Control of the Field Herbicide Dissipation by Cover Crop Mulch in Conservation Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Soil Sampling, and Processing
2.2. Herbicide Extraction and Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Dissipation Kinetics of S-Metolachlor in Soils under Conventional Tillage and Non-Tillage
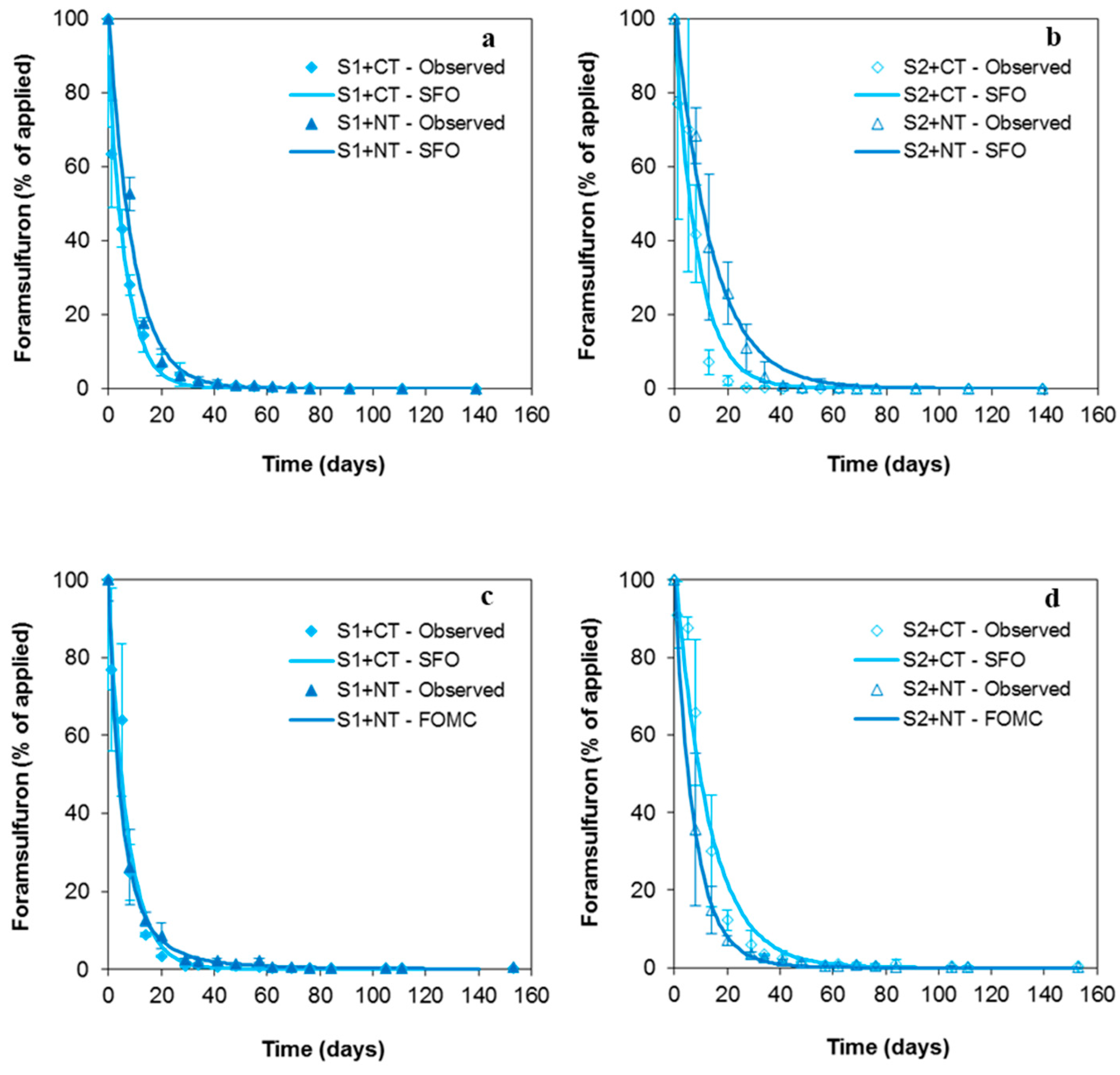
3.2. Dissipation Kinetics of Foramsulfuron in Soils under Conventional Tillage and Non-Tillage
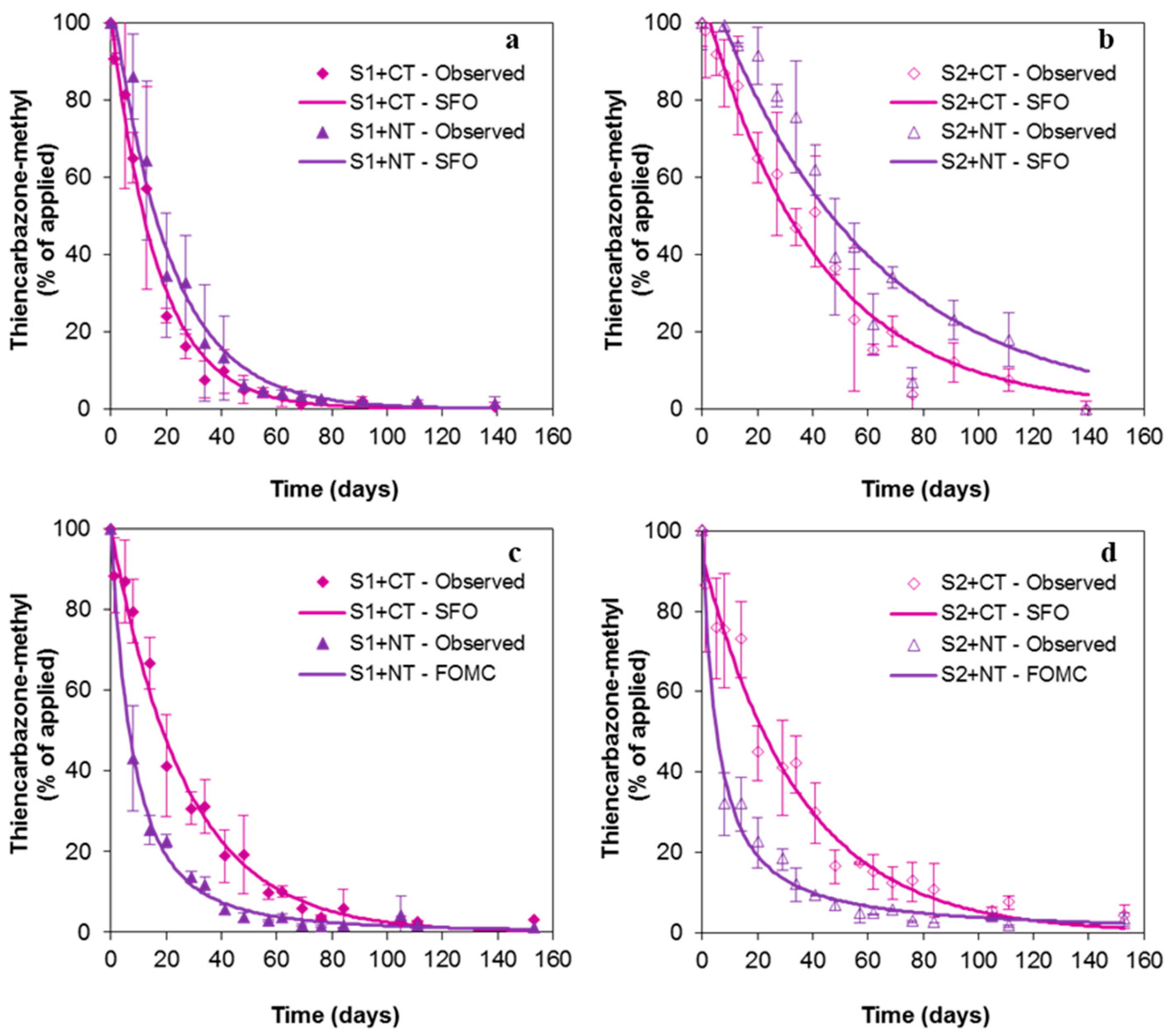
3.3. Dissipation Kinetics of Thiencarbazone-Methyl in Soils under Conventional Tillage and Non-Tillage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaraman, S.; Dang, Y.P.; Naorem, A.; Page, K.L.; Dalal, R.C. Conservation agriculture as a system to enhance ecosystem services. Agriculture 2021, 11, 718. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Perego, A.; Rocca, A.; Cattivelli, V.; Tabaglio, V.; Fiorini, A.; Barbieri, S.; Schillaci, C.; Chiodini, M.E.; Brenna, S.; Acutis, M. Agro-environmental aspects of conservation agriculture compared to conventional systems: A 3-year experience on 20 farms in the Po valley (Northern Italy). Agric. Syst. 2019, 168, 73–87. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R.; Kienzle, J. Overview of the worldwide spread of conservation agriculture. Field Actions Sci. Rep. 2015, 8, 8. Available online: https://journals.openedition.org/factsreports/3966 (accessed on 15 November 2023).
