W.; Doughty, R.; Yang, J.; Zhou, D.; Zhang, L.; Xiao, X. Evolution of light use efficiency models: Improvement, uncertainties, and implications. Agric. For. Meteorol. 2022, 317, 108905. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. How the Distribution of Photon Delivery Impacts Crops in Indoor Plant Environments: A Review. Sustainability 2023, 15, 4645. [Google Scholar] [CrossRef]
- Fiorucci, A.-S.; Fankhauser, C. Plant Strategies for Enhancing Access to Sunlight. Curr. Biol. 2017, 27, R931–R940. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- van Iersel, M.W.; Weaver, G.; Martin, M.T.; Ferrarezi, R.S.; Mattos, E.; Haidekker, M. A chlorophyll fluorescence-based biofeedback system to control photosynthetic lighting in controlled environment agriculture. J. Am. Soc. Hortic. Sci. 2016, 141, 169–176. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Lighting from Top and Side Enhances Photosynthesis and Plant Performance by Improving Light Usage Efficiency. Int. J. Mol. Sci. 2022, 23, 2448. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Yudina, L.; Zolin, Y.; Popova, A.; Sukhov, V. Development, Verification, and Analysis of Simple Mathematical Model of Lettuce Productivity under Different Light Conditions. Horticulturae 2023, 9, 1259. [Google Scholar] [CrossRef]
- Jin, W.; Formiga Lopez, D.; Heuvelink, E.; Marcelis, L.F.M. Light use efficiency of lettuce cultivation in vertical farms compared with greenhouse and field. Food Energy Secur. 2023, 12, e391. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research Progress in Improving Photosynthetic Efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Romero-Troncoso, R.d.J.; Guevara-Gonzalez, R.G.; Millan-Almaraz, J.R. Instrumentation in Developing Chlorophyll Fluorescence Biosensing: A Review. Sensors 2012, 12, 11853–11869. [Google Scholar] [CrossRef]
- Wacker, K.; Kim, C.; van Iersel, M.W.; Sidore, B.; Pham, T.; Haidekker, M.; Seymour, L.; Ferrarezi, R.S. Development of an Automated Low-Cost Multispectral Imaging System to Quantify Canopy Size and Pigmentation. Sensors 2024, 24, 5515. [Google Scholar] [CrossRef]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of Growth, Water Use Efficiency, Chlorophyll Fluorescence, and Stomatal Characteristics of Lettuce Plants to Light Intensity. J. Plant Growth Regul. 2021, 40, 2191–2207. [Google Scholar] [CrossRef]
- He, J.; Jawahir, N.K.B.; Qin, L. Quantity of supplementary LED lightings regulates photosynthetic apparatus, improves photosynthetic capacity and enhances productivity of Cos lettuce grown in a tropical greenhouse. Photosynth Res. 2021, 149, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- He, C.; Davies, F.T., Jr.; Lacey, R.E. Separating the effects of hypobaria and hypoxia on lettuce: Growth and gas exchange. Physiol. Plant 2007, 131, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Paradiso, R.; Moscatello, S.; Saccardo, F.; Battistelli, A. Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment. Plants 2023, 12, 804. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2020, 185, 34–48. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-l. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Keller, B.; Zimmermann, L.; Rascher, U.; Matsubara, S.; Steier, A.; Muller, O. Toward predicting photosynthetic efficiency and biomass gain in crop genotypes over a field season. Plant Physiol. 2022, 188, 301–317. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y.; Tang, J. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and Physiological Stress Responses of Lettuce to Different Intensities of Continuous Light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]

| Nutrients (mg/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO3−-N | NH4+-N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Mo | Zn |
| 77 | 3 | 10 | 100 | 62 | 42 | 57 | 0.29 | 0.16 | 1.63 | 0.62 | 0.03 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palsha, P.L.; van Iersel, M.W.; Dickson, R.W.; Seymour, L.; Yelton, M.; Qin, K.; Ferrarezi, R.S. Strategic Light Use Efficiency Optimization of Hydroponic Lettuce Exposed to Different Photosynthetic Photon Flux Densities. Agronomy 2024, 14, 2281. https://doi.org/10.3390/agronomy14102281
Palsha PL, van Iersel MW, Dickson RW, Seymour L, Yelton M, Qin K, Ferrarezi RS. Strategic Light Use Efficiency Optimization of Hydroponic Lettuce Exposed to Different Photosynthetic Photon Flux Densities. Agronomy. 2024; 14(10):2281. https://doi.org/10.3390/agronomy14102281
Chicago/Turabian StylePalsha, Peyton Lou, Marc W. van Iersel, Ryan William Dickson, Lynne Seymour, Melanie Yelton, Kuan Qin, and Rhuanito Soranz Ferrarezi. 2024. "Strategic Light Use Efficiency Optimization of Hydroponic Lettuce Exposed to Different Photosynthetic Photon Flux Densities" Agronomy 14, no. 10: 2281. https://doi.org/10.3390/agronomy14102281
APA StylePalsha, P. L., van Iersel, M. W., Dickson, R. W., Seymour, L., Yelton, M., Qin, K., & Ferrarezi, R. S. (2024). Strategic Light Use Efficiency Optimization of Hydroponic Lettuce Exposed to Different Photosynthetic Photon Flux Densities. Agronomy, 14(10), 2281. https://doi.org/10.3390/agronomy14102281