- Thierfelder, C.; Matemba-Mutasa, R.; Bunderson, W.T.; Mutenje, M.; Nyagumbo, I.; Mupangwa, W. Evaluating manual conservation agriculture systems in southern Africa. Agr. Ecosyst. Environ. 2016, 222, 112–124. [Google Scholar] [CrossRef]
- Choudhary, M.; Ghasal, P.C.; Kumar, S.; Yadav, R.P.; Singh, S.; Meena, V.S.; Bisht, J.K. Conservation agriculture and climate change: An overview. In Conservation Agriculture: An Approach to Combat Climate Change in Indian Himalaya, 1st ed.; Bisht, J.K., Meena, V.S., Mishra, P.K., Pattanayak, A., Eds.; Springer: Singapore, 2016; pp. 1–37. [Google Scholar] [CrossRef]
- Ravichandran, M.; Samiappan, S.C.; Pandiyan, R.; Velu, R.K. Improvement of crop and soil management practices through mulching for enhancement of soil fertility and environmental sustainability: A review. J. Exp. Biol. Agric. Sci. 2022, 10, 4. [Google Scholar] [CrossRef]
- Alletto, L.; Coquet, Y.; Benoit, P.; Heddadj, D.; Barriuso, E. Tillage management effects on pesticide fate in soils. A review. Agron. Sustain. Dev. 2010, 30, 367–400. [Google Scholar] [CrossRef]
- Aslam, S.; Iqbal, A.; Lafolie, F.; Recous, S.; Benoit, P.; Garnier, P. Mulch of plant residues at the soil surface impact the leaching and persistence of pesticides: A modelling study from soil columns. J. Contam. Hydrol. 2018, 214, 54–64. [Google Scholar] [CrossRef]
- Aslam, S.; Iqbal, A.; Deschamps, M.; Recous, S.; Garnier, P.; Benoit, P. Effect of rainfall regimes and mulch decomposition on the dissipation and leaching of S-metolachlor and glyphosate: A soil column experiment. Pest Manag. Sci. 2015, 71, 278–291. [Google Scholar] [CrossRef]
- Bedos, C.; Alletto, L.; Durand, B.; Fanucci, O.; Brut, A.; Bourdat-Deschamps, M.; Giuliano, S.; Loubet, B.; Ceschia, E.; Benoit, P. Observed volatilization fluxes of S-metolachlor and benoxacor applied on soil with and without crop residues. Environ. Sci. Pollut. Res. 2017, 24, 3985–3996. [Google Scholar] [CrossRef]
- Wołejko, E.; Kaczyński, P.; Łozowicka, B.; Wydro, U.; Borusiewicz, A.; Hrynko, I.; Konecki, R.; Snarska, K.; Dec, D.; Malinowski, P. Dissipation of S-metolachlor in plant and soil and effect on enzymatic activities. Environ. Monit. Assess. 2017, 189, 355. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Garnier, P.; Rumpel, C.; Parent, S.E.; Benoit, P. Adsorption and desorption behavior of selected pesticides as influenced by decomposition of maize mulch. Chemosphere 2013, 91, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Cassigneul, A.; Benoit, P.; Bergheaud, V.; Dumeny, V.; Etiévant, V.; Goubard, Y.; Maylin, A.; Justes, E.; Alletto, L. Fate of glyphosate and degradates in cover crop residues and underlying soil: A laboratory study. Sci. Total Environ. 2016, 545–546, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Cassigneul, A.; Benoit, P.; Nobile, C.; Bergheaud, V.; Dumeny, V.; Etiévant, V.; Maylin, A.; Justes, E.; Alletto, L. Behaviour of S-metolachlor and its oxanilic and ethanesulfonic acids metabolites under fresh vs. partially decomposed cover crop mulches: A laboratory study. Sci. Total Environ. 2018, 631–632, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.V.; Odero, D.C.; MacDonald, G.E.; Ferrell, J.A.; Sellers, B.A.; Wilson, P.C. Field dissipation of S-metolachlor in organic and mineral soils used for sugarcane production in Florida. Weed Technol. 2020, 34, 362–370. [Google Scholar] [CrossRef]
- Aslam, S.; Benoit, P.; Chabauty, F.; Bergheaud, V.; Geng, C.; Vieublé-Gonod, L.; Garnier, P. Modelling the impacts of maize decomposition on glyphosate dynamics in mulch. Eur. J. Soil Sci. 2014, 65, 231–247. [Google Scholar] [CrossRef]
- Rampoldi, E.A.; Hang, S.; Barriuso, E. The fate of glyphosate in crop residues. Soil Sci. Soc. Am. J. 2011, 75, 553–559. [Google Scholar] [CrossRef]
- Cueff, S.; Alletto, L.; Dumény, V.; Benoit, P.; Pot, V. Adsorption and degradation of the herbicide nicosulfuron in a stagnic Luvisol and Vermic Umbrisol cultivated under conventional or conservation agriculture. Environ. Sci. Pollut. Res. 2021, 28, 15934–15946. [Google Scholar] [CrossRef]
- Locke, M.A.; Zablotowicz, R.M.; Bauer, P.J.; Steinriede, R.W.; Gaston, L.A. Conservation cotton production in the southern United States: Herbicide dissipation in soil and cover crops. Weed Sci. 2005, 53, 717–727. [Google Scholar] [CrossRef]
- Yousefi, M.; Kamkar, B.; Gherekhloo, J.; Faez, R. Sulfosulfuron persistence in soil under different cultivation systems of wheat (Triticum aestivum). Pedosphere 2016, 26, 666–675. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Locke, M.A.; Gaston, L.A. Tillage and cover effects on soil microbial properties and fluometuron degradation. Biol. Fert. Soils 2007, 44, 27–35. [Google Scholar] [CrossRef]
- Bedmar, F.; Gimenez, D.; Costa, J.L.; Daniel, P.E. Persistence of acetochlor, atrazine, and S-metolachlor in surface and subsurface horizons of 2 typic argiudolls under no-tillage. Environ. Toxicol. Chem. 2017, 36, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Kouame, K.B.-J.; Savin, M.C.; Willett, C.D.; Bertucci, M.B.; Butts, T.R.; Grantz, E.; Roma-Burgos, N. S-metolachlor persistence in soil as influenced by within-season and inter-annual herbicide use. Environ. Adv. 2022, 9, 100318. [Google Scholar] [CrossRef]
- Si, Y.; Takagi, K.; Iwasaki, A.; Zhou, D. Adsorption, desorption and dissipation of metolachlor in surface and subsurface soils. Pest Manag. Sci. 2009, 65, 956–962. [Google Scholar] [CrossRef]
- Alletto, L.; Benoit, P.; Bolognési, B.; Couffignal, M.; Bergheaud, V.; Dumény, V.; Longueval, C.; Barriuso, E. Sorption and mineralisation of S-metolachlor in soils from fields cultivated with different conservation tillage systems. Soil Till. Res. 2013, 128, 97–103. [Google Scholar] [CrossRef]
- Gul, P.; Ahmad, K.S.; Gul, M.M. Herbicide thiencarbazone-methyl pedospheric disposition through sorption and degradation mechanisms in heterogenous soils. Environ. Earth Sci. 2020, 79, 478. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Sparks, D.L. Methods of Soil Analysis. Part 3: Chemical Methods; SSSA Series; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Douibi, M.; Krishtammagari, A.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Mulching vs. organic soil amendment: Effects on adsorption-desorption of herbicides. Sci. Total Environ. 2023, 892, 164749. [Google Scholar] [CrossRef]
- FOCUS, FOrum for Co-Ordination of Pesticide Fate Models and Their Use Guidance. Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration. The Final Report of the Work Group on Degradation Kinetics of FOCUS: Sanco/10058/2005, Version 2. 2006. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 1 March 2024).
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance S-metolachlor exluding the assessment of the endocrine disrupting properties. EFSA J. 2023, 21, 7851. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Ordax, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. The role of two organic amendments to modify the environmental fate of S-metolachlor in agricultural soils. Environ. Res. 2021, 195, 110871. [Google Scholar] [CrossRef]
- Carpio, M.J.; Rodríguez-Cruz, M.S.; García-Delgado, C.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Mobility monitoring of two herbicides in amended soils: A field study for modeling applications. J. Environ. Manag. 2020, 260, 110161. [Google Scholar] [CrossRef] [PubMed]
- Willkommen, S.; Pfannerstill, M.; Ulrich, U.; Guse, B.; Fohrer, N. How weather conditions and physico-chemical properties control the leaching of flufenacet, diflufenican, and pendimethalin in a tile-drained landscape. Agr. Ecosyst. Environ. 2019, 278, 107–116. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Singh, N.; Singh, S.B. Effect of fly ash amendment on metolachlor and atrazine degradation and microbial activity in two soils. Environ. Monit. Assess. 2016, 188, 482. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of organic residues on pesticide behavior in soils: A Review of laboratory research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Peña, D.; Albarrán, A.; Gómez, S.; Fernández-Rodríguez, D.; Manuel Rato-Nunes, J.; López-Piñeiro, A. Effects of olive mill wastes with different degrees of maturity on behaviour of S-metolachlor in three soils. Geoderma 2019, 348, 86–96. [Google Scholar] [CrossRef]
- Rusinamhodzi, L. Crop rotations and residue management in conservation agriculture. In Conservation Agriculture, 1st ed.; Farooq, M., Siddique, K.H.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 21–37. [Google Scholar] [CrossRef]
- Douibi, M. Coupling Pesticides Fate Modelling and Conservation Cropping Systems to Reduce the Agriculture Footprints on Ecosystems. Ph.D. Thesis, University of Salamanca, Salamanca, Spain, 2023. Available online: http://hdl.handle.net/10261/350430 (accessed on 18 March 2024).
- Cao, P.; Wang, X.; Liu, F.; Zhao, E.; Han, L. Dissipation and residue of S-metolachlor in maize and soil. Bull. Environ. Contam. Toxicol. 2008, 80, 391–394. [Google Scholar] [CrossRef]
- Zemolin, C.R.; Avila, L.A.; Cassol, G.V.; Massey, J.H.; Camargo, E.R. Environmental fate of S-Metolachlor: A review. Planta Daninha 2014, 32, 655–664. [Google Scholar] [CrossRef]
- Douibi, M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Sustainable agricultural practices influence S-metolachlor, foramsulfuron and thiencarbazone-methyl degradation and their metabolites formation. Sci. Total Environ. 2024, 945, 174039. [Google Scholar] [CrossRef]
- Rice, P.; Anderson, T.; Coats, J. Degradation and persistence of metolachlor in soil: Effects of concentration, soil moisture, soil depth, and sterilization. Environ. Toxicol. Chem. 2002, 21, 2640–2648. [Google Scholar] [CrossRef]
- Gish, T.; McKee, L.; Kustas, W. Solar radiation, relative humidity, and soil water effects on metolachlor volatilization. Environ. Sci. Technol. 2005, 39, 5219–5226. [Google Scholar] [CrossRef]
- Gish, T.J.; Prueger, J.H.; Kustas, W.P.; Daughtry, C.S.T.; McKee, L.G.; Russ, A.; Hatfield, J.L. Soil moisture and metolachlor volatilization observations over three years. J. Environ. Qual. 2009, 38, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Prueger, J.H.; Hatfield, J.L.; Sauer, T.J. Field-scale metolachlor volatilization flux estimates from broadcast and banded application methods in Central Iowa. J. Environ. Qual. 1999, 28, 75–81. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance foramsulfuron. EFSA J. 2016, 14, 4421. [Google Scholar]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance thiencarbazone-methyl. EFSA J. 2013, 11, 3270. [Google Scholar]

| Treatment | Fitting Model | k (Days−1) α/β 1 | DT50 (Days) 2,3 | DT90 (Days) | χ2 | r2 |
|---|---|---|---|---|---|---|
| First experimental year | ||||||
| S1+CT | SFO | 1.70 | 30.0 ± 0.5 a | 99.6 ± 2.8 a | 12.7 | 0.956 |
| S1+NT | FOMC | 4.26/54.0 | 9.50 ± 0.3 d | 38.7 ± 1.4 d | 7.50 | 0.996 |
| S2+CT | FOMC | 3.46/96.2 | 21.3 ± 1.0 b | 90.8 ± 2.9 b | 11.3 | 0.974 |
| S2+NT | FOMC | 6.12/122 | 14.7 ± 0.8 c | 55.9 ± 2.30 c | 11.3 | 0.984 |
| Second experimental year | ||||||
| S1+CT | SFO | 1.52 | 20.9 ± 1.8 b | 69.5 ± 3.3 b | 14.0 | 0.968 |
| S1+NT | SFO | 0.64 | 3.60 ± 0.2 c | 11.9 ± 0.2 c | 9.40 | 0.996 |
| S2+CT | FOMC | 3.23/126 | 30.2 ± 2.2 a | 131 ± 4 a | 13.0 | 0.947 |
| S2+NT | FOMC | 1.37/2.52 | 1.70 ± 0.1 d | 11.0 ± 0.3 d | 6.80 | 0.998 |
| Treatment | Fitting Model | k (Days−1) α/β 1 | DT50 (Days) 2,3 | DT90 (Days) | χ2 | r2 |
|---|---|---|---|---|---|---|
| First experimental year | ||||||
| S1+CT | SFO | 0.75 | 4.50 ± 0.1 b | 14.9 ± 0.2 a | 14.7 | 0.920 |
| S1+NT | SFO | 0.92 | 6.30 ± 0.2 ab | 20.9 ± 0.6 a | 14.3 | 0.978 |
| S2+CT | SFO | 0.90 | 6.00 ± 1.0 ab | 20.0 ± 7.8 a | 14.3 | 0.893 |
| S2+NT | SFO | 1.13 | 9.50 ± 1.6 a | 31.6 ± 5.2 a | 12.9 | 0.982 |
| Second experimental year | ||||||
| S1+CT | SFO | 0.80 | 5.00 ± 0.2 b | 16.4 ± 0.7 a | 14.9 | 0.960 |
| S1+NT | FOMC | 2.81/13.1 | 3.70 ± 1.3 b | 16.6 ± 3.3 a | 4.80 | 1.000 |
| S2+CT | SFO | 1.09 | 8.80 ± 2.3 a | 29.3 ± 7.6 a | 14.6 | 0.960 |
| S2+NT | FOMC | 13.4/96.8 | 5.10 ± 0.8 b | 18.1 ± 0.3 a | 5.70 | 1.000 |
| Treatment | Fitting Model | k (Days−1) α/β 1 | DT50 (Days) 2,3 | DT90 (Days) | χ2 | r2 |
|---|---|---|---|---|---|---|
| First experimental year | ||||||
| S1+CT | SFO | 1.23 | 11.6 ± 2.8 c | 38.4 ± 9.3 c | 13.4 | 0.986 |
| S1+NT | SFO | 1.33 | 14.4 ± 2.2 bc | 47.8 ± 7.5 bc | 14.8 | 0.972 |
| S2+CT | SFO | 1.68 | 28.6 ± 3.0 ab | 95.1 ± 10.0 ab | 13.7 | 0.939 |
| S2+NT | SFO | 1.84 | 39.5 ± 5.4 a | 131 ± 18 a | 13.7 | 0.830 |
| Second experimental year | ||||||
| S1+CT | SFO | 1.46 | 18.7 ± 0.4 b | 62.1 ± 1.2 a | 8.40 | 0.986 |
| S1+NT | FOMC | 2.20/17.7 | 6.60 ± 0.5 c | 32.7 ± 1.7 b | 8.60 | 0.996 |
| S2+CT | SFO | 1.60 | 24.3 ± 0.8 a | 80.8 ± 4.7 a | 10.4 | 0.978 |
| S2+NT | FOMC | 1.15/6.30 | 5.20 ± 2.1 c | 40.2 ± 9.5 b | 14.5 | 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douibi, M.; Carpio, M.J.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Control of the Field Herbicide Dissipation by Cover Crop Mulch in Conservation Agriculture. Agronomy 2024, 14, 2284. https://doi.org/10.3390/agronomy14102284
Douibi M, Carpio MJ, Rodríguez-Cruz MS, Sánchez-Martín MJ, Marín-Benito JM. Control of the Field Herbicide Dissipation by Cover Crop Mulch in Conservation Agriculture. Agronomy. 2024; 14(10):2284. https://doi.org/10.3390/agronomy14102284
Chicago/Turabian StyleDouibi, Marwa, María José Carpio, M. Sonia Rodríguez-Cruz, María J. Sánchez-Martín, and Jesús M. Marín-Benito. 2024. "Control of the Field Herbicide Dissipation by Cover Crop Mulch in Conservation Agriculture" Agronomy 14, no. 10: 2284. https://doi.org/10.3390/agronomy14102284
APA StyleDouibi, M., Carpio, M. J., Rodríguez-Cruz, M. S., Sánchez-Martín, M. J., & Marín-Benito, J. M. (2024). Control of the Field Herbicide Dissipation by Cover Crop Mulch in Conservation Agriculture. Agronomy, 14(10), 2284. https://doi.org/10.3390/agronomy14102284








